Abstract
A deficit in brain serotonin is thought to be associated with deteriorated stress coping behaviour, affective disorders and exaggerated violence. We challenged this hypothesis in mice with a brain-specific serotonin depletion caused by a tryptophan hydroxylase 2 (TPH2) deficiency. We tested TPH2-deficient (Tph2−/–) animals in two social situations. As juveniles, Tph2−/− mice displayed reduced social contacts, whereas ultrasonic vocalizations (USVs) were unchanged within same-sex same-genotype pairings. Interestingly, juvenile females vocalized more than males across genotypes. Sexually naive adult males were exposed to fresh male or female urine, followed by an interaction with a conspecific, and re-exposed to urine. Although Tph2−/− mice showed normal sexual preference, they were hyper-aggressive towards their interaction partners and did not vocalize in response to sexual cues. These results highlight that central serotonin is essential for prosocial behaviour, especially USV production in adulthood, but not for sexual preference.
Keywords: serotonin, TPH2, ultrasonic vocalization, social behaviour, aggression
1. Introduction
A deficit in central serotonin (5-HT) is thought to be associated with deteriorated stress coping behaviour, affective disorders and exaggerated violence [1]. In rodents, genetically or pharmacologically induced 5-HT reduction in the central nervous system (CNS) is related to a loss of avoidance behaviour and increased aggression [2,3], and is suggested to cause the loss of sexual preference [4,5]. However, a detailed analysis of social communication deficits associated with the observed behavioural alterations [6] across development is still missing.
We recently generated mice that are constitutively deficient for the rate-limiting enzyme of 5-HT synthesis, tryptophan hydroxylase 2 (TPH2), which is expressed solely in 5-HT-producing cells within the CNS. Mice lacking TPH2 are almost completely devoid of brain 5-HT (less than 4% of wild-type levels), exhibit growth retardation during the first weeks of life but are vital and do not show obvious malfunctions in adulthood, probably owing to compensatory mechanisms evoked by the lifelong absence of 5-HT [7,8]. Here, we used these mice to investigate if central 5-HT is essential for social behaviour and communication in non-violent conditions, i.e. juvenile interaction and sexual behaviour at adult age. Our data reveal an important role of central 5-HT for the expression of (a)social, but not sexual behaviours.
2. Material and methods
Behavioural tests were conducted in wild-type (Tph2+/+), heterozygous (Tph2+/−) and homozygous (Tph2−/−) mutant mice [7] on a highly social C57BL/6N background [2] (see details in the electronic supplementary material). Offspring from Tph2+/− breeding couples were weaned around postnatal day (PND) 21, group housed and maintained on a 12 : 12 light/dark cycle (lights off at 18.00 for juvenile or at 06.00 for adult mice) with standard chow and water ad libitum.
Juvenile social interaction was investigated after 1 day of single housing around PND25 in a new cage. An unfamiliar naive animal of the same sex, age and genotype was introduced after 1 min. Behaviour and ultrasonic vocalizations (USVs) were measured for 5 min under red light to reduce the stress level.
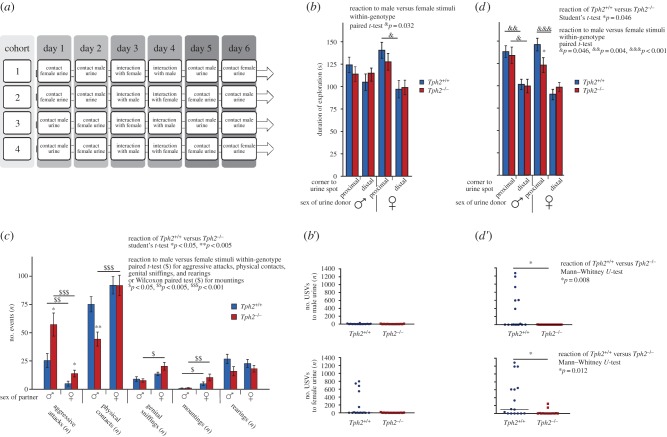
Sexually naive males at 15 weeks of age were singly housed for three weeks in a female-free husbandry room. Behavioural tests were conducted under red light during the dark phase following a modified protocol [9]. On day1 and day2, Tph2+/+ and Tph2−/− mice were cross-balanced exposed for 5 min (figure 2a) to a new cage containing 50 µl of either fresh male or female urine, collected from FVB/N mice a maximum of 4 h earlier. On day3 and day4, animals had 1 min of habituation to a new cage followed by 15 min of cross-balanced interaction with either a male or oestrus-synchronized female FVB/N mouse (group housed, three months of age). Finally, on day5 and day6, sexually experienced mice were re-exposed to urine of each sex. Avisoft Bioacoustics (Germany) and Viewer2 software (Biobserve, Germany) were used to analyse USV emission and behaviour (see details in the electronic supplementary material).
Figure 2.
Social behaviour in adult males. (a) Cross-balanced protocol with four cohorts (n = 3–4 per genotype). (b–d) Behaviour of male Tph2+/+ (blue dots and bars (left), n = 14) and Tph2−/− mice (red dots and bars (right), n = 14) before (b,b′), during (c) and after (d,d′) social interaction. (b,b′,d,d′) Reactions to sexual stimuli exposition (male or female urine) are shown for behaviour (mean ± s.e.m., b,d) and ultrasonic vocalizations (USVs; single dot per animal, median line, b′,d′). Behavioural patterns during social interaction to a male or female partner are shown (mean ± s.e.m., c). Asterisk, dollar and ampersand symbols indicate statistically significant differences between genotypes, between the interaction to male and female partners, and between exploration of proximal and distal corners during urine exposition, respectively. (Online version in colour.)
For comparing social behaviour and USV production between genotypes two-way ANOVAs for repeated measurements with the between-subject factors ‘genotype’ and ‘sex’ were calculated, followed by least significant difference (LSD) post hoc tests when appropriate. To analyse urine preference and social behaviour during dyadic interactions, paired and unpaired t-tests, or non-parametric tests (Mann–Whitney U-test and Wilcoxon paired test) were performed. A p-value of less than 0.05 was considered statistically significant. The Kolmogorov–Smirnov test was used to evaluate if groups meet the Gaussian distribution. Accordingly, parametric (for Gaussian distribution) and non-parametric (for non-Gaussian distribution) tests were used for the analysis.
3. Results
(a). Juvenile social interaction
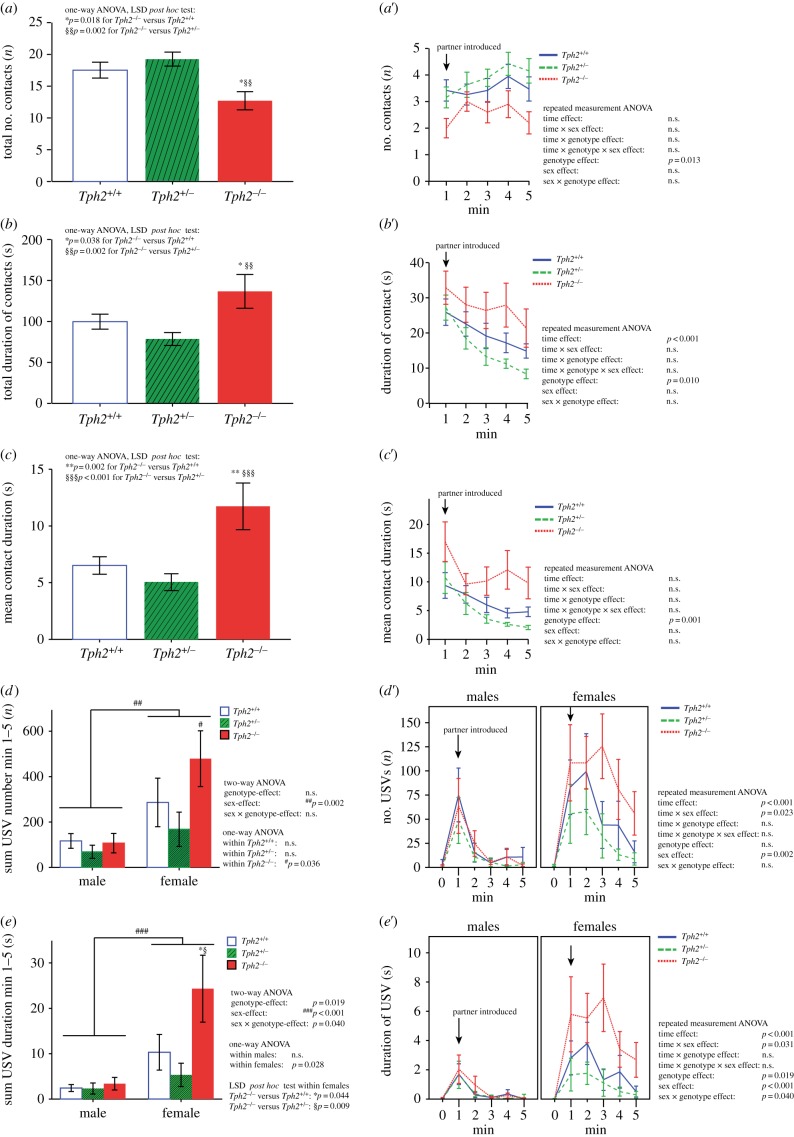
To evaluate the impact of central 5-HT deficiency on juvenile mice, we first investigated social behaviour and USV production at PND25 in Tph2+/+, Tph2+/− and Tph2−/− mice during interaction with an unfamiliar conspecific of the same genotype, sex and age. Genotypes differed in juvenile social interaction behaviour, with significant genotype differences being detectable in all three parameters determined, namely number of social interactions (ANOVA, F2,48 = 5.660; p = 0.006; figure 1a), total contact duration (ANOVA, F2,48 = 5.657; p = 0.006; figure 1b) and average contact duration (ANOVA, F2,48 = 8.715; p = 0.001; figure 1c). Specifically, juvenile Tph2−/− displayed fewer social contacts than Tph2+/− (LSD post hoc, p = 0.002) and Tph2+/+ (LSD post hoc, p = 0.018) littermates. However, total contact duration was higher in juvenile Tph2−/− than in Tph2+/− (LSD post hoc, p = 0.002) and Tph2+/+ (LSD post hoc, p = 0.038) pairs. This is due to the fact that the average contact duration was longer in juvenile Tph2−/− than Tph2+/− (LSD post hoc, p < 0.001) and Tph2+/+ (LSD post hoc, p = 0.002) littermates. In all three parameters determined, Tph2+/− and Tph2+/+ did not differ from each other. The genotype-dependent alterations in juvenile social interaction behaviour were also seen in a more detailed temporal analysis (figure 1a′–c′). Sex had no effect on juvenile social interaction.
Figure 1.
Juvenile interaction of Tph2+/+, Tph2+/− and Tph2−/− mouse pairs. (a) Total number of physical contacts, (b) total contact duration, (c) mean contact duration and (a′–c′) the respective time-dependent change of all parameters of Tph2+/+ (n = 19, open blue bars or solid line), Tph2+/− (n = 19, hatched green bars or dashed line) and Tph2−/− (n = 10, filled red bars or dotted line) male–male and female–female pairs (mean ± s.e.m.). (d) Total number, (e) total duration and (d′,e′) the respective time-dependent change of ultrasonic vocalizations (USVs) of Tph2+/+ (n = 9/8), Tph2+/− (n = 9/8) and Tph2−/− (n = 4/5) male–male/female–female pairs (mean ± s.e.m.). Asterisk and section symbols indicate statistically significant differences between genotypes (*Tph2−/− versus Tph2+/+; §Tph2−/− versus Tph2+/−); hash symbols indicate statistically significant differences between sexes. (Online version in colour.)
In contrast to social contacts, genotype affected total duration of USV emission (ANOVA, F2,43 = 4.414; p = 0.019; figure 1e), but not total number of USVs (ANOVA, F2,43 = 2.390; p = 0.106; figure 1d). Furthermore, during juvenile social interactions sex had a strong impact on USV emission, with females producing more USVs (ANOVA, F1,43 = 11.648; p = 0.002; figure 1d) and calling for longer (ANOVA, F1,43 = 16.287; p < 0.001; figure 1e) than males. In females, differences in total calling time were also genotype-dependent (ANOVA, F1,43 = 4.411; p = 0.028): Tph2−/− females spent more time vocalizing than Tph2+/− (LSD post hoc, p = 0.009) and Tph2+/+ (LSD post hoc, p = 0.044), whereas in males, no differences were observed. The sex-dependent difference in USV pattern was confirmed in a more detailed temporal analysis (figure 1d′,e′): while males exhibited a fast drop in USV number and total calling time after the first minute of interaction, females of all genotypes showed a blunted decrement.
(b). Adult social interaction
To evaluate the impact of central 5-HT deficiency on adult social behaviour, we analysed 15-week-old Tph2+/+ and Tph2−/− male mice before (naive), during and after their first social (male–male) or sexual (male–female) interaction in a cross-balanced manner (figure 2a).
(i). Urine exposure
When being exposed to a drop of female urine, naive Tph2+/+, but not Tph2−/− male mice spent more time in the corner with the female urine spot than in the opposite corner (paired t-test, t13 = 2.394; p = 0.032 and t13 = 1.736; p = 0.106, respectively), yet genotypes did not differ in the time spent in proximity to the female urine spot (t-test, t26 = 1.001; p = 0.326; figure 2b). After the first sexual experience Tph2−/− male mice still did not display a preference for the side with the female urine spot, in contrast to Tph2+/+ animals (paired t-test, t13 = 1.972; p = 0.070 and t13 = 4.751; p < 0.001, respectively). Furthermore, after sexual experience Tph2−/− mice spent less time in proximity to the female urine spot than Tph2+/+ mice (t-test, t26 = 2.099; p = 0.046; figure 2d).
Irrespective of the genotype, no side preference was evoked by male urine in socially naive males (figure 2b). However, after social interaction both genotypes displayed a preference for the side containing male urine (paired t-test, t13 = 3.490; p = 0.004 and t13 = 2.205; p = 0.046, respectively), with similar time spent in proximity to male urine (t-test, t26 = 0.371; p = 0.714; figure 2d).
Before social interaction, some male Tph2+/+, but no Tph2−/− mice emitted USVs when exposed to female urine, with both genotypes emitting no USV in response to male urine (U-test; n.s.; figure 2b′). After social interaction, however, Tph2+/+ male mice emitted USVs to both male and female urine, whereas male Tph2−/− mice were almost silent in response to both stimuli (U-test, U = 41.5; p = 0.008 and U = 44.5; p = 0.012, respectively; figure 2d′).
(ii). Social interaction
Compared with Tph2+/+ male mice, Tph2−/− male mice displayed more aggressive attacks on both partners during male–male and male–female social interaction (t-test, t21.153 = −2.672; p = 0.014 and t26 = −2.401; p = 0.024, respectively), with attacks on males occurring more often in both Tph2+/+and Tph2−/− mice (Wilcoxon paired test, t13 = 3.363; p = 0.005 and t13 = 4.130; p = 0.001, respectively; figure 2c). Additionally, fewer physical contacts with males were observed for Tph2−/− in comparison with Tph2+/+mice (t-test, t26 = 21.153; p = 0.002). Furthermore, Tph2−/− but not Tph2+/+ mice preferred females over males, exhibiting more physical contacts and genital sniffings (paired t-test, t13 = −4.891; p < 0.001 and t13 = −3.267; p = 0.006, respectively). Finally, both Tph2+/+ and Tph2−/− mice displayed a mounting preference towards females (paired t-test, t13 = 2.796; p = 0.015 and t13 = 3.223; p = 0.007, respectively), with some mountings of male partners occurring in both genotypes. Non-social behaviour (e.g. rearing) did not differ between genotypes.
Finally, Tph2−/− male mice exhibited altered correlations between different social and sexual behaviours which were obvious for Tph2+/+ males (see electronic supplementary material, table S1), indicating that the loss of central 5-HT disconnects affective social behaviour (mounting, attacks) and contrasts peaceful (physical) contacts with aggressive approaches (attacks).
4. Discussion
Here, we investigated how lifelong depletion in brain 5-HT affects social and sexual behaviour in juvenile and adult Tph2-deficient mice. During social interaction, juvenile Tph2−/− mouse pairs displayed a reduced number but longer duration of physical contacts in comparison with Tph2+/+ and Tph2+/− mice, indicating alterations in juvenile social behaviour. Analysis of ultrasonic communication revealed a sexual dimorphism in USV production during development, but did not reveal an overall genotype effect, highlighting that 5-HT depletion does not affect the ability of either male or female juvenile mice to vocalize.
In adulthood, neither naive nor experienced Tph2−/− male mice showed a preference for female urine in contrast to Tph2+/+ animals, whereas the behaviour towards male urine did not differ between the genotypes, with no preference in naive conditions and clear preference after first social experience. Importantly, both male and female urine did not evoke USV in Tph2−/− mice, whereas experienced Tph2+/+ mice produced USVs to male and female urine (courtship syllables).
While environmental exploration during dyadic interactions was identical in both genotypes irrespective of the partner, social behaviour was highly influenced by the partner's sex in both genotypes. Similar to Tph2+/+ mice, Tph2−/− males showed less aggression towards and more mountings and sniffing of female than male partners. However, Tph2−/− had fewer contacts with other males in comparison with Tph2+/+ mice and with their own response to females. Thus, a loss of sexual preference, as was suggested in recent publications [4,5], could not be verified in our cross-balanced study.
Furthermore, Tph2−/− mice were hyper-aggressive to both sexes in comparison with Tph2+/+ mice, which is in line with reports of increased aggression of Tph2−/− males to male intruders [2,10]. Surprisingly, Liu et al. [4] did not report any peculiarities in the aggressive behaviour of Tph2−/− mice during male–male or male–female interactions. Potentially, these discrepancies between the two studies could be a consequence of non-balanced experimental protocols (in reference [4]) that can enhance the experience-biased reactions during tests [11,12].
In summary, we conclude that central 5-HT activity is essential for control of aggression and fine-tuning of prosocial behaviour, but does not affect sexual preference.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Ethics statement
Procedures were approved by the ethical committee of the local government (LAGeSo, Berlin and Regierungspräsidium Gießen, Germany).
Data availability
The data is available via the supplementary files submitted along with the manuscript.
Funding statement
German Research Foundation (Deutsche Forschungsgemeinschaft) to M.W. (DFG_WO1732/1-1) and N.A. (DFG_Al1197/5–1); Russian Science Foundation to N.A. (no. 14-50-00069).
Author contributions
D.B. and M.W. conceived and designed the experiments; D.B. performed the experiments; D.B., M.F. and K.H. analyzed the data; M.W. contributed reagents/materials/analysis tools; D.B., N.A. and M.W. wrote the paper; M.W., N.A. and M.B. gave final approval of the manuscript.
Competing interests
The authors have no competing interests.
References
- 1.Chen GL, Miller GM. 2012. Advances in tryptophan hydroxylase-2 gene expression regulation: new insights into serotonin–stress interaction and clinical implications. Am. J. Med. Genet. B Neuropsychiatry Genet. B 159, 152–171. ( 10.1002/ajmg.b.32023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, Alenina N. 2012. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl. Psychiatry 2, e122 ( 10.1038/tp.2012.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez SP, Gaspar P. 2012. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology 62, 144–154. ( 10.1016/j.neuropharm.2011.08.049) [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Jiang Y, Si Y, Kim JY, Chen ZF, Rao Y. 2011. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature 472, 95–99. ( 10.1038/nature09822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S, Liu Y, Rao Y. 2013. Serotonin signaling in the brain of adult female mice is required for sexual preference. Proc. Natl Acad. Sci. USA 110, 9968–9973. ( 10.1073/pnas.1220712110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wöhr M, Scattoni ML. 2013. Behavioural methods used in rodent models of autism spectrum disorders: current standards and new developments. Behav. Brain Res. 251, 5–17. ( 10.1016/j.bbr.2013.05.047) [DOI] [PubMed] [Google Scholar]
- 7.Alenina N, et al. 2009. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc. Natl Acad. Sci. USA 106, 10 332–10 337. ( 10.1073/pnas.0810793106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutknecht L, et al. 2012. Impacts of brain serotonin deficiency following Tph2 inactivation on development and raphe neuron serotonergic specification. PLoS ONE 7, e43157 ( 10.1371/journal.pone.0043157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wöhr M, Roullet FI, Hung AY, Sheng M, Crawley JN. 2011. Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS ONE 6, e20631 ( 10.1371/journal.pone.0020631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kane MJ, Angoa-Perez M, Briggs DI, Sykes CE, Francescutti DM, Rosenberg DR, Kuhn DM. 2012. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS ONE 7, e48975 ( 10.1371/journal.pone.0048975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. 2001. The use of behavioral test batteries: effects of training history. Physiol. Behav. 73, 705–717. ( 10.1016/S0031-9384(01)00528-5) [DOI] [PubMed] [Google Scholar]
- 12.Bartolomucci A, Fuchs E, Koolhaas JM, Ohl F. 2009. Acute and chronic social defeat: stress protocols and behavioral testing. In Mood and anxiety related phenotypes in mice: characterization using behavioral tests (ed. Gould TD.), pp. 261–275. Humana Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is available via the supplementary files submitted along with the manuscript.