Abstract
Mitochondrial genes are part of the oxidative phosphorylation pathway and important for energy production. Although evidence for positive selection at the mitochondrial level exists, few studies have investigated the link between amino acid changes and phenotype. Here we test the hypothesis that differences in two life-history related traits, migratory distance between spawning and foraging areas and larval phase duration, are associated with divergent selection within the mitochondrial ATP6 gene in anguillid eels. We compare amino acid changes among 18 species with the sequence of the putative ancestral species, believed to have shown short migratory distance and larval phase duration. We find positive correlations between both life-history related traits and (i) the number of amino acid changes and (ii) the strength of the combined physico-chemical and structural changes at positions previously identified as candidates for positive selection. This supports a link between genotype and phenotype driven by positive selection at ATP6.
Keywords: anguillid eels, ATP6, positive selection
1. Introduction
The mitochondrial genome (mitogenome) consists of 13 coding genes directly involved in the oxidative phosphorylation pathway and the production of energy (ATP) [1]. Although the mitogenome historically has been assumed to evolve neutrally [2], multiple studies show evidence for positive selection acting on mitochondrial genes in mammals [3,4] and fishes (e.g. [5]). So far, however, few studies have provided solid evidence for positive selection by demonstrating direct links between amino acid changes and phenotype.
Freshwater eels (Anguilla spp.) constitute excellent candidates for investigating positive selection at mitogenomes and its effect on phenotype. The genus encompasses several species [6], which furthermore show high effective population sizes (e.g. [7,8]). Thus, selection is likely to be a stronger evolutionary force than genetic drift in these species. Positive selection at the mitochondrial ATP6 gene has been shown between the American (Anguilla rostrata) and European eel (Anguilla anguilla) based on McDonald–Kreitman tests [8,9]. Moreover, evidence for positive diversifying selection has been reported at codon 52 in ATP6 across all species of freshwater eels, whereas such consistent patterns of selection are not present at other mitochondrial genes [8].
ATP6 is a subunit of the F0-proton channel in ATP synthase, the fifth and final complex of the oxidative phosphorylation chain which catalyses synthesis of ATP from ADP [1]. Changes within ATP6 have been associated with differences in metabolism and selection linked to energetics [3,4,10]. In freshwater eels, selection may be linked to their migratory loops [8,11]. These involve spawning migration (from freshwater or coastal regions to the ocean) of highly variable distances in different species and consequently also a highly variable larval phase, as larvae rely on ocean currents for advection back to the continental feeding grounds [6,11]. Thus, energy production linked to the spawning migration, or migration or metabolism during the larval stage, may underlie selection at ATP6.
The ancestor of all current eel species presumably lived in southeast Asia or Africa [6,11] around 14–18 Ma [8,12]. As most of the species found there show a short migratory loop, this is believed to be the ancestral state [11,13]. If length of spawning migration and larval phase duration underlie directional selection at ATP6, this should be reflected in more fixed non-synonymous changes and changes of stronger physico-chemical and structural importance at key positions in species with longer migratory loops. We test this by assessing the correlation of amino acid changes from the putative ancestral sequence with migratory distance or larval phase duration of species.
2. Material and methods
Twenty-one mitogenome sequences were downloaded from GenBank: 18 species of freshwater eels (GenBank accession nos NC_002707, NC_006531–NC_006547) and three species of Serrivomeridae, the family most closely related to Anguillidae [14] (NC_013436, NC_013627–NC_013628). Sequences were aligned in geneious pro v. 5.4.6 [15] and the 13 coding genes were extracted and concatenated. The ancestral sequence of all freshwater eels was reconstructed using the Ancestral State Reconstruction software (ASR) [16] implemented in the hyphy package on the DataMonkey server (http://www.datamonkey.org/dataupload.php) (electronic supplementary material, note S1). Synonymous and non-synonymous changes and dN/dS between the putative ancestral sequence and each species were calculated using DnaSP v. 5.1 [17].
Larval phase duration (estimated by age at metamorphosis of larvae based on Sr/Ca ratio increments in otoliths) and migratory distance to the spawning ground were obtained from the literature (electronic supplementary material, table S1). Mean migratory distances were based on estimates from Aoyama [11] or estimated in the case of the New Zealand eel (Anguilla differenbachii) (electronic supplementary material, note S2). When several estimates were available, the average among studies was used (see electronic supplementary material, note S2 and table S1). For the European and American eels, significant discrepancies exist between studies [18]. Hence, the mean estimates from Wang & Tzeng [19] were chosen, as those estimates were derived from the largest sample sizes to date and accord well with studies that have estimated larval phase duration based on ocean current transportation [20].
Correlations between genotype and either migratory distance or larval phase duration were tested using linear regression or Spearman rank analysis in past [21]. All data were checked for normality prior to parametric statistical analysis and a logarithmic transformation was conducted if necessary (electronic supplementary material, table S2). A possible correlation between migratory distance and larval phase duration was tested to avoid pseudoreplication using both the extant species' trait values and an analysis of independent contrasts (CAIC) correcting for phylogenetic relatedness (electronic supplementary material, note S3) [22].
Genotype was represented by different datasets: (i) total non-synonymous (amino acid changing) and synonymous changes and dN/dS considering all 13 genes, (ii) total amino acid changes at ATP6 and (iii) within five amino acid positions fixed between the American and European eels possibly under positive selection [8,9]. Differences in properties of amino acid replacements between the ancestral and species sequences were assessed using 11 physico-chemical and four structural properties [23]. The numerical differences of properties were summed to produce an overall score. This was conducted for datasets 2 and 3 and for codon position 52, which shows evidence for diversifying selection across the entire phylogeny of freshwater eels [8].
3. Results and discussion
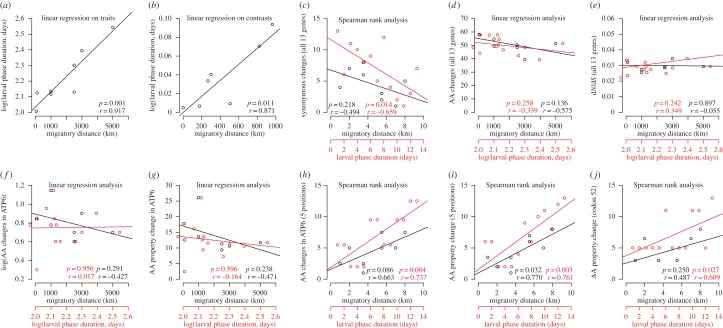
Larval phase duration and migratory distance were highly correlated even when correcting for phylogenetic relationships (figure 1a,b), making us unable to make reliable inferences about which of these two traits selection is acting upon.
Figure 1.
Correlation analyses. Each plot shows data along with the best-fitted line, correlation coefficient and p-values. When multiple plots are present the colours match the x-axis. Number of changes and property scores represent the overall difference between the current species and the putative ancestral sequence. AA denotes amino acid (non-synonymous substitutions).
Number of amino acid changes and their overall difference in physico-chemical and structural properties within ATP6 did not correlate with either trait (figure 1f,g). However, when analysing the positions previously found to be involved in positive selection between A. anguilla and A. rostrata [8,9], significant positive correlations were observed. Currently, there is no high-resolution three-dimensional structure for ATP synthase available, which precludes prediction of the exact functional importance of the five positions. However, the positions are likely to cover important roles for protein function in the ATP6 gene in freshwater eels. Three of the positions (83, 105 and 195) are situated in transmembrane domains (electronic supplementary material, figure S1) that are highly conserved in mammals [3], and are thus likely to cover important regions. Position 195 is located in the last transmembrane domain, where mutation is associated with severe decrease in the rate of ATP production in humans [10]. Finally, positions 43 and 52 are found in a region likely to be under positive selection in mammals [3].
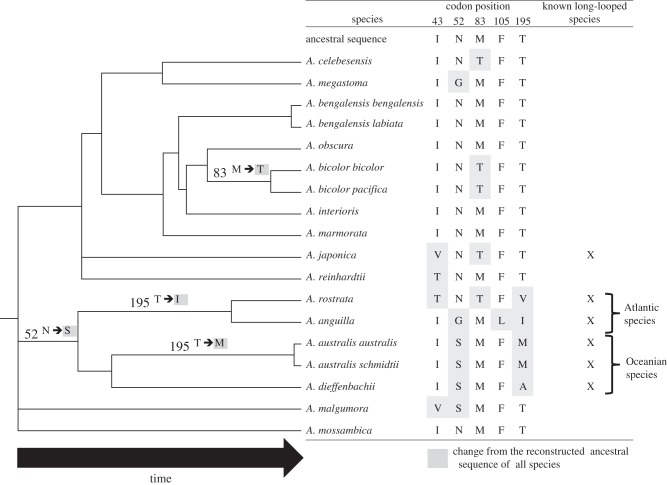
A significant correlation was found between the amino acid property score and duration of larval phase at codon 52. However, the association was mainly driven by A. anguilla (figure 1j, table 1). This position has been shown to be under positive diversifying selection across the 18 freshwater eels [8] and may represent an important site for protein function. However, it seems likely that positive selection involves multiple sites. This is further supported by results at the five candidate positions in ATP6 [8,9]. Within these sites, both the number and property score of derived amino acids show significant positive correlations with the examined traits (figure 1h,i). Interestingly, at these positions the derived amino acids relative to the ancestral reconstructed sequence are highly polymorphic between species with long migratory loops. This is also the case between long-looped Atlantic and Oceanian species that are sister clades (e.g. [8,12]; figure 2). This suggests that evolution at these sites is not related to the phylogenetic relationship and that the observed changes may be adaptive, occurring after species divergence. Moreover, the immediate ancestral nodes of the current long-looped Atlantic and Oceanian species also show multiple changes within the five candidate positions (figure 2). As these ancestors likely showed long migratory loops this result further supports positive selection within ATP6.
Table 1.
Estimates for each species of migratory distances, larval phase duration and genetic change from the putative ancestral sequence. For calculations and references, see electronic supplementary material, note S1 and table S1.
| overall number of changes (all 13 genes) |
number of amino acid changes in ATP6 |
overall amino acid property change in ATP6 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| species | migratory distance mean (km) | larval phase duration mean (days) | synonymous | non-synonymous | overall dN/dS (all 13 genes) | all sites | five sitesa | all sites | five sitesa | codon 52b |
| A. celebesensis | 50 | 101.50 | 522.17 | 58.17 | 0.0333 | 7 | 1 | 17.822 | 3.332 | 0 |
| A. megastoma | NA | NA | 534.42 | 63.92 | 0.0355 | 7 | 1 | 16.158 | 3.656 | 3.656 |
| A. bengalensis bengalensis/ A. nebulosa nebulosa | NA | NA | 555.75 | 54.92 | 0.0308 | 9 | 0 | 16.118 | 0 | 0 |
| A. bengalensis labiata/ A. nebulosa labiata | NA | 117.50 | 554.00 | 57.67 | 0.0292 | 9 | 0 | 16.118 | 0 | 0 |
| A. obscura | NA | NA | 566.00 | 45.17 | 0.0235 | 5 | 0 | 11.109 | 0 | 0 |
| A. bicolor bicolor | NA | 92.60 | 631.50 | 48.17 | 0.0221 | 6 | 1 | 13.619 | 3.332 | 0 |
| A. bicolor pacifica | 1000 | 136.25 | 619.00 | 54.67 | 0.0255 | 6 | 1 | 13.619 | 3.332 | 0 |
| A. interioris | NA | NA | 539.17 | 46.17 | 0.0254 | 6 | 0 | 13.670 | 0 | 0 |
| A. marmorata | 1000 | 130.77 | 532.00 | 49.33 | 0.0274 | 14 | 0 | 26.073 | 0 | 0 |
| A. japonica | 2500 | 134.56 | 512.67 | 50.33 | 0.0293 | 4 | 2 | 9.397 | 4.555 | 0 |
| A. reinhardtii | NA | 144.50 | 511.00 | 46.67 | 0.0274 | 4 | 1 | 11.602 | 4.663 | 0 |
| A. rostrata | 2500 | 200.00 | 507.50 | 48.5 | 0.0285 | 5 | 3 | 11.355 | 9.228 | 0 |
| A. anguilla | 5000 | 350.20 | 524.00 | 51.33 | 0.0293 | 5 | 3 | 11.712 | 9.585 | 3.656 |
| A. australis australis | NA | 181.59 | 478.67 | 42.33 | 0.0249 | 4 | 2 | 7.275 | 5.148 | 1.816 |
| A. australis schmidtii | NA | NA | 473.17 | 38.83 | 0.0267 | 4 | 2 | 7.275 | 5.148 | 1.816 |
| A. dieffenbachii | 3000 | 248.00 | 354.00 | 39.33 | 0.0344 | 8 | 2 | 10.619 | 3.919 | 1.816 |
| A. malgumora/ A. borneensis | 100 | 133.00 | 537.83 | 57.83 | 0.0320 | 7 | 2 | 12.591 | 3.039 | 1.816 |
| A. mossambica | NA | 102.10 | 556.83 | 44.17 | 0.0235 | 2 | 0 | 2.446 | 0 | 0 |
aCodon positions 43, 52, 83, 105 and 195, candidates for positive selection between Atlantic eels (8,9).
bCandidate for diversifying selection across all species of freshwater eels (8).
Figure 2.
Amino acid evolution in freshwater eels within the five candidate positions investigated. Ancestral changes are shown along the respective phylogenetic branches while current genotypes are shown in the table. The phylogeny is identical to that of Jacobsen et al. [8] using a posterior threshold of 0.90 to determine phylogenetic relationships.
The pattern shown here could also be the outcome of relaxed purifying selection in long-looped species if they have experienced higher genetic drift. As such, evolution in the candidate positions would be slightly deleterious instead of adaptive. However, this is not supported here, as analyses of dN/dS do not show significant correlations with the length of the migratory loop (figure 1e). Moreover, the results are not related to substitution rate. In fact, species with smaller migratory loops show the highest number of substitutions from the ancestral sequence, supporting a higher substitution rate (figure 1c,d). This may reflect the fact that generation time is positively associated with food availability in eels [24], potentially leading to faster generation time and thereby substitution rate in highly productive tropical areas (e.g. [25]), such as those inhabited by most short-looped species (see electronic supplementary material, note S2 for discussion of other possible biases).
Overall, the pattern found in this study is in accordance with the hypothesis that positive selection at a few key positions drove divergence from an ancestral species with a short migratory loop [11]. According to this scenario, species with longer migratory loops show more amino acid changes and higher overall physico-chemical and structural change at ATP6 relative to the putative ancestral sequence. As changes in the migratory loop are likely crucial for understanding speciation in freshwater eels [8,11,13], selection at ATP6 may be particularly important for understanding their evolution. Selection may further lead to cytonuclear incompatibilities, as proposed for Atlantic eels [9], which could lead to reproductive isolation. Consequently, ATP6 may be a key gene underlying speciation in freshwater eels. This suggests that selection at mitochondrial genes may generally be important in groups of organisms characterized by large interspecific differences in migratory properties.
Supplementary Material
Acknowledgements
We thank handling editor Paul Sniegowski and referee Konstantin Popadin and one anonymous referee for constructive comments and suggestions.
Ethical statement
All data used in this paper belong to already published research.
Data accessibility
Reconstructed ancestral sequences and information about estimated larval phase duration and migratory distances are available as electronic supplementary material.
Funding statement
We acknowledge funding from the Danish Council for Independent Research, Natural Sciences (grant no. 09-072120) and Elisabeth og Knud Petersen's Foundation.
Author contributions
M.W.J. conceived and designed the study and conducted the analyses. M.W.J., M.M.H. and J.M.P. wrote the manuscript.
Competing interests
The authors have no competing interests.
References
- 1.Saraste M. 1999. Oxidative phosphorylation at the fin de siecle. Science 283, 1488–1493. ( 10.1126/science.283.5407.1488) [DOI] [PubMed] [Google Scholar]
- 2.Ballard JWO, Whitlock MC. 2004. The incomplete natural history of mitochondria. Mol. Ecol. 13, 729–744. ( 10.1046/j.1365-294X.2003.02063.x) [DOI] [PubMed] [Google Scholar]
- 3.da Fonseca RR, Johnson WE, O'Brien SJ, Ramos MJ, Antunes A. 2008. The adaptive evolution of the mammalian mitochondrial genome. BMC Genom. 9, 119 ( 10.1186/1471-2164-9-119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontanillas P, Depraz A, Giorgi MS, Perrin N. 2005. Nonshivering thermogenesis capacity associated to mitochondrial DNA haplotypes and gender in the greater white-toothed shrew, Crocidura russula. Mol. Ecol. 14, 661–670. ( 10.1111/j.1365-294X.2004.02414.x) [DOI] [PubMed] [Google Scholar]
- 5.Garvin MR, Bielawski JP, Gharrett AJ. 2011. Positive Darwinian selection in the piston that powers proton pumps in complex I of the mitochondria of Pacific salmon. PLoS ONE 6, e24127 ( 10.1371/journal.pone.0024127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tesch F. 2003. The eel. Oxford, UK: Blackwell Science. [Google Scholar]
- 7.Tseng MC, Kao HW, Hung YH, Lee TL. 2012. A study of genetic variations, population size, and population dynamics of the catadromous Japanese eel Anguilla japonica (Pisces) in northern Taiwan. Hydrobiologia 683, 203–216. ( 10.1007/s10750-011-0958-z) [DOI] [Google Scholar]
- 8.Jacobsen MW, Pujolar JM, Gilbert MTP, Moreno-Mayar JV, Bernatchez L, Als TD, Lobon-Cervia J, Hansen MM. 2014. Speciation and demographic history of Atlantic eels (Anguilla anguilla and A. rostrata) revealed by mitogenome sequencing. Heredity 113, 432–442. ( 10.1038/hdy.2014.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagnaire PA, Normandeau E, Bernatchez L. 2012. Comparative genomics reveals adaptive protein evolution and a possible cytonuclear incompatibility between European and American eels. Mol. Biol. Evol. 29, 2909–2919. ( 10.1093/molbev/mss076) [DOI] [PubMed] [Google Scholar]
- 10.Kucharczyk R, Ezkurdia N, Couplan E, Procaccio V, Ackerman SH, Blondel M, di Rago JP. 2010. Consequences of the pathogenic T9176C mutation of human mitochondrial DNA on yeast mitochondrial ATP synthase. Biochim. Biophys. Acta 1797, 1105–1112. ( 10.1016/j.bbabio.2009.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoyama J. 2009. Life history and evolution of migration in catadromous eels (genus Anguilla). Aqua-BioSci. Monogr. 2, 1–42. See http://www.terrapub.co.jp/onlinemonographs/absm/. [Google Scholar]
- 12.Santini F, Kong X, Sorensen L, Carnevale G, Mehta RS, Alfaro ME. 2013. A multi-locus molecular timescale for the origin and diversification of eels (order: Anguilliformes). Mol. Phylogenet. Evol. 69, 884–894. ( 10.1016/j.ympev.2013.06.016) [DOI] [PubMed] [Google Scholar]
- 13.Aoyama J, Wouthuyzen S, Miller MJ, Inagaki T, Tsukamoto K. 2003. Short-distance spawning migration of tropical freshwater eels. Biol. Bull. 204, 104–108. ( 10.2307/1543500) [DOI] [PubMed] [Google Scholar]
- 14.Johnson GD, Ida H, Sakaue J, Sado T, Asahida T, Miya M. 2012. A ‘living fossil’ eel (Anguilliformes: Protanguillidae, fam. nov.) from an undersea cave in Palau. Proc. R. Soc. B 279, 934–943. ( 10.1098/rspb.2011.1289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BioMatters. 2012. Geneious version 5.4.6 See http://www.geneious.com.
- 16.Pond SLK, Frost SDW. 2005. Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol. Biol. Evol. 22, 1208–1222. ( 10.1093/molbev/msi105) [DOI] [PubMed] [Google Scholar]
- 17.Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452. ( 10.1093/bioinformatics/btp187) [DOI] [PubMed] [Google Scholar]
- 18.McCleave JD. 2008. Contrasts between spawning times of Anguilla species estimated from larval sampling at sea and from otolith analysis of recruiting glass eels. Mar. Biol. 155, 249–262. ( 10.1007/s00227-008-1026-8) [DOI] [Google Scholar]
- 19.Wang CH, Tzeng WN. 2000. The timing of metamorphosis and growth rates of American and European eel leptocephali: a mechanism of larval segregative migration. Fish. Res. 46, 191–205. ( 10.1016/S0165-7836(00)00146-6) [DOI] [Google Scholar]
- 20.Kettle AJ, Haines K. 2006. How does the European eel (Anguilla anguilla) retain its population structure during its larval migration across the North Atlantic Ocean? Can. J. Fish. Aquat. Sci. 63, 90–106. ( 10.1139/cjz-2013-0303) [DOI] [Google Scholar]
- 21.Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 9 See http://palaeo-electronica.org/2001_2001/past/issue2001_2001.htm.) [Google Scholar]
- 22.Purvis A, Rambaut A. 1995. Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. Comput. Appl. Biosci. 11, 247–251. ( 10.1093/bioinformatics/11.3.247) [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Kang X. 2012. Grading amino acid properties increased accuracies of single point mutation on protein stability prediction. BMC Bioinform. 13, 44 ( 10.1186/1471-2105-13-44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollestad LA. 1992. Geographic variation in age and length at metamorphosis of maturing European eel: environmental effects and phenotypic plasticity. J. Anim. Ecol. 61, 41–48. ( 10.2307/5507) [DOI] [Google Scholar]
- 25.Arai T, Chino N. 2013. Timing of maturation of a tropical eel, Anguilla bicolor bicolor in Malaysia. J. Appl. Ichthyol. 29, 271–273. ( 10.1111/jai.12040) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Reconstructed ancestral sequences and information about estimated larval phase duration and migratory distances are available as electronic supplementary material.