Abstract
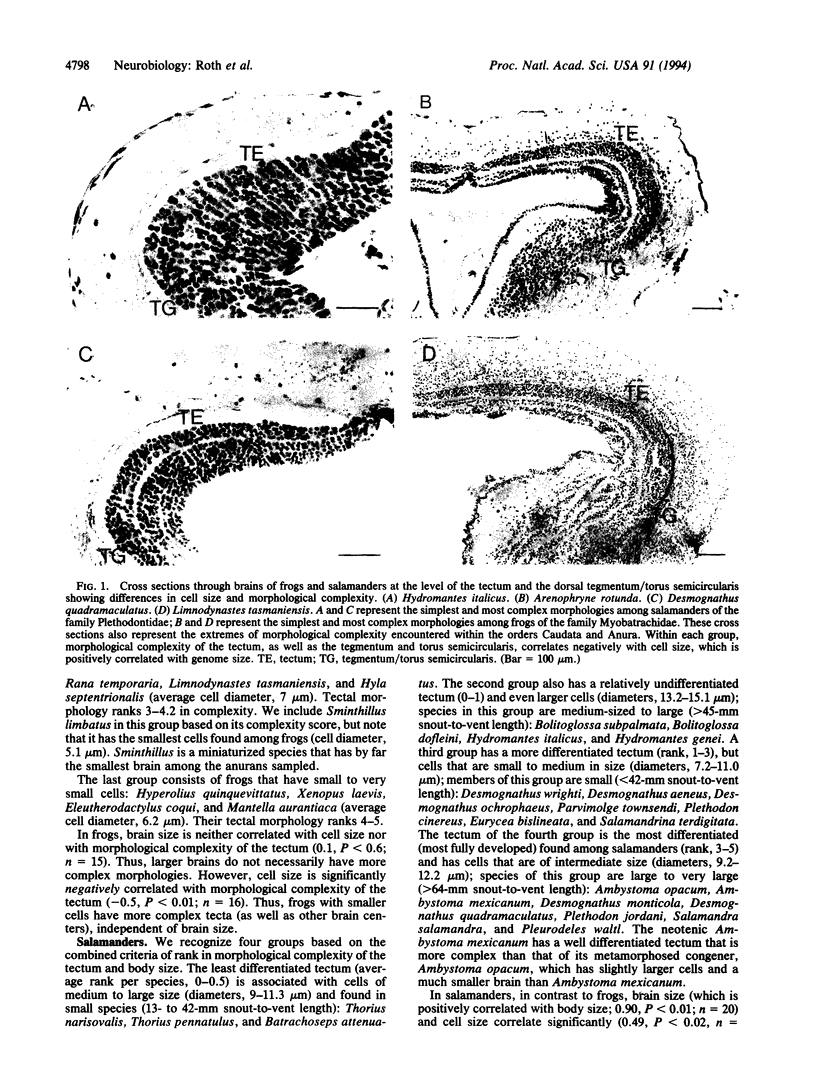
The morphological organization of the brain of frogs and salamanders varies greatly in the degree to which it is subdivided and differentiated. Members of these taxa are visually oriented predators, but the morphological complexity of the visual centers in the brain varies interspecifically. We give evidence that the morphological complexity of the amphibian tectum mesencephali, the main visual center, can be predicted from knowledge of cell size, which varies greatly among these taxa. Further, cell size is highly correlated with genome size. Frogs with small cells have more complex morphologies of the tectum than do those with large cells independent of body and brain size. In contrast, in salamanders brain-body size relationships also are correlated with morphological complexity of the brain. Small salamanders with large cells have the simplest tecta, whereas large salamanders with small cells exhibit the most complex tectal morphologies. Increases in genome, and consequently cell size, are associated with a decrease in the differentiation rate of nervous tissue, which leads to the observed differences in brain morphology. On the basis of these findings we hypothesize that important features of the structure of the brain can arise independently of functional demands, from changes at a lower level of organismal organization--in this case increase in genome size, which induces simplification of brain morphology.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavalier-Smith T. Nuclear volume control by nucleoskeletal DNA, selection for cell volume and cell growth rate, and the solution of the DNA C-value paradox. J Cell Sci. 1978 Dec;34:247–278. doi: 10.1242/jcs.34.1.247. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature. 1980 Apr 17;284(5757):601–603. doi: 10.1038/284601a0. [DOI] [PubMed] [Google Scholar]
- Hally M. K., Rasch E. M., Mainwaring H. R., Bruce R. C. Cytophotometric evidence of variation in genome size of desmognathine salamanders. Histochemistry. 1986;85(3):185–192. doi: 10.1007/BF00494802. [DOI] [PubMed] [Google Scholar]
- Horner H. A., Macgregor H. C. C value and cell volume: their significance in the evolution and development of amphibians. J Cell Sci. 1983 Sep;63:135–146. doi: 10.1242/jcs.63.1.135. [DOI] [PubMed] [Google Scholar]
- KLUVER H., BARRERA E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. 1953 Oct;12(4):400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- Naujoks-Manteuffel C., Manteuffel G. Origins of descending projections to the medulla oblongata and rostral medulla spinalis in the urodele Salamandra salamandra (amphibia). J Comp Neurol. 1988 Jul 8;273(2):187–206. doi: 10.1002/cne.902730205. [DOI] [PubMed] [Google Scholar]
- Neary T. J., Northcutt R. G. Nuclear organization of the bullfrog diencephalon. J Comp Neurol. 1983 Jan 20;213(3):262–278. doi: 10.1002/cne.902130303. [DOI] [PubMed] [Google Scholar]
- Olmo E. Nucleotype and cell size in vertebrates: a review. Basic Appl Histochem. 1983;27(4):227–256. [PubMed] [Google Scholar]
- Orgel L. E., Crick F. H. Selfish DNA: the ultimate parasite. Nature. 1980 Apr 17;284(5757):604–607. doi: 10.1038/284604a0. [DOI] [PubMed] [Google Scholar]
- Pagel M., Johnstone R. A. Variation across species in the size of the nuclear genome supports the junk-DNA explanation for the C-value paradox. Proc Biol Sci. 1992 Aug 22;249(1325):119–124. doi: 10.1098/rspb.1992.0093. [DOI] [PubMed] [Google Scholar]
- Potter H. D. Structural characteristics of cell and fiber populations in the optic tectum of the frog (Rana catesbeiana). J Comp Neurol. 1969 Jun;136(2):203–231. doi: 10.1002/cne.901360207. [DOI] [PubMed] [Google Scholar]
- Roth G., Naujoks-Manteuffel C., Grunwald W. Cytoarchitecture of the tectum mesencephali in salamanders: a Golgi and HRP study. J Comp Neurol. 1990 Jan 1;291(1):27–42. doi: 10.1002/cne.902910104. [DOI] [PubMed] [Google Scholar]
- Roth G., Nishikawa K. C., Naujoks-Manteuffel C., Schmidt A., Wake D. B. Paedomorphosis and simplification in the nervous system of salamanders. Brain Behav Evol. 1993;42(3):137–170. doi: 10.1159/000114147. [DOI] [PubMed] [Google Scholar]
- Szarski H. Cell size and nuclear DNA content in vertebrates. Int Rev Cytol. 1976;44:93–111. doi: 10.1016/s0074-7696(08)61648-4. [DOI] [PubMed] [Google Scholar]
- Szarski H. Cell size and the concept of wasteful and frugal evolutionary strategies. J Theor Biol. 1983 Nov 21;105(2):201–209. doi: 10.1016/s0022-5193(83)80002-2. [DOI] [PubMed] [Google Scholar]
- Thexton A. J., Wake D. B., Wake M. H. Tongue function in the salamander Bolitoglossa occident alis. Arch Oral Biol. 1977;22(6):361–366. doi: 10.1016/0003-9969(77)90057-7. [DOI] [PubMed] [Google Scholar]