Abstract
Anthropogenic accumulation of metals such as manganese is a well-established health risk factor for vertebrates. By contrast, the long-term impact of these contaminants on invertebrates is mostly unknown. Here, we demonstrate that manganese ingestion alters brain biogenic amine levels in honeybees and fruit flies. Furthermore, we show that manganese exposure negatively affects foraging behaviour in the honeybee, an economically important pollinator. Our findings indicate that in addition to its direct impact on human health, the common industrial contaminant manganese might also have indirect environmental and economical impacts via the modulation of neuronal and behavioural functions in economically important insects.
Keywords: dopamine, biogenic amines, Apis mellifera, Drosophila melanogaster, fruit fly
1. Introduction
The possible impact of environmental contaminants on human health is often measured only in terms of its direct impact on human biology [1]. By contrast, their possible indirect impact on human health via negative effects on other organisms is often ignored. Consequently, some environmental pollutants could have a significant effect on human health by affecting, for example, pollinators of important food crops even when present at levels that are not considered toxic.
Understanding the possible negative impact of metals such as manganese on insects could be important when considering the alarming reports on the continual loss of insect pollinators [2], which include the honeybee [3]. As honeybees bring nectar and pollen back to the nest where it is concentrated before being consumed [4,5], this can lead to the accumulation of contaminants such as metals in both honey and bee tissues [5,6]. Previous studies have shown that some metals can affect the responsiveness of honeybees to sucrose [7] without an impact on their visitation rates of contaminated flowers [8,9]. These data suggest that in areas where metals are present in nectars, bees are likely to carry them back to their hive. For example, elements such as selenium, aluminium and nickel can have an impact on behaviours of honeybees, bumblebees and other pollinators [7–10]. By contrast, the impacts of common anthropogenic metal pollutants such as manganese on bee health are not as well understood, despite their well-known effects on the physiology of plants [11] and vertebrates [12].
Previous work indicated that exposure to Mn2+ affects feeding behaviour of bees and flies [13,14] and is associated with changes in their brain transcriptome [15]. In addition, the concentration coefficient for Mn2+, defined as tissue accumulation relative to amounts consumed, is higher for honeybees than that of all other metals studied to date [16]. Because excessive Mn2+ levels have been found in commercial honeys, and its levels in honeys reflect the levels seen in the immediate environment [16,17], relatively small increases in environmental levels of Mn2+ could lead to significantly higher accumulation of this metal in honeybee tissues relative to other metal ions. As exposure to excessive Mn2+ levels affect biogenic amine signalling in the mammalian brain [18], and biogenic amines are key modulators of honeybee foraging [19], we investigated the possible impact of dietary Mn2+ on brain aminergic signalling pathways and foraging behaviour in honeybees.
2. Material and methods
We quantified levels of octopamine, dopamine and serotonin from the brains of honeybees (Apis mellifera) and fruit flies (Drosophila melanogaster) fed differing levels of Mn2+ using high-pressure liquid chromatography as described elsewhere [20]. Mn2+ was supplied in either 1.5 M sucrose (bees) or standard Drosophila medium (flies) over a period of 4 days. We tracked the individual bees treated with Mn2+ using an RFID system that allowed us to track foraging activity throughout the lifespan. See electronic supplement material for additional details.
3. Results and discussion
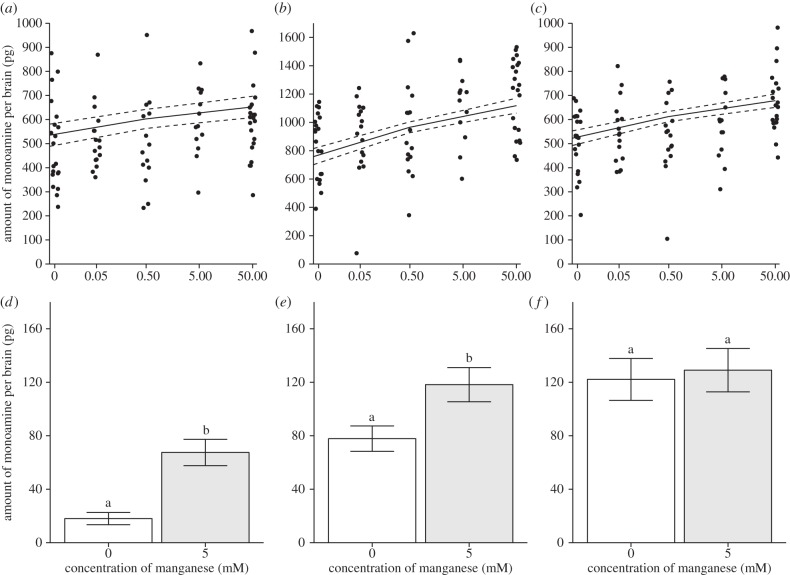
We found that consumption of Mn2+ by honeybees leads to a dose-dependent increase in brain levels of octopamine, dopamine and serotonin (figure 1a–c and table 1). These findings disagree with previous reports in mammalian models and the fruit fly, which showed Mn2+ caused dopaminergic neurotoxicity and reduced levels of dopamine in the brain [21–23]. To confirm that our current observations were not unique to the honeybee, we treated fruit flies with sub-toxic levels of Mn2+ and examined its impact on biogenic amine levels. As in the honeybee, we found that ingestion of 5 mM Mn2+ by Drosophila caused an increase in brain levels of octopamine and dopamine, but not serotonin (figure 1a–f). Together, these results indicate that exposure to Mn2+ at levels that are considered safe for humans can still affect insect behaviour.
Figure 1.
Relationship between Mn2+ treatments and biogenic amine levels. Linear mixed regression estimates (95% CIs) and data for (a) octopamine, (b) dopamine and (c) serotonin in honeybee brains as a function of Mn2+ levels (see table 1 for statistics). Levels of (d) octopamine (t34 = −4.5631, p = 0.00006, n = 18), (e) dopamine (t34 = −2.5393, p = 0.0159, n = 18) and (f) serotonin (t31 = −0.3063, p = 0.7614, n = 18) from fly brains. Data are presented as mean ±s.e. Different letters above bars denote statistical difference.
Table 1.
Linear-mixed regression models for the effect of Mn2+ exposure on biogenic amine levels in honeybees.
| fixed effect | Mn2+ concentration |
||||
|---|---|---|---|---|---|
| dependent variable | d.f. | estimate | s.e. | F | p-value |
| octopamine | 1 | 33.04 | 11.48 | 8.238 | 0.00525 |
| dopamine | 1 | 97.16 | 20.61 | 21.904 | 0.00001 |
| serotonin | 1 | 43.11 | 10.42 | 16.894 | 0.00009 |
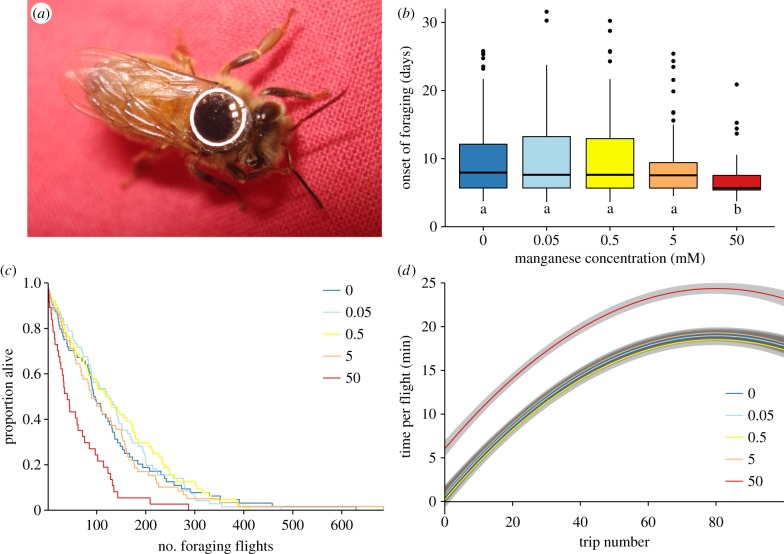
As increased biogenic amines in the honeybee brain are associated with precocious foraging [24,25], we next used the tracking of individual bees to study the effects of Mn2+ treatment on the ontogeny of bee foraging (figure 2a). Similar to our previous report [14], here we found that honeybees treated with 50 mM Mn2+ showed a precocious transition from in-hive behaviours to foraging (χ2 = 25.4636, d.f. = 4, p < 0.0001; figure 2b; electronic supplementary material, figure S1A). Surprisingly, precocious foragers completed significantly fewer foraging trips over their lifetime (χ2 = 17.6, d.f. = 1, p < 0.0001; figure 2c; electronic supplementary material, figure S1B), which suggests that long-term exposure of beehives to Mn2+ could negatively affect colony fitness. Furthermore, although all treatment groups increased the length of their foraging trips over time, the initial trips of 50 mM Mn2+-treated bees were significantly longer (F6,393 = 85.55, p < 0.000001, R2 = 0.51, figure 2d; electronic supplementary material, figure S1C).
Figure 2.
Effects of Mn2+ on honeybee foraging. (a) Honeybee forager tagged with an RFID transponder. (b) Boxplots show age at onset of foraging for bees treated with 0–50 mM Mn2+ (χ2 = 25.4634, d.f. = 4, p < 0.0001). Different lower case letters below bars denote statistically different groups. (c) Kaplan–Meier survival curves showing the number of foraging trips completed by honeybees treated with 0–50 mM Mn2+ between onset of foraging and death (χ2 = 17.6, d.f. = 1, p < 0.0001). (d) Polynomial regression of the relationship between time spent outside the hive per foraging trip and number of foraging trips taken (F6,393 = 85.55, p < 0.001, R2 = 0.51).
Our studies support a model in which Mn2+ treatment leads to an early transition to foraging by increasing brain aminergic signalling, which is in agreement with previous studies of biogenic amines in honeybees [19]. However, our findings are also in contrast to previous studies in mammals [18] and Drosophila [21], which indicated that Mn2+ exposure leads to dopaminergic neuronal loss, and overall reduced levels of dopamine in the brain. We do not yet understand the main reason for the differences between our current findings and previous reports. Noteworthy, the Mn2+ doses we have used in our studies were far below previously reported neurotoxic levels [21]. As a result, these previously published data together with our current findings suggest that the interaction of Mn2+ with biogenic amine signalling and behaviour comprises two phases: exposure to low Mn2+ levels leads to an increase in biogenic amine synthesis but, once above the neurotoxic threshold, it leads to a reduction in biogenic amine levels.
Our finding that Mn2+ treatment leads to extended initial foraging trips suggest that Mn2+-induced precocious foraging might be associated with decreased navigational abilities or lower physical fitness. As increase in time spent on individual foraging flights has previously been linked to declining health and decreased navigational abilities of foragers [26], our findings further support the hypothesis that exposure to even low levels of Mn2+ could affect the long-term health of bees.
As Mn2+ induces precocious foraging and the foraging performance of precocious foragers is significantly lower than typical-age foragers [27], our data indicate that in addition to the increased environmental pressures from parasites, pathogens, insecticides and modern agricultural practices on the health of pollinators [2], it is important to consider other potential anthropogenic factors such as metal pollution as possible risk factors. Consequently, better understanding of these factors would lead to improved risk assessment, and improved management practices of pollinators and other beneficial invertebrates.
Supplementary Material
Acknowledgements
A. Taylor developed the RFID monitoring software. Photo insert used in figure 2a was from N. Even. We thank G. E. Robinson, B. G. Fanson and members of the Ben-Shahar laboratory for valuable feedback on earlier versions of the manuscript.
Data accessibility
All data has been deposited in Dryad (doi:10.5061/dryad.9j0j8).
Author contributions
E.S., Y.B.-S. and A.B.B. designed experiments, analysed data and wrote the paper. E.S., C.J.P. and A.L.M. conducted experiments.
Funding statement
This project was funded by Australian Research Council grant no. DP0986021 (A.B.B.) and Children's Discovery Institute grant no. MD-II-2009-170 (Y.B.-S.).
Conflict of interests
We have no competing interests.
References
- 1.Briggs D. 2003. Environmental pollution and the global burden of disease. Br. Med. Bull. 68, 1–24. ( 10.1093/bmb/ldg019) [DOI] [PubMed] [Google Scholar]
- 2.Gallai N, Salles J-M, Settele J, Vaissière BE. 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68, 810–821. ( 10.1016/j.ecolecon.2008.06.014) [DOI] [Google Scholar]
- 3.Hoshiba H, Sasaki M. 2008. Perspectives of multi-modal contribution of honeybee resources to our life. Entomol. Res. 38, S15–S21. ( 10.1111/j.1748-5967.2008.00170.x) [DOI] [Google Scholar]
- 4.Silici S, Uluozlu OD, Tuzen M, Soylak M. In press. Honeybee and honey as monitors for heavy metal contamination near the thermal power plants in Mugla, Turkey. Toxicol. Ind. Health. ( 10.1177/0748233713503393) [DOI] [PubMed] [Google Scholar]
- 5.Celli G, Maccagnani B. 2003. Honey bees as bioindicators of environmental pollution. Bull. Insectol. 56, 137–139. [Google Scholar]
- 6.Leita L, Muhlbachova G, Cesco S, Barbattini R, Mondini C. 1996. Investigation of the use of honey bees and honey bee products to assess heavy metals contamination. Environ. Monit. Assess. 43, 1–9. ( 10.1007/BF00399566) [DOI] [PubMed] [Google Scholar]
- 7.Hladun KR, Smith BH, Mustard JA, Morton RR, Trumble JT. 2012. Selenium toxicity to honey bee (Apis mellifera L.) pollinators: effects on behaviors and survival. PLoS ONE 7, 1–10. ( 10.1371/journal.pone.0034137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hladun KR, Parker DR, Tran KD, Trumble JT. 2013. Effects of selenium accumulation on phytotoxicity, herbivory, and pollination ecology in radish (Raphanus sativus L.). Environ. Pollut. 172, 70–75. ( 10.1016/j.envpol.2012.08.009) [DOI] [PubMed] [Google Scholar]
- 9.Quinn CF, et al. 2011. Selenium accumulation in flowers and its effects on pollination. New Phytol. 192, 727–737. ( 10.1111/j.1469-8137.2011.03832.x) [DOI] [PubMed] [Google Scholar]
- 10.Meindl GA, Ashman TL. 2013. The effects of aluminum and nickel in nectar on the foraging behavior of bumblebees. Environ. Pollut. 177, 78–81. ( 10.1016/j.envpol.2013.02.017) [DOI] [PubMed] [Google Scholar]
- 11.Clemens S. 2006. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88, 1707–1719. ( 10.1016/j.biochi.2006.07.003) [DOI] [PubMed] [Google Scholar]
- 12.Valko M, Morris H, Cronin MTD. 2005. Metals, toxicity and oxidative stress. Curr. Med. Chem. 12, 1161–1208. ( 10.2174/0929867053764635) [DOI] [PubMed] [Google Scholar]
- 13.Orgad S, Nelson H, Segal D, Nelson N. 1998. Metal ions suppress the abnormal taste behavior of the Drosophila mutant malvolio. J. Exp. Biol. 201, 115–120. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Shahar Y, Dudek NL, Robinson GE. 2004. Phenotypic deconstruction reveals involvement of manganese transporter malvolio in honey bee division of labor. J. Exp. Biol. 207, 3281–3288. ( 10.1242/jeb.01151) [DOI] [PubMed] [Google Scholar]
- 15.Whitfield CW, Ben-Shahar Y, Brillet C, Leoncini I, Crauser D, Leconte Y, Rodriguez-Zas S, Robinson GE. 2006. Genomic dissection of behavioral maturation in the honey bee. Proc. Natl Acad. Sci. USA 103, 16 068–16 075. ( 10.1073/pnas.0606909103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashed MN, Soltan ME. 2004. Major and trace elements in different types of Egyptian mono-floral and non-floral bee honeys. J. Food Compos. Anal. 17, 725–735. ( 10.1016/j.jfca.2003.10.004) [DOI] [Google Scholar]
- 17.Van der Steen JJM, de Kraker J, Grotenhuis T. 2012. Spatial and temporal variation of metal concentrations in adult honeybees (Apis mellifera L.). Environ. Monit. Assess. 184, 4119–4126. ( 10.1007/s10661-011-2248-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda A. 2003. Manganese action in brain function. Brain Res. Rev. 41, 79–87. ( 10.1016/S0165-0173(02)00234-5) [DOI] [PubMed] [Google Scholar]
- 19.Barron AB, Schulz DJ, Robinson GE. 2002. Octopamine modulates responsiveness to foraging-related stimuli in honey bees (Apis mellifera). J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 188, 603–610. ( 10.1007/s00359-002-0335-5) [DOI] [PubMed] [Google Scholar]
- 20.Søvik E, Cornish JL, Barron AB. 2013. Cocaine tolerance in honey bees. PLoS ONE 8, e64920 ( 10.1371/journal.pone.0064920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonilla-Ramirez L, Jimenez-Del-Rio M, Velez-Pardo C. 2011. Acute and chronic metal exposure impairs locomotion activity in Drosophila melanogaster: a model to study Parkinsonism. Biometals 24, 1045–1057. ( 10.1007/s10534-011-9463-0) [DOI] [PubMed] [Google Scholar]
- 22.Komura J, Sakamoto M. 1992. Effects of manganese forms on biogenic amines in the brain and behavioral alterations in the mouse: long-term oral administration of several manganese compounds. Environ. Res. 44, 34–44. ( 10.1016/S0013-9351(05)80017-9) [DOI] [PubMed] [Google Scholar]
- 23.Roth JA, Li Z, Sridhar S, Khoshbouei H. 2013. The effect of manganese on dopamine toxicity and dopamine transporter (DAT) in control and DAT transfected HEK cells. Neurotoxicology 35, 121–128. ( 10.1016/j.neuro.2013.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagener-Hulme C, Kuehn JC, Schulz DJ, Robinson GE. 1999. Biogenic amines and division of labor in honey bee colonies. J. Comp. Physiol. A 184, 471–479. ( 10.1007/s003590050348) [DOI] [PubMed] [Google Scholar]
- 25.Schulz DJ, Barron AB, Robinson GE. 2002. A role for octopamine in honey bee division of labor. Brain Behav. Evol. 60, 350–359. ( 10.1159/000067788) [DOI] [PubMed] [Google Scholar]
- 26.Wolf S, McMahon DP, Lim KS, Pull CD, Clark SJ, Paxton RJ, Osborne JL. 2014. So near and yet so far: harmonic radar reveals reduced homing ability of nosema infected honeybees. PLoS ONE 9, e103989 ( 10.1371/journal.pone.0103989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry CJ, Søvik E, Myerscough MR, Barron AB. In press. Rapid behavioral maturation accelerates failure of stressed honey bee colonies. Proc. Natl Acad. Sci. USA, 201422089 ( 10.1073/pnas.1422089112) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data has been deposited in Dryad (doi:10.5061/dryad.9j0j8).