Abstract
Objectives
To compare participation rates and clinical effectiveness of sedated endoscopy (sEGD) versus unsedated transnasal endoscopy (uTNE) for esophageal assessment and Barrett's esophagus (BE) screening in a population-based cohort.
Methods
Prospective, randomized, controlled trial in a community population. Subjects, ≥ 50 years of age who previously completed validated gastrointestinal symptom questionnaires were randomized (stratified by age, sex and reflux symptoms) to one of three screening techniques (either sEGD, or uTNE in a mobile research van (muTNE), or uTNE in a hospital outpatient endoscopy suite (huTNE)) and invited to participate.
Results
209 of 459 (46%) subjects agreed to participate (muTNE n=76, huTNE n=72, sEGD n=61). Participation rates were numerically higher in the unsedated arms of muTNE (47.5%) and huTNE (45.7%) compared to the sEGD arm (40.7%), but were not statistically different (p=0.27). Complete evaluation of the esophagus was similar using muTNE (99%), huTNE (96%) and sEGD (100%) techniques (p=0.08). Mean recovery times (minutes) were longer for sEGD (67.3) compared to muTNE (15.5) and huTNE (18.5) (p<0.001). Approximately, 80% of uTNE subjects were willing to undergo the procedure again in future. 29% and 7.8% of participating subjects had esophagitis and BE respectively.
Conclusions
Mobile van and clinic uTNE screening had comparable clinical effectiveness with similar participation rates and safety profile to sEGD. Evaluation time with uTNE was significantly shorter. Prevalence of BE and esophagitis in community subjects ≥ 50 years of age was substantial. Mobile and outpatient unsedated techniques may provide an effective alternative strategy to sEGD for esophageal assessment and BE screening.
Keywords: Esophageal adenocarcinoma, Barrett's esophagus, transnasal endoscopy, screening, mobile van screening
Introduction
The incidence rate of esophageal adenocarcinoma (EAC) has rapidly risen over the past 3 decades, exceeding that of melanoma, lung, colon and breast cancers(1). Five year survival remains below 20% for EAC cases diagnosed after the onset of symptoms, while early stage EAC has a 5-year survival exceeding 80%(2). Barrett's esophagus (BE) is the strongest and only known precursor of EAC. However, cancer progression rates in clinically diagnosed BE are as low as 0.33% per year(3), hence endoscopic surveillance alone may not have an impact on survival rates from EAC(4). Moreover, it is estimated that only a third of patients with BE in the population are detected clinically, with the rest remaining undiagnosed, despite the substantial increase in the use of endoscopy (5). Indeed, up to 90% of patients who present with EAC do not have a previous diagnosis of BE(6), despite its presence on histology, an indication of the underlying challenge for early detection.
Screening for BE in high-risk individuals coupled with curable endotherapy of early neoplasia may represent an alternative strategy to reduce mortality from this lethal cancer. This was endorsed by recent guidelines from gastroenterology societies (7, 8). However, several questions remain to be addressed before such an approach can be considered or implemented. To this date, the true population prevalence of BE in the United States remains unknown partly due to the lack of suitable screening tests to replace sedated endoscopy (sEGD) for use in the community. Current BE risk prediction models are derived from patients seen at referral centers undergoing endoscopy for clinical indications and may not be representative for use at a population level (9, 10). Finally, The lack of real data on participation rates and yield of different screening modalities for assessment in the community limits the validity of assumptions utilized in modeling studies which have found screening to be cost effective(11).
Unsedated transnasal endoscopy (uTNE) has been proposed as an acceptable and accurate alternative to sEGD for detection of BE(12, 13). The feasibility and acceptability of this technique was demonstrated in a pilot randomized trial in Olmsted County(14). A survey study in this population showed the potential for increased participation if screening was provided closer to home(15). The EndoSheath® transnasal esophagoscope (Vision-Sciences Inc., Orangeburg, New York) has recently become available. It utilizes a novel disposable sheath that encases the endoscope and completely isolates it from patient contact, obviating the need for decontamination and reprocessing facilities. It utilizes a more compact processing system allowing for easy portability, rendering it suitable for widespread use. Initial reports on the clinical utility of this device in an office-based setting have been encouraging(16).
The comparative clinical effectiveness of uTNE compared to sEGD in community screening for BE specifically participation rates, diagnostic yield, tolerability, and safety has not been studied before. While prior studies have used either uTNE alone or in tandem with sEGD (12, 16); this does not allow a simultaneous and systematic comparison of both approaches separately. Furthermore, the clinical utility and effectiveness of using a mobile van unit for screening with uTNE closer to the patient's home also remains unknown.
The aims of our study were: 1. To assess participation rates in BE screening using uTNE in a mobile van unit (muTNE), uTNE in a hospital endoscopy unit (huTNE) and conventional sEGD; and 2. To compare the clinical effectiveness of these techniques in a population based randomized trial.
Methods
Trial Design and Settings
This was a prospective, randomized, controlled trial conducted in Olmsted County, MN. The study took place between April 1st 2011 and October 30th 2013. The Olmsted County population comprises approximately 144,000 persons of whom 86% are white (2010 US Census data). County residents receive their medical care almost exclusively from two group practices: the Mayo Medical Centre and Olmsted Medical Center. The Mayo Clinic has maintained a common medical record system with its two affiliated hospitals. The system was further developed by the Rochester Epidemiology Project (REP), which created indices for the records of other providers of medical care to local residents. Annually, over 80% of the entire population is attended by one or both of these two practices, and nearly everyone is seen at least once during any given 4-year period(17). Therefore, the REP medical records linkage system provides an enumeration of the population from which samples can be drawn. All participants gave informed consent and the study received approval from the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. The trial was registered on clinicaltrials.gov (identifier: NCT01288612).
Participants
Using the REP resources, age- and gender-stratified random samples of Olmsted County residents were previously drawn, and those subjects were mailed validated gastrointestinal symptom questionnaires from 1988 to 2009 (5, 18, 19). Those population-based surveys have allowed identification of a cohort of community subjects, well characterized by the frequency of reflux symptoms, without history of endoscopic evaluation and not known to have BE.
Subjects 50 years or older in this cohort (n= 1157) were randomized - stratified by age, sex, and reflux symptoms- and assigned to muTNE, huTNE, or sEGD groups. Within each group, patients were sorted in a random order, and an initial sample of 450 potential participants was obtained (150 per group) to contact for recruitment to the study. Medical chart review was performed to check for exclusion criteria, namely: history of progressive dysphagia; known Zenker's or epiphrenic diverticulum; history of recurrent epistaxis; moved out of Olmsted County or deceased; significant illness that may impair ability to complete questionnaires (e.g. metastatic cancer, major stroke, and dementia); and coagulopathy.
Successive patients who met the inclusion and exclusion criteria in each of the 3 groups were initially sent generic invitation letters asking if “they agree to be contacted by phone in two weeks' time to inform them about a research study run by Mayo Clinic”. Subjects who explicitly declined to be contacted regarding research were excluded from the study, while others who agreed or did not respond after 2 weeks were considered eligible to be contacted. Details of patient flow are shown in figure 2.
Figure 2.
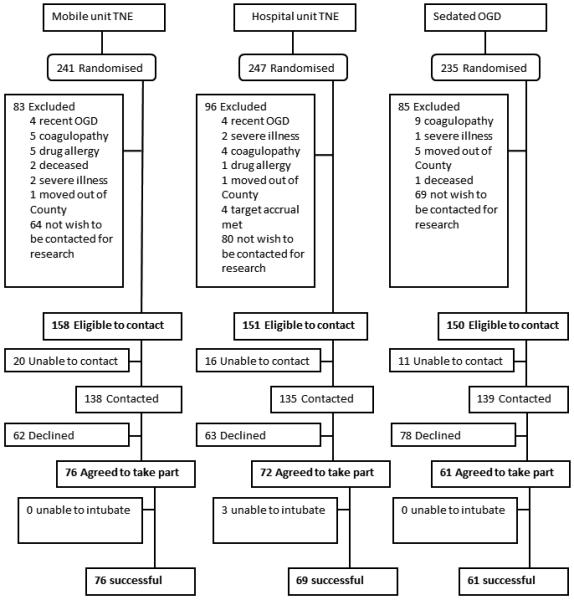
Study flowchart describing patient identification and recruitment.
Eligible patients received a telephone call from a research coordinator who was blinded to the primary hypothesis of the study. One of 3 standardized telephone scripts were used depending on the group assignment. Subjects were only offered the technique that they had been randomized to. These scripts included information on the rationale of the study and details on the procedure and its potential adverse effects. In each group, the order for contacting patients was randomized. Patients who declined participation or could not be reached by telephone after 3 attempts were not further contacted. Patients in all 3 groups were treated and followed up in the same manner.
Interventions
Prior to endoscopic procedures, participants filled out the GERQ questionnaire(18), a validated measure of reflux and somatic symptoms. Reflux symptoms were classified into: frequent (≥ one episode/week of heartburn or acid regurgitation); infrequent (< one episode/week of heartburn or acid regurgitation); or absent (No Heartburn or acid regurgitation).
The sEGD procedures were performed using a conventional high definition endoscope (GIF-180, Olympus America, Center Valley, PA) under conscious sedation with intravenous midazolam and fentanyl. uTNE was performed using the EndoSheath® transnasal esophagoscope (TNE-5000, Vision Sciences, Orangeburg, NY). The working length is 650 mm with 140° up & 215° down tip deflection and 120° field of view. The insertion diameter is 4.7 mm when using a sheath with no working channel, and this increases to 5.4 mm or 5.8 mm when using a sheath containing 1.5 mm or 2.1 mm working channel, respectively. All procedures were performed by expert gastroenterologists, who had performed over 1000 upper gastrointestinal endoscopic examinations. Topical anesthetic aerosol spray (Benzocaine 20%; Topex®, Sultan Healthcare Inc., Hackensack, NJ, USA) was applied to the posterior pharynx and nasal spray mixture (Phenylephrine 1.0%, Leader®, Cardinal Health, Dublin, Ohio, USA; and Lidocaine 4%, Hospira Inc., Lake Forest, IL, USA) was applied to the patient's nares (6–8 sprays) 10–15 minutes prior to uTNE.
The uTNE procedure was performed with the patient lying in the left lateral position. The endoscope was introduced into the right or left nares and advanced into the proximal esophagus under direct vision. Prior to every procedure, the esophagoscope was placed inside a new, sterile sheath. Following the procedure, the esophagoscope was removed from the sheath, which is then discarded, and the endoscope was cleaned with a 70% ethyl alcohol wipe after every use.
The muTNE group underwent the research procedures in a mobile research vehicle (MRV) that is staffed by a registered nurse. The MRV contains two exam rooms with cardio respiratory monitoring equipment, a private area for patient interviews and audiovisual technology for patient education (figure 1). Eligible subjects were divided into 4 groups geographically in Olmsted County (on the basis of residential address: northwest, northeast, southwest and southeast quadrants,) and were approached consecutively by group. Screening exams in this group were conducted in the exam room space, in sets of 5–6 subjects to maximize efficiency. Once 5 subjects consented to participate in this arm of the study, the MRV was driven to a geographical location in close proximity to the subjects' residence and stationed in a suitable area with convenient parking. A maximum of 6 screening procedures were conducted in one muTNE session over a 4–5 hour period. Fifteen sessions were conducted to screen 76 subjects.
Figure 1.

Mobile research vehicle: A) outside view, B) view of exam room inside
Biopsies were taken from any endoscopically suspected BE and from the GE junction and squamous mucosa in all subjects. The length of BE segment was defined using the Prague criteria(20). All participants received a telephone call from the research coordinator 1 and 30 days after the procedure, to complete validated tolerability scales(21) and adverse events questionnaires.
Outcome Measures
The primary outcome measure was participation rate. This was estimated as the proportion of subjects who agreed to undergo esophageal assessment in the three groups (muTNE, huTNE, sEGD) out of those who were eligible to be contacted for participation in screening. Additional analysis was performed to identify clinical and demographic factors associated with higher participation rates in screening.
The secondary outcome measure was clinical effectiveness. This was evaluated by assessing both the quality and yield of esophageal assessment with muTNE, huTNE and sEGD. All parameters were recorded by the trial coordinator for all procedures.
Endpoints for procedure quality were: rate of successful intubation (the ability to traverse the upper esophageal sphincter and visualize the esophageal mucosa) classified as successful or unsuccessful; rate of complete evaluation (visualization of the whole esophagus and identification of landmarks: squamocolumnar junction, gastroesophageal junction [upper margin of gastric folds with stomach deflated] and the diaphragmatic hiatus) classified as complete = all three landmarks identified, incomplete = some landmarks identified, or unsuccessful; rate of successful acquisition of biopsies scored as successful = all biopsies obtained, incomplete = some biopsies are obtained, or unsuccessful = no biopsies can be obtained; duration of procedure in minutes (time from the beginning of procedure [initiation of sedation or local anesthesia] to extubation, and time from extubation to discharge [recovery time]); tolerability (validated pain scales(21) - where “0” is none and “10” is severe - were used to assess the degree of pain, choking, gagging, and anxiety experienced during the procedure, overall tolerance was rated on a scale from 0–10, where “0” is good and “10” is poor tolerance); acceptability (proportion of patients willing to undergo the procedure again in the future); safety (adverse events including pain, abdominal discomfort, bleeding, perforation, or need for hospitalization) was assessed 1 and 30 days after the procedures in all subjects.
Endpoints for procedure yield included: reflux esophagitis (graded using the Los Angeles classification(22)); suspected BE (presence of columnar mucosa at least 1 cm length in the tubular esophagus); and confirmed BE (presence of intestinal metaplasia with goblet cells in biopsies on Hematoxylin and Eosin stains). All patients with suspected BE in the muTNE and huTNE groups were offered a “confirmatory” clinical sEGD with histology in 2 weeks' time. Histology was interpreted by a gastrointestinal pathologist blinded to the cohort assignment.
Randomization and Sample Size Estimation
Subjects eligible to be contacted were randomly assigned to one of three procedures (muTNE, huTNE and sEGD) stratified by sex, presence of GERD, and age group (≥65 vs. <65). The assignments were generated via a computer algorithm (SAS®) by the study statistician and sent to the study coordinator via an EXCEL spreadsheet (Excel 2010, Microsoft, Redmond, WA).
In a prior pilot study done by us (14), the participation rate for using sEGD was 37% compared with 47% for uTNE. A sample size of 150 subjects (eligible to be invited for participation) in each group provided 80% power to detect a 20% increase in screening participation using uTNE (thought to be clinically significant) over sEGD, using a two sample test of proportions at an alpha level of 0.0125, accounting for four comparisons (1. sEGD versus huTNE, 2. sEGD versus muTNE, 3. sEGD versus huTNE and muTNE combined, and 4. huTNE versus muTNE).
Statistical Analysis
Pair wise comparisons of participation proportions in the three study arms were assessed. These proportions were compared using a chi square test (or a Fisher's exact test as appropriate). Logistic regression analysis was utilized to identify factors predicting participation. Several logistic regression models were examined, each one including the procedure group and one of a fixed (a-priori selected) set of predictor variables to identify factors associated with participation in screening. We assessed the following a-priori selected variables: age, gender, education level, employment, marital status, reflux symptoms, use of PPI drugs, randomization group, SSC score, and Charlson comorbidity index.
The performance characteristics and yield of muTNE, huTNE, and sEGD were summarized either as proportions (95% confidence intervals) for categorical characteristics, or as mean (standard deviation), or median (interquartile range) depending on their distribution, for continuous characteristics. These characteristics were compared across the three groups using a Kruskal-Wallis test for the continuous characteristics and a chi-square test for categorical characteristics. Given the need for multiple comparisons to assess this hypothesis, statistical significance was defined using an alpha level of 0.01 (adjusting for testing multiple measures of performance). The statistical software (SAS® version 9.3, SAS Institute, Cary NC) was used for analysis.
Results
Participation Rates and Predictors
459 subjects were eligible to be contacted and invited for screening in their allocated study arm (muTNE n=158, huTNE n=151, sEGD n=150). 43 (9.4%) had frequent reflux symptoms (≥1/week), while 197 (42.9%) had infrequent (<1/week) and 219 (47.7%) had no reflux symptoms in this cohort. Baseline characteristics were comparable among the three groups (table 1).
Table 1.
Baseline characteristics of subjects who were invited for screening in their allocated study arm
| Variable | muTNE (N=158) | huTNE (N=151) | sEGD (N=150) |
|---|---|---|---|
| Mean age, years (SD) | 64 (10) | 64 (10) | 65 (9) |
| Male Sex, N (%) | 71 (44.9%) | 69 (45.7%) | 69 (46.0%) |
| Education N (%) | |||
| Less than high school | 7 (4.6%) | 3 (2.0%) | 2 (1.4%) |
| High school or some college | 72 (47.1%) | 76 (52.0%) | 77 (53.8%) |
| College/professional training | 74 (48.4%) | 67 (45.9%) | 64 (44.8%) |
| Employment N (%) | |||
| Employed | 56 (36.6%) | 63 (43.2%) | 48 (33.6%) |
| Unemployed/homemaker | 11 (7.2%) | 8 (5.5%) | 8 (5.6%) |
| Retired | 86 (56.2%) | 75 (51.4%) | 87 (60.8%) |
| Marital status N (%) | |||
| Married | 116 (73.9%) | 118 (78.2%) | 115 (77.2%) |
| Not married (single, widowed, divorced, separated) | 41 (26.1%) | 33 (21.8%) | 34 (22.8%) |
| Reflux symptoms N (%) | |||
| Heartburn or acid regurgitation >= 1/week | 15 (9.5%) | 16 (10.6%) | 12 (8.0%) |
| Heartburn or acid regurgitation < 1 / week | 68 (43.0%) | 67 (44.4%) | 62 (41.3%) |
| No Heartburn or acid regurgitation | 75 (47.5%) | 68 (45.0%) | 76 (50.7%) |
| Current use PPI drugs N (%) | 28 (18.2%) | 23 (15.5%) | 25 (16.9%) |
| SSC score, mean (SD) | 0.8 (0.6) | 0.7 (0.5) | 0.8 (0.6) |
| Charlson comorbidity index, mean (SD) | 0.9 (1.5) | 1.0 (1.5) | 1.2 (1.9) |
(n=459), PPI, proton pump inhibitors; SSC, somatic symptom checklist; sEGD, sedated endoscopy; muTNE, mobile unitTNE; huTNE, hospital unitTNE; SD, standard deviation; N, number.
Of the 459 subjects contacted, 209 (46%) agreed to undergo study procedures (muTNE n=76, huTNE n=72, sEGD n=61). The mean (+/− SD) age of this cohort of “participants” was 65 years (+/− 9) and 45.9% were males. 29 (13.9%) had frequent reflux symptoms, 86 (41.1%) had infrequent and 90 (43%) had no reflux symptoms in this cohort Baseline characteristics were also comparable among the three groups of participants (table 2).
Table 2.
Baseline characteristics of subjects participating in the three arms of the randomized trial
| Variable | muTNE (N=76) | huTNE (N=72) | sEGD (N=61) |
|---|---|---|---|
| Mean age (years), mean (SD) | 65 (9) | 64 (9) | 66 (9) |
| Male Sex N (%) | 32 (42.1%) | 35 (48.6%) | 29 (47.5%) |
| White ethnicity N (%) | 73 (96.0%) | 72 (100%) | 61 (100%) |
| BMI mean (SD) | 29.0 (5.6) | 30.5 (13.9) | 28.8 (5.8) |
| Waist to hip ratio, mean (SD) | 0.91 (0.09) | 0.92 (0.11) | 0.91 (0.09) |
| Education N (%) | |||
| Less than high school | 2 (2.7%) | 1 (1.4%) | 1 (1.7%) |
| High school or some college | 37 (49.3%) | 40 (55.6%) | 33 (55.0%) |
| College/professional training | 36 (48.0%) | 31 (43.1%) | 26 (43.3%) |
| Employment N (%) | |||
| Employed | 23 (30.7%) | 29 (40.3%) | 17 (28.3%) |
| Unemployed/homemaker | 6 (8.0%) | 6 (8.3%) | 5 (8.3%) |
| Retired | 46 (61.3%) | 37 (51.4%) | 38 (63.3%) |
| Marital status N (%) | |||
| Married | 57 (76.0%) | 62 (86.1%) | 42 (70.0%) |
| Not married (single, widowed, divorced, separated) | 18 (24.0%) | 10 (13.9%) | 18 (30.0%) |
| Frequency of reflux symptoms N (%) | |||
| ≥1/week | 11 (14.5%) | 10 (13.8%) | 8 (13.1%) |
| <1/week | 26 (34.2%) | 32 (44.4%) | 28 (45.9%) |
| None | 38 (50%) | 27 (37.5%) | 25 (41.0%) |
| Current use PPI drugs N (%) | 15 (19.7%) | 9 (12.5%) | 11 (18.0%) |
| Current aspirin use N (%) | 38 (50.0%) | 37 (51.4%) | 36 (59.0%) |
| SSC score, mean (SD) | 0.8 (0.6) | 0.8 (0.6) | 0.8 (0.5) |
| Charlson comorbidity index, mean (SD) | 0.9 (1.3) | 0.9 (1.4) | 1.3 (2.0) |
(n=209). PPI, proton pump inhibitors; SSC, somatic symptom checklist; sEGD, sedated endoscopy; muTNE, mobile unitTNE; huTNE, hospital unitTNE; SD, standard deviation; N, number.
Though participation rates were numerically higher in the unsedated arms of muTNE (48.1%) and huTNE (47.7%) compared to the sEGD arm (40.7%), these differences were not statistically significant on pair wise comparisons (sEGD vs. muTNE, p=0.25; sEGD vs. huTNE, p=0.42; sEGD vs. muTNE or huTNE, p=0.27; muTNE vs. huTNE, p=0.82). Factors which independently predicted higher participation rates included presence of frequent reflux symptoms (OR 2.94, 95%CI 1.47–5.88,) and being either unemployed or a homemaker (OR 2.48, 95%CI 1.07–5.77). Age, gender, education level, marital status, current PPI use, SSC score and Charlson co morbidity index were not associated with significant differences in participation rates (table 3).
Table 3.
Proportions and predictors of participation in screening.
| Variable | Proportion of participants | OR for participation (95%CI)a | P valuea |
|---|---|---|---|
| Age b | 1.09 (0.90,1.31) | 0.39 | |
| Sex | |||
| Male | 45.4% | 1.0 (ref) | |
| Female | 44.0% | 0.94 (0.65,1.36) | 0.75 |
| Education | |||
| Less than high school | 33.3% | 0.60 (0.17,2.06) | 0.41 |
| High school or some college | 48.0% | 1.17 (0.80,1.71) | 0.42 |
| College/professional training | 44.0% | 1.0 (ref) | |
| Employment | |||
| Employed | 40.7% | 1.0 (ref) | |
| Unemployed/homemaker | 63.0% | 2.48 (1.07,5.77) | 0.03 |
| Retired | 47.6% | 1.34 (0.90,2.00) | 0.15 |
| Marital status | |||
| Married | 45.6% | 1.23 (0.79,1.90) | 0.36 |
| Not married (single, widowed, divorced, separated) | 40.7% | 1.0 (ref) | |
| Reflux symptoms | |||
| Heartburn or acid regurgitation >= 1 / week | 67.4% | 2.94 (1.47,5.88) | 0.002 |
| Heartburn or acid regurgitation < 1 / week | 43.6% | 1.10 (0.75,1.63) | 0.62 |
| None | 41.1% | 1.0 (ref) | |
| Current use PPI drugs | |||
| PPI user | 46.0% | 1.02 (0.62,1.68) | 0.93 |
| PPI non-user | 45.4% | 1.0 (ref) | |
| Randomization group | |||
| muTNE | 48.1% | 1.32 (0.84,2.07) | 0.23 |
| huTNE | 47.7% | 1.23 (0.78,1.94) | 0.38 |
| sEGD | 40.7% | 1.0 (ref) | |
| SSC score c | 1.01 (0.73,1.41) | 0.93 | |
| Charlson comorbidity index d | 0.99 (0.89,1.12) | 0.94 | |
multiple variable model.
per 10 years of age.
per unit score.
per unit of the index.
OR, odds ratio; CI, confidence interval; PPI, proton pump inhibitors; SSC, somatic symptom checklist; sEGD, sedated endoscopy; muTNE, mobile unitTNE; huTNE, hospital unitTNE
Quality and Yield of Endoscopic Assessment
Complete evaluation of the esophagus (intubation, visualization of the mucosa and landmark identification) was comparable using muTNE (n=75, 98.7%), huTNE (n=69, 95.8%) and sEGD (n=61, 100%) techniques (p=0.08). However, successful biopsy acquisition was lower in the muTNE (n=60, 79%) and huTNE (n=60, 83.3%) groups compared to sEGD (n=61, 100%) (p=0.001), due to inability to advance the TNE scope with the biopsy sheath through narrow nasal passages and patient intolerance. Recovery times (from extubation to discharge) were significantly longer following sEGD (67.3 +/−5.9) compared to muTNE (15.5 +/−2.8) and huTNE (18.5 +/− 4.2) techniques (p<0.001).
Though the overall tolerability scores (mean +/−SD) for sEGD was better than muTNE and huTNE (0.4 +/−0.6 vs. 1.9 +/−2.2 and 2.2 +/−2.2 respectively, p<0.001), uTNE was well tolerated. Moreover, a substantial majority of patients undergoing muTNE and huTNE were willing to undergo TNE again (78.9% and 83.3%, respectively, compared to 93% with sEGD). No serious adverse events were reported in any of the three arms (table 4).
Table 4.
Quality and safety parameters in the three study arms.
| Variable | muTNE (n=76) | huTNE (n=72) | sEGD (n=61) | P value§ |
|---|---|---|---|---|
| Rate of successful intubation | 76 (100%) | 69 (95.8%) | 61 (100%) | 0.06 |
| Rate of complete evaluation | ||||
| Complete | 75 (98.7%) | 69 (95.8%) | 61 (100%) | 0.08 |
| Incomplete* | 1 (1.3%) | 0 (0%) | 0 (0%) | |
| Unsuccessful** | 0 (0%) | 3 (4.2%) | 0 (0%) | |
| Rate of successful acquisition of biopsies from the oesophagus | 60 (79.0%) | 60 (83.3%) | 61 (100%) | 0.001 |
| Duration of procedure | ||||
| Mean duration of procedure (SD). | 8.5 (2.5) | 8.0 (2.7) | 9.3 (1.6) | <0.001 |
| Mean duration from extubation to discharge (SD) | 15.5 (2.8) | 18.5 (4.2) | 67.3 (5.9) | <0.001 |
| Tolerability scores | ||||
| Pain | 3.2 (8.8) | 2.7 (2.3) | 0.1 (0.5) | <0.001 |
| Choking | 0.6 (1.9) | 0.8 (1.8) | 0 (0) | <0.001 |
| Gagging | 1.3 (2.3) | 1.2 (2.4) | 0.1 (0.6) | <0.001 |
| Anxiety | 2.8 (2.8) | 2.3 (2.2) | 0.8 (1.5) | <0.001 |
| Overall tolerance | 1.9 (2.2) | 2.2 (2.2) | 0.4 (0.6) | <0.001 |
| N (%) willing to have procedure again in future | 60 (78.9%) | 60 (83.3%) | 57 (93.4%) | 0.001 |
| Patient reported procedure related adverse events | ||||
| Sore throat | 12 (15.8%) | 10 (13.8%) | 8 (13.1%) | 0.97 |
| Bloating | 3 (4.0%) | 4 (5.6%) | 2 (3.3%) | 0.83 |
| Epistaxis (self-limiting nose bleed) | 1 (1.3%) | 1 (1.4%) | 0 (0%) | 1.00 |
| Nasal pain | 1 (1.3%) | 0 (0%) | 0 (0%) | 1.00 |
| Abdominal/chest discomfort | 2 (2.7%) | 4 (5.6%) | 4 (6.6%) | 0.51 |
| Headache | 4 (5.3%) | 1 (1.4%) | 0 (0%) | 0.16 |
| Serious adverse events (bleeding, perforation, hospitalization) | 0 (0%) | 0 (0%) | 0 (0%) | |
Data presented as number (percentage) or mean (+/− standard deviation). sEGD, sedated endoscopy; muTNE, mobile unit TNE; huTNE, hospital unit TNE; SD, standard deviation; N, number..
Fisher's Exact test (discrete variables) or Kruskal-Wallis test (quantitative variables)
unable to intubate with biopsy sheath, but able to intubate with non-biopsy sheath.
unable to intubate with either biopsy or non-biopsy sheath.
There was no difference in procedure yield between the three study arms with regards to suspected (p=0.37) and confirmed (p=0.44) BE. However, the yield for erosive esophagitis was higher in the huTNE and sEGD arms compared to muTNE (p=0.003). Overall, 16 subjects (5 females and 11 males) were diagnosed with BE (7.8%). Median segment length (using Prague classification) was C0 (range 0–8) M2 (range 1–10). One patient was found to have low grade dysplasia and one patient was diagnosed with high grade dysplasia. The rates of detection of manifestations of GERD-induced esophageal injury in subjects with and without reflux symptoms are shown in table 5. Erosive esophagitis was seen in several subjects with (35%, 17% LA Grade B, C) and without reflux symptoms (23%, 10% LA grade B, C).
Table 5.
The rates of detection of manifestations of GERD-induced esophageal injury in subjects with and without reflux symptoms.
| Findings | Frequent reflux ≥1/week (n=29) | Infrequent reflux <1/week (n=86) | No reflux (n=90) | P value§ |
|---|---|---|---|---|
| Normal GE junction | 13 (44.8%) | 44 (51.2%) | 59 (65.6%) | 0.06 |
| Hiatus hernia present | 22 (75.9%) | 43 (50.0%) | 58 (64.4%) | 0.03 |
| Mean (SD) Hiatus hernia length (cm) | 2.4 (1.7) | 1.4 (1.6) | 1.9 (2.1) | 0.03 |
| Suspected BE on endoscopy | 7 (24.1%) | 17 (19.8%) | 14 (15.6%) | 0.53 |
| Confirmed BE on histology | 6 (20.7%) | 6 (7.0%) | 4 (4.4%) | 0.03 |
| Erosive Esophagitis | 11 (37.9%)* | 29 (33.7%) ** | 21 (23.3%) *** | 0.18 |
Data presented as number of patients (percentage) or mean (+/−SD, standard deviation).
Fisher's Exact test (discrete variables) or Kruskal-Wallis test (quantitative variables).
11 patients [Grade A= 5, Grade B= 4, Grade C=2].
29 patients [Grade A=15, Grade B=13, Grade C=1].
21 patients [Grade A=12, Grade B=8, Grade C=1].
Discussion
We report results from the first large prospective randomized trial evaluating novel approaches to assess GERD complications (including BE) in a population cohort in the United States. In this study we have demonstrated comparable participation rates, clinical effectiveness and safety of unsedated TNE (performed in both the hospital and in a mobile setting) to sedated EGD for the assessment of GERD complications. Though the participation rates were numerically higher in the unsedated techniques (muTNE and huTNE) compared to sEGD, the difference was not statistically significant. The patient's visit was significantly shorter using uTNE techniques with adequate tolerability and acceptability. Patients with frequent heartburn or acid regurgitation were significantly more likely to participate in screening compared to those with infrequent or no reflux symptoms. The overall proportion of subjects in the study population who had BE was 7.8%, with 4.4% of subjects without reflux symptoms being diagnosed with BE.
In this study, subjects were randomized and offered their allocated screening test only, as opposed to offering all three to every patient (with the patient choosing the technique). This design was chosen in order to obtain comparative and valid estimates of participation rates for each one of the three techniques separately. Results from this study are concordant with the previous pilot trial comparing uTNE, video capsule endoscopy (VCE) and sEGD screening (14). Though VCE was the most acceptable in this study, given its suboptimal accuracy for the detection of BE (23), this technique was not assessed in the current study.
Development of non-invasive screening tests over the past few years have led to renewed interest in screening for BE and EAC. The Cytosponge™ is a capsule cell-sampling device, coupled with a biomarker(24). In a primary care study involving 12 UK general practices, 18% of invited subjects agreed to take part. The sensitivity and specificity of the test for the detection of Barrett's epithelium was 73.3% and 93.8% (24). 3.0% of participants (15/501) had a confirmed diagnosis of BE. Cytosponge™ screening was also found to be cost-effective in a recent economic modeling study (25). This device is promising given its low cost and potential widespread applicability. Nonetheless, it is a non-endoscopic technique and hence will still need a confirmatory endoscopy.
BE prevalence rates between 6–25% have been reported from U.S. referral center cohorts (26, 27). Population studies from Europe have reported lower prevalence (1.3%–1.6%) for BE (28, 29). This may reflect differences in risk factors such as obesity, Helicobacter pylori and differences in endoscopic and histological assessment(30). The reasons for higher BE prevalence in our study (7.6%) compared to the Cytosponge™ study are not fully clear. Participants in both studies had comparable sociodemographic characteristics but all patients in the Cytosponge™ study had history of reflux symptoms or receiving acid suppressive medication prescriptions. Participation rates in the current study were substantially higher than in the Cytosponge™ study. Prevalence of BE while higher in those with frequent and infrequent reflux symptoms (10%), was also substantial on those without reflux symptoms (4.4%).
Unsedated TNE has been shown to be an accurate alternative to sEGD for the diagnosis of BE (12, 13). However, comparative data on technical success rate, tolerability and acceptability of uTNE with respect to sEGD in the population are lacking. While uTNE screening was found to be more cost effective than screening using sEGD(11), assumptions such as a 95% participation rate in screening were used. Our study provides the first real data on participation and completion rates of uTNE in the community, in addition to demonstrating the comparable clinical effectiveness of uTNE to sEGD. Although a substantial majority of patients who underwent muTNE and huTNE were willing to undergo the test again in future if necessary (78.9% and 83.3%, respectively), acceptability of sEGD was significantly higher (93.4%, p=0.001). It is likely that the use of sedation for sEGD may explain this as it makes the procedure more comfortable to patients, and likely impairs memory and re-collection of the event.
Mobile screening vans have been found to be effective instruments in increasing population participation in screening (in and outside the U.S.) for cervical(31), colon(32), breast(33) and lung(34) cancer with no decrement in accuracy, in a cost-effective manner(35). A survey study conducted by us previously suggested that participation in BE screening may increase by the use of mobile screening(15). Therefore we elected to evaluate this technique by incorporating the portable and disposable EndoSheath system in a mobile research van,. We did not find a statistically significant increase in uptake rates between the hospital and mobile TNE arms. This may be a result of the excellent access to medical care and endoscopy in Olmsted County and should be tested in more medically underserved populations with limited access to screening tests. Despite this, an important finding of our study was the reduction in recovery and overall patient visit duration achieved by using the muTNE and huTNE techniques compared to sEGD, without any substantial decrement in yield, tolerability and safety. Furthermore, uTNE can be performed by non-gastroenterology physicians as well as by physician extenders raising the possibility of this being a truly community based screening tool (36).
Successful biopsy acquisition remains a limitation of the current uTNE device. This was lower in the muTNE (79%) and huTNE (83.3%) groups compared to sEGD (100%) (p=0.001), primarily due to inability to intubate the esophagus with the larger biopsy sheath. In those cases, the endoscopist switched to the thinner channel-free sheath to enable successful intubation and visualization of the esophagus, without biopsies being taken. If screening is to be implemented in practice, then the issue of biopsy acquisition using uTNE becomes less important because all subjects with endoscopically suspected BE on uTNE screening will likely require a clinical sEGD for surveillance biopsies(7).
There was a higher yield of erosive esophagitis with the hospital based techniques compared to the mobile technique. The reasons for this remain unclear, in particular, as the proportion of subjects with reflux symptoms and other baseline characteristics were comparable across the three study arms. Better visualization of the esophagus in studies conducted in the hospital may be a potential explanation.
This study provides an insight into the potential subset of undetected BO in the community(37) and helped us to determine the acceptability of screening in a previously untested segment of the population. Given the demographic make- up of Olmsted County, our study sample is representative of the US white population, which is the likely target population for future screening efforts for BO and OAC. Although response bias is a possibility because the cohort consisted of responders to previous health questionnaires, this is unlikely because of the lack of difference between responders and non-responders that has been demonstrated in this population(38).
In conclusion, in this prospective randomized population based study utilizing state-of-the-art novel minimally invasive endoscopic techniques in a hospital and mobile setting, we demonstrate comparable clinical effectiveness, safety and participation rates with uTNE. Data from this study may help inform future economic and risk stratification modeling studies. BE and esophagitis prevalence in the community is substantial, in both those with and without reflux symptoms.
Study Highlights.
What is current knowledge?
Screening for BE with unsedated transnasal endoscopy (uTNE) has been found to be cost effective, but assumptions such as a 95% participation rate and up to 15% prevalence rates were used.
The comparative effectiveness of esophageal assessment by uTNE compared to sedated endoscopy is not known.
Existing uTNE devices are not suitable for widespread use in the community setting due to limited portability and need for decontamination equipment.
Mobile screening vans have increased participation in screening for cervical, colon, breast, and lung cancers with no decrement in accuracy.
What is new here?
The clinical effectiveness of uTNE is comparable to that of sedated endoscopy, enabling the use of uTNE in BE screening of high risk individuals.
uTNE screening using the novel EndoSheath® (portable and disposable) esophagoscope is effective and acceptable both in the hospital and community settings
Overall participation in uTNE screening was substantial (but lower than previously assumed), and prevalence of BE in our population-based study sample was 7.8%.
Mobile van screening for BE and esophageal adenocarcinoma (EAC) may be an effective alternative to hospital or clinic-based techniques.
Acknowledgments
Funding: Funded by NIH grant (RC4DK090413) and the American College of Gastroenterology Career Development Award.
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Financial Support: Supported in part by the National Institutes of Health (RC4DK090413) and the National Center for Advancing Translational Sciences (UL TROOO135). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of National Institutes of Health. Dr. S. S. Sami is funded by an Olympus-Core National Endoscopy Research Fellowship grant, Core charity, United Kingdom
Potential competing interests: Dr. K. Ragunath received research grant funding from Intromedic Ltd (Seoul, South Korea) and Olympus Keymed (United Kingdom). Other authors have no conflicts to declare.
Abbreviations
- BE
Barrett's Esophagus
- EAC
esophageal adenocarcinoma
- GERD
gastroesophageal reflux disease
- GERQ
gastroesophageal reflux questionnaire
- huTNE
unsedated transnasal endoscopy in hospital outpatient endoscopy suite
- MRV
mobile research vehicle
- muTNE
unsedated transnasal endoscopy in mobile research van
- REP
Rochester Epidemiology Project
- sEGD
sedated endoscopy
- uTNE
unsedated transnasal endoscopy
Footnotes
Guarantor of the article: Dr. Prasad Iyer accepts full responsibility for the conduct of the study.
Specific Author Contributions: Dr. S. S. Sami contributed to the acquisition of data; analysis; interpretation of data; and drafted the manuscript.
Kelly T. Dunagan contributed to the acquisition of data; analysis
Michele L. Johnson contributed to the acquisition of data; analysis
Cathy D. Schleck contributed to acquisition of data and data analysis
Alan R. Zinsmeister contributed to the acquisition of data; statistical analysis
Louis-Michel Wongkeesong contributed to acquisition of data, critical revision of the manuscript for important intellectual content
Kenneth K. Wang contributed to critical revision of the manuscript for important intellectual content
David A. Katzka contributed to acquisition of data, critical revision of the manuscript for important intellectual content
Krish Ragunath contributed to critical revision of the manuscript for important intellectual content.
Prasad G. Iyer contributed to study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; obtained funding; study supervision.
References
- 1.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das A, Singh V, Fleischer DE, et al. A Comparison of Endoscopic Treatment and Surgery in Early Esophageal Cancer: An Analysis of Surveillance Epidemiology and End Results Data. Am J Gastroenterol. 2008;103:1340–1345. doi: 10.1111/j.1572-0241.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- 3.Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut. 2012;61:970–6. doi: 10.1136/gutjnl-2011-300730. [DOI] [PubMed] [Google Scholar]
- 4.Corley DA, Mehtani K, Quesenberry C, et al. Impact of Endoscopic Surveillance on Mortality From Barrett's Esophagus-Associated Esophageal Adenocarcinomas. Gastroenterology. 2013;145:312–319.e1. doi: 10.1053/j.gastro.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung KW, Talley NJ, Romero Y, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett's esophagus: a population-based study. Am J Gastroenterol. 2011;106:1447–55. doi: 10.1038/ajg.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett's esophagus in esophageal adenocarcinoma: A systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2013 doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 8.Spechler SJ, Sharma P, Souza RF, et al. American gastroenterological association technical review on the management of Barrett's esophagus. Gastroenterology. 2011;140:e18–e52. doi: 10.1053/j.gastro.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barrett's esophagus among men. Am J Gastroenterol. 2013;108:353–62. doi: 10.1038/ajg.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thrift AP, Garcia JM, El-Serag HB. A Multibiomarker Risk Score Helps Predict Risk for Barrett's Esophagus. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nietert PJ, Silverstein MD, Mokhashi MS, et al. Cost-effectiveness of screening a population with chronic gastroesophageal reflux. Gastrointest Endosc. 2003;57:311–8. doi: 10.1067/mge.2003.101. [DOI] [PubMed] [Google Scholar]
- 12.Shariff MK, Bird-Lieberman EL, O'Donovan M, et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett's esophagus. Gastrointest Endosc. 2012;75:954–61. doi: 10.1016/j.gie.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Jobe BA, Hunter G, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett's esophagus: a randomized and blinded comparison. Am J Gastroenterol. 2006;101:2693–703. doi: 10.1111/j.1572-0241.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 14.Chang JY, Talley NJ, Locke GR, 3rd, et al. Population screening for barrett esophagus: a prospective randomized pilot study. Mayo Clinic Proceedings. 2011;86:1174–80. doi: 10.4065/mcp.2011.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta M, Beebe TJ, Dunagan KT, et al. Screening for Barrett's Esophagus: Results from a Population-Based Survey. Dig Dis Sci. 2014 Mar 21; doi: 10.1007/s10620-014-3092-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peery AF, Hoppo T, Garman KS, et al. Feasibility, safety, acceptability, and yield of office-based, screening transnasal esophagoscopy (with video) Gastrointest Endosc. 2012;75:945–953. doi: 10.1016/j.gie.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 18.Locke GR, Talley NJ, Weaver AL, et al. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc. 1994;69:539–47. doi: 10.1016/s0025-6196(12)62245-9. [DOI] [PubMed] [Google Scholar]
- 19.Halder SL, Locke GR, 3rd, Schleck CD, et al. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133:799–807. doi: 10.1053/j.gastro.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392–9. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 21.Zaman A, Hahn M, Hapke R, et al. A randomized trial of peroral versus transnasal unsedated endoscopy using an ultrathin videoendoscope. Gastrointest Endosc. 1999;49:279–284. doi: 10.1016/s0016-5107(99)70001-5. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 23.Bhardwaj A, Hollenbeak CS, Pooran N, et al. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett's esophagus in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2009;104:1533–1539. doi: 10.1038/ajg.2009.86. [DOI] [PubMed] [Google Scholar]
- 24.Kadri SR, Lao-Sirieix P, O'Donovan M, et al. Acceptability and accuracy of a nonendoscopic screening test for Barrett's oesophagus in primary care: cohort study. BMJ. 2010;341:c4372. doi: 10.1136/bmj.c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benaglia T, Sharples LD, Fitzgerald RC, et al. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett's esophagus. Gastroenterology. 2013;144:62–73 e6. doi: 10.1053/j.gastro.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 26.Gerson L. Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology. 2002;123:461–467. doi: 10.1053/gast.2002.34748. [DOI] [PubMed] [Google Scholar]
- 27.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–7. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett's esophagus in the general population: An endoscopic study. Gastroenterology. 2005;129:1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 29.Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and barrett's oesophagus in the general population: The Loiano-Monghidoro study. Gut. 2008;57:1354–1359. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 30.Rex DK, Shaw M, Wong R. Prevalence of Barrett's Esophagus. Gastroenterology. 2006;130:1373–1374. doi: 10.1053/j.gastro.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Mauad EC, Nicolau SM, Gomes UA, et al. Can mobile units improve the strategies for cervical cancer prevention? Diagn Cytopathol. 2010;38:727–30. doi: 10.1002/dc.21287. [DOI] [PubMed] [Google Scholar]
- 32.Anderson DW, Goldberg PA, Algar U, et al. Mobile colonoscopic surveillance provides quality care for hereditary nonpolyposis colorectal carcinoma families in South Africa. Colorectal Dis. 2007;9:509–14. doi: 10.1111/j.1463-1318.2006.01172.x. [DOI] [PubMed] [Google Scholar]
- 33.Reuben DB, Bassett LW, Hirsch SH, et al. A randomized clinical trial to assess the benefit of offering on-site mobile mammography in addition to health education for older women. Am J Roentgenol. 2002;179:1509–14. doi: 10.2214/ajr.179.6.1791509. [DOI] [PubMed] [Google Scholar]
- 34.Sone S, Takashima S, Li F, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet. 1998;351:1242–5. doi: 10.1016/S0140-6736(97)08229-9. [DOI] [PubMed] [Google Scholar]
- 35.O'Malley AS, Lawrence W, Liang W, et al. Feasibility of mobile cancer screening and prevention. J Health Care Poor Underserved. 2002;13:298–319. [PubMed] [Google Scholar]
- 36.Alashkar B, Faulx AL, Hepner A, et al. Development of a Program to train Physician Extenders to Perform Transnasal Esophagoscopy and Screen for Barrett's Esophagus'. Clin Gastroenterol Hepatol. 2013;12:785–92. doi: 10.1016/j.cgh.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cameron AJ, Zinsmeister AR, Ballard DJ, et al. Prevalence of columnar-lined (Barrett's) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology. 1990;99:918–22. doi: 10.1016/0016-5085(90)90607-3. [DOI] [PubMed] [Google Scholar]
- 38.Bendtsen F, Skovgaard LT, Sorensen TI, et al. Agreement among multiple observers on endoscopic diagnosis of esophageal varices before bleeding. Hepatology. 1990;11:341–7. doi: 10.1002/hep.1840110302. [DOI] [PubMed] [Google Scholar]


