Abstract
The consequences of sexual interactions extend beyond the simple production of offspring. These interactions typically entail direct effects on female fitness, but may also impact the life histories of later generations. Evaluating the cross-generational effects of sexual interactions provides insights into the dynamics of sexual selection and conflict. Such studies can elucidate whether offspring fitness optima diverge across sexes upon heightened levels of sexual interaction among parents. Here, we found that, in Drosophila melanogaster, components of reproductive success in females, but not males, were contingent on the nature of sexual interactions experienced by their mothers. In particular, maternal sexual interactions with non-sires enhanced female fecundity in the following generation. This highlights the importance of non-sire influences of sexual interactions on the expression of offspring life histories.
Keywords: indirect genetic effects, interacting phenotypes, Drosophila melanogaster, sexual selection, sexual conflict, parental effects
1. Introduction
In obligately sexually reproducing species, one thing is fact—individuals cannot contribute to the next generation unless they mate with a member of the opposite sex. While this requires cooperation, there is also scope for evolutionary conflicts to emerge between the sexes [1]. As such, the consequences of sexual interactions extend far beyond the simple production of offspring. Both direct and indirect effects brought about by sexual interactions may have profound effects—both on the lifetime reproductive fitness of the males and females involved, and that of their offspring [1–6].
Evaluating the cross-generational effects of sexual interactions can address several questions pertaining to the evolution of sexual conflict. These include whether the costs of mating in females can be compensated by increases in offspring fitness [7–12], whether heightened exposure to sexual interactions in one generation can have differential effects on the fitness of each sex in subsequent generations [13,14], whether such responses are mediated by pre-copulatory or post-copulatory effects [5], and whether mating with a particular male benefits offspring unrelated to that male (e.g. non-sire effects; [6]).
Here, we address these questions by probing the transgenerational consequences of sexual interactions in Drosophila melanogaster with a design enabling us to trace male-mediated effects to sires and non-sires. Our experiment expands on a previous study in fruit flies, which suggested that the receipt of additional seminal fluid proteins by mothers can increase the reproductive success of daughters [4], and addresses several aspects of that study that were questioned [15]. Our findings support the previous work—bouts of mating in the previous generation enhance the reproductive success of daughters—and provide several novel insights. First, when mothers mate multiply, positive effects on the fitness of their daughters can be invoked by mating with males that were not the sires of these daughters; second, the physical act of mating and/or ejaculate-mediated effects can explain this pattern; and third, there are no observable transgenerational effects of mating on male fitness.
2. Material and methods
Full methods can be found in the electronic supplementary material. The focal flies were sourced from an outbred laboratory-reared population of D. melanogaster, fixed for a recessive autosomal mutation encoding brown eyes (LHM-bw). We also sourced flies from a wild-type outbred population (CH). This CH population provided ‘tester’ males, as described below. We also generated lines of flies of standardized genotype, by crossing two near-isogenic lines that each expressed the recessive brown eye mutation. These crosses sourced standardized heterozygote tester flies.
Focal LHM-bw mothers were collected as virgins and exposed to the sexual treatment when 4 days old. They were transferred to vials in groups of eight, with ad libitum access to live yeast. Twelve 4 day old LHM-bw males were then added to each vial, and males and females provided with a 2 h opportunity to mate (females mate once during this period, see the electronic supplementary material). Males were then removed from the mating vials, and each respective group of mothers was randomly allocated to one level of a sexual treatment (out of five levels in total; table 1) lasting 10 days. In total, 70 maternal vials (=replicates), each containing a group of eight mothers were allocated among the treatment levels in two independent sampling blocks (table 1).
Table 1.
Mating treatment levels used to test transgenerational effects of sexual interactions in Drosophila melanogaster.
| level | treatment level name | no. males per replicate (first 2 h) | no. males per replicate (subsequent 10 days) | treatment level description | no. replicates (vials containing 8 mothers each) | no. mothers treated | no. male offspring assayed | no. female offspring assayed |
|---|---|---|---|---|---|---|---|---|
| 1 | ‘baseline’ | 12 LHM-bw | — | baseline (single mating): focal mothers received no further exposure to males. This level is referred to as baseline, because all females in the experiment first experienced this 2 h exposure to males (one mating) prior to their subsequent assignment to the other levels | 11 | 88 | 90 | 93 |
| 2 | ‘pre-cop only’ | 12 LHM-bw | 12 cauterized LHM-bw | baseline plus additional pre-copulatory effects: focal mothers were exposed to a new group of 12 LHM-bw virgin males that had their genitals cauterized using a fine tungsten wire probe connected to a 6V, 1A power source, leaving them able to harass the females but unable to mate with them. This procedure precludes copulation by males without impairing their pre-copulatory sexual behaviour (see the electronic supplementary material) | 13 | 104 | 113 | 115 |
| 3 | ‘pre + post-cop’ | 12 LHM-bw | 12 LHM-bw | baseline plus additional pre-copulatory and post-copulatory effects (multiple mating one genetic background): prospective mothers were exposed to a group of 12 LHM-bw virgin males whose genitals were fully intact. Mothers assigned to this level therefore experienced effects of ongoing male pre-copulatory activity, several copulations, and post-copulatory effects associated with the receipt of multiple ejaculates from males from the LHM-bw genetic background | 13 | 104 | 107 | 114 |
| 4 | ‘pre-cop interacting phenotypes’ | 12 LHM-bw | 6 LHM-bw plus 6 cauterized CH | baseline plus additional pre-copulatory and post-copulatory effects (multiple mating one genetic background and additional pre-copulatory effects from second genetic background): each replicate of prospective mothers was exposed to a group of males consisting of six LHM-bw virgin males and six CH cauterized virgin males. Thus, females in this level were subjected to pre-copulatory effects by males from the two genetic backgrounds but post-copulatory effects (receipt of ejaculates) from just the LHM-bw genetic background | 16 | 128 | 122 | 136 |
| 5 | ‘pre + post-cop interacting phenotypes’ | 12 LHM-bw | 6 LHM-bw + 6 CH | baseline plus additional pre-copulatory and post-copulatory effects (multiple mating two genetic backgrounds): each group of eight prospective mothers was exposed to six L LHM-bw virgin males and six CH virgin males. This level was used to test for transgenerational effects arising from exposure (at the pre- and post-copulatory stages) to a higher diversity of male genotypes (sourced from two distinct global populations), compared with level 3. Level 5 and the use of the brown-eye morphological marker allowed us to inspect transgenerational effects that are in essence indirect genetic effects on offspring fitness traits. That is, while mothers in treatment level 5 produced a mixture of brown- (sired by LHM-bw males) and red-eyed flies (sired by CH males), we only assayed reproductive success of the brown-eyed offspring (i.e. those produced by LHM-bw females and sired by LHM-bw, not by CH males). Thus, any effects seen in the offspring generated in level 5, compared with 3, would arise from mothers interacting sexually with males from different populations and not by Mendelian genetic effects tied to the sire | 17 | 136 | 119 | 131 |
| total | 70 | 560 | 551 | 589 |
On the fifth and tenth days of the treatment, we collected eggs laid by each group of mothers, so that effects of maternal age could be examined. Flies were transferred to new vials and eggs laid over a period of 32 h by each group of eight focal mothers were collected and transferred to vials at a maximum density of 25 per vial. These vials, denoted ‘juvenile vials’, were the vials in which the focal offspring were reared. The number of pupal cases that subsequently formed in each of these vials was recorded 8 days after the laying period and used to estimate juvenile viability for each cohort of mothers, in both the 5 and 10 day age class. Focal sons and daughters were collected as virgins, and stored by sex in groups of 10 until they entered the offspring fitness assays when 4 days old.
Each daughter (n = 589) was provided an individual vial. One 3 to 4 day old (mean = 3.6, s.e. = 0.02) male from a brown-eyed standardized heterozygote tester line (SHL-bw) was added to each vial for 2 h to ensure single mating (see the electronic supplementary material), and then removed. Daughters were then allowed to oviposit for 40 h, across two vials (20 h per vial), and the number of eggs laid across the 40 h period counted (fecundity). The number of adult offspring that eclosed from each of these vials was recorded 14 days later (productivity). The proportion of eggs ultimately producing adults provided a measure of egg-to-adult viability (fertility). Virgin focal sons (all LHM-bw, n = 551) were assayed in sperm competition trials as the second mates (i.e. in P2 position) of tester line (SHL-bw) females who had previously mated with an SHL-wt male ([16]; and see the electronic supplementary material).
We ran multilevel linear and generalized linear-mixed models in R v. 3.0.3 [17]. Explanatory variables were (i) treatment level (fixed factor with five levels—see table 1), (ii) maternal age at oviposition (fixed factor with two levels) and (iii) larval viability of the juvenile vial, a covariate, centred after log transformation (n = 134 vials). In addition, all models included (iv) block (two levels), entered as a random factor to account for the multilevel structure of the data, and (v) replicate, a random factor accounting for vial-sharing effects of the focal flies during juvenile development.
Response variables in individual analyses were (i) daughters' productivity (n = 589 daughters), (ii) daughters' fecundity (probability of egg laying, n = 589 daughters; analysis of number of eggs, n = 465 daughters), (iii) daughters' fertility (n = 465 daughters) and (iv) son's fertilization success under sperm competition (n = 527 sons). See the electronic supplementary material for further details about the analyses.
3. Results
Daughter fecundity depended on the sexual treatment experienced by their mothers (LRT = 13.6269, p = 0.0086, figure 1). The fecundity of daughters sired by LHM-bw males was enhanced when the multiply-mated mothers hosted ejaculates of LHM-bw and CH males inside their reproductive tract (level 5, table 1), compared with when multiple-mated mothers hosted only ejaculates from LHM-bw males (Tukey's test: levels 5 versus 2, p = 0.0266, standardized effect size d = 0.51 (0.22, 0.8); levels 5 versus 3, p = 0.0204, d = 0.49 (0.2, 0.78)). Daughter fertility (egg-to-adult viability of daughters' offspring) was not affected by the sexual treatment, and this lack of effect suggests that daughter fertility will not be involved in any functional trade-off with daughter fecundity.
Figure 1.
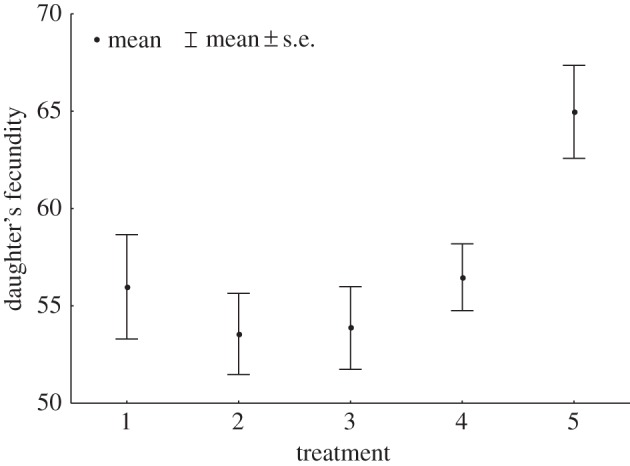
Effects of maternal sexual history on daughter's fecundity. Daughters produced in treatment level 5 had higher fecundity than those produced in levels 2 and 3 (table 1).
Daughter productivity differed among treatment levels (likelihood ratio test, LRT = 12.031, p = 0.017), with daughters whose mothers were exposed to pre-copulatory only (level 4, table 1), or pre- and post-copulatory interactions (level 5), with males from a second population, exhibiting higher productivity than daughters whose mothers were exposed to just a one-off mating encounter with LHM-bw males (Tukey's test: treatment level 1 versus 4, p = 0.006, d = 0.36 (95% CI, 0.1, 0.63); level 1 versus 5, p = 0.039, d = 0.43 (0.16, 0.7); figure 2).
Figure 2.
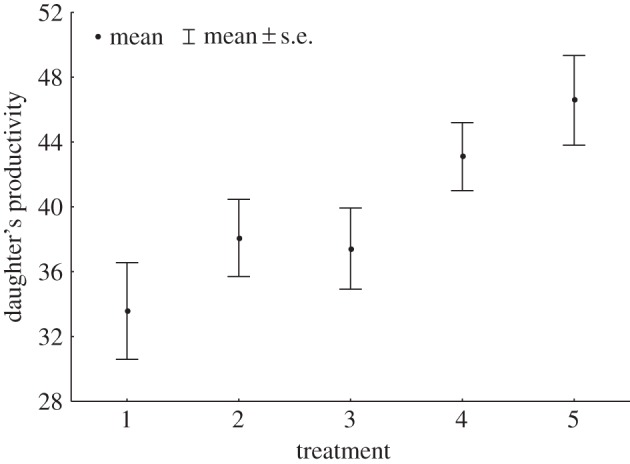
Effects of maternal sexual history on daughter's productivity. Daughters from mothers in treatment levels 4 and 5 produced a higher number of adults than those in the baseline treatment level 1 (table 1).
The fertilization success of sons, assessed in the sperm competition trials, was not influenced by the sexual treatment (LRT = 1.81, p = 0.77). Other results are presented in the electronic supplementary material.
4. Discussion
Studies of sexual conflict have typically focused on measuring the direct costs of mating for females, and less so on measuring downstream effects on offspring fitness. However, trajectories of sexually antagonistic coevolution are shaped by effects of sexual interactions on the fitness of subsequent generations. Studying the cross-generational consequences of sexual interactions might thus provide key insights into understanding the evolution or resolution of sexual conflict.
We found that daughters of mothers who had interacted sexually with the males from two distinct populations exhibited increased reproductive success relative to mothers interacting with the males of just one population (LHM-bw). This result cannot be attributed to Mendelian-inherited sire effects, because all of the daughters assayed were unambiguously sired by LHM-bw population males. Our results can therefore not be explained by good genes or genetic incompatibility processes.
There is increasing evidence suggesting the ejaculate as mediator of non-genetically transmitted transgenerational effects in both invertebrates and vertebrates [4,6,10,18–21]. Our results might be driven by ejaculate-mediated effects tied to the receipt of additional ejaculates from non-sires. Mechanisms responsible for these indirect genetic effects could include differential provisioning of the egg cytoplasm or ejaculate-induced changes to the egg epigenome. Indeed, ejaculate-mediated effects are consistent with the results of a previous study in D. melanogaster [4]. However, we cannot exclude other potential mechanisms. For instance, the reported effects could potentially be induced through the physical act of copulating with genetically divergent males of two distinct populations, through longer mating bouts, or by sexual transfer of microbial communities, attributable to males of the second population.
In addition, we found that there are no observable transgenerational effects of mating on the component of male fitness measured (P2, the fertilization success of the last male to mate in competitive situations), which suggests that cross-generational effects are sex-specific. While sex-specific transgenerational effects might contribute to the dynamics of sexual conflict, we note that P2 is inherently high in fruit flies, and this might limit the power to detect variance in this trait induced by transgenerational effects.
We also note that the transgenerational benefits of mating observed here might not be cost free, and could be involved in life-history trade-offs, with increases in reproductive output coming at a cost to survival. The existence of indirect costs associated with sexual interactions was recently established in D. melanogaster; ongoing exposure by mothers to male pre-copulatory interactions drives transgenerational reductions in offspring survival and accelerated ageing [5]. That study, in conjunction with our results, suggests that there are four main classes of effects that need to be considered when assessing the economics of sexual interactions and conflict. Two of these are traditionally investigated—direct costs and genetic benefits—whereas two have been generally overlooked—transgenerational (indirect) costs [5] and indirect genetic effects [6].
Supplementary Material
Acknowledgements
We thank Belinda Williams for culturing the flies and running the assays, Scarlette Baccini for help in the laboratory, Ian Stewart for designing the cautery and two anonymous reviewers for their comments on a previous version of the manuscript.
Data accessibility
Data are available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.p9h8g.
Author contributions
F.G.G. and D.K.D. collaborated on each component of the study, from conception through to publication.
Funding statement
This study was supported by a UWA RCA (FGG, DKD) and the Australian Research Council (DP0985859, DP1092897, DP120103205) to both F.G.G. and D.K.D. F.G.G. was also supported by the Spanish Ministry of Economy through the Ramon y Cajal programme and grant (CGL2012-34685, co-funded by the European Regional Development Fund), and the Spanish Severo Ochoa Programme (SEV-2012-0262).
Competing interests
We declare no competing interests.
References
- 1.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Slatyer RA, Mautz BS, Backwell PRY, Jennions MD. 2012. Estimating genetic benefits of polyandry from experimental studies: a meta-analysis. Biol. Rev. 87, 1–33. ( 10.1111/j.1469-185X.2011.00182.x) [DOI] [PubMed] [Google Scholar]
- 3.Wigby S, Chapman T. 2005. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15, 316–321. ( 10.1016/j.cub.2005.01.051) [DOI] [PubMed] [Google Scholar]
- 4.Priest NK, Roach DA, Galloway LF. 2008. Cross-generational fitness benefits of mating and male seminal fluid. Biol. Lett. 4, 6–8. ( 10.1098/rsbl.2007.0473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowling DK, Williams BR, Garcia-Gonzalez F. 2014. Maternal sexual interactions affect offspring survival and ageing. J. Evol. Biol. 27, 88–97. ( 10.1111/jeb.12276) [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Gonzalez F, Simmons LW. 2007. Paternal indirect genetic effects on offspring viability and the benefits of polyandry. Curr. Biol. 17, 32–36. ( 10.1016/j.cub.2006.10.054) [DOI] [PubMed] [Google Scholar]
- 7.Parker GA. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum MS, Blum NA.), pp. 123–166. New York, NY: Academic Press. [Google Scholar]
- 8.Cordero C, Eberhard WG. 2003. Female choice of sexually antagonistic male adaptations: a critical review of some current research. J. Evol. Biol. 16, 1–6. ( 10.1046/j.1420-9101.2003.00506.x) [DOI] [PubMed] [Google Scholar]
- 9.Head ML, Hunt J, Jennions MD, Brooks R. 2005. The indirect benefits of mating with attractive males outweigh the direct costs. PLoS Biol. 3, 289–294. ( 10.1371/journal.pbio.0030289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Gonzalez F, Simmons LW. 2010. Male-induced costs of mating for females compensated by offspring viability benefits in an insect. J. Evol. Biol. 23, 2066–2075. ( 10.1111/j.1420-9101.2010.02065.x) [DOI] [PubMed] [Google Scholar]
- 11.Rundle H, Ödeen A, Mooers A. 2007. An experimental test for indirect benefits in Drosophila melanogaster. BMC Evol. Biol. 7, 36 ( 10.1186/1471-2148-7-36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart AD, Hannes AM, Mirzatuny A, Rice WR. 2008. Sexual conflict is not counterbalanced by good genes in the laboratory Drosophila melanogaster model system. J. Evol. Biol. 21, 1808–1813. ( 10.1111/j.1420-9101.2008.01593.x) [DOI] [PubMed] [Google Scholar]
- 13.Fedorka KM, Mousseau TA. 2004. Female mating bias results in conflicting sex-specific offspring fitness. Nature 429, 65–67. ( 10.1038/nature02492) [DOI] [PubMed] [Google Scholar]
- 14.Pischedda A, Chippindale AK. 2006. Intralocus sexual conflict diminishes the benefits of sexual selection. PLoS Biol. 4, e356 ( 10.1371/journal.pbio.0040356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long TAF, Stewart AD, Miller PM. 2009. Potential confounds to an assay of cross-generational fitness benefits of mating and male seminal fluid. Biol. Lett. 5, 26–27. ( 10.1098/rsbl.2008.0325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Gonzalez F, Evans JP. 2011. Fertilization success and the estimation of genetic variance in sperm competitiveness. Evolution 65, 746–756. ( 10.1111/j.1558-5646.2010.01127.x) [DOI] [PubMed] [Google Scholar]
- 17.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org. [Google Scholar]
- 18.Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. 2014. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc. Natl Acad. Sci. USA 111, 2200–2205. ( 10.1073/pnas.1305609111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane M, Robker RL, Robertson SA. 2014. Parenting from before conception. Science 345, 756–760. ( 10.1126/science.1254400) [DOI] [PubMed] [Google Scholar]
- 20.Kumar M, Kumar K, Jain S, Hassan T, Dada R. 2013. Novel insights into the genetic and epigenetic paternal contribution to the human embryo. Clinics 68, 5–14. ( 10.6061/clinics/2013(Sup01)02) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crean AJ, Kopps AM, Bonduriansky R. 2014. Revisiting telegony: offspring inherit an acquired characteristic of their mother's previous mate. Ecol. Lett. 17, 1545–1552. ( 10.1111/ele.12373) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.p9h8g.


