Abstract
Background
Statins have proven efficacy in the reduction of cardiovascular events, but the financial impact of its widespread use can be substantial.
Objective
To conduct a cost-effectiveness analysis of three statin dosing schemes in the Brazilian Unified National Health System (SUS) perspective.
Methods
We developed a Markov model to evaluate the incremental cost-effectiveness ratios (ICERs) of low, intermediate and high intensity dose regimens in secondary and four primary scenarios (5%, 10%, 15% and 20% ten-year risk) of prevention of cardiovascular events. Regimens with expected low-density lipoprotein cholesterol reduction below 30% (e.g. simvastatin 10mg) were considered as low dose; between 30-40%, (atorvastatin 10mg, simvastatin 40mg), intermediate dose; and above 40% (atorvastatin 20-80mg, rosuvastatin 20mg), high-dose statins. Effectiveness data were obtained from a systematic review with 136,000 patients. National data were used to estimate utilities and costs (expressed as International Dollars - Int$). A willingness-to-pay (WTP) threshold equal to the Brazilian gross domestic product per capita (circa Int$11,770) was applied.
Results
Low dose was dominated by extension in the primary prevention scenarios. In the five scenarios, the ICER of intermediate dose was below Int$10,000 per QALY. The ICER of the high versus intermediate dose comparison was above Int$27,000 per QALY in all scenarios. In the cost-effectiveness acceptability curves, intermediate dose had a probability above 50% of being cost-effective with ICERs between Int$ 9,000-20,000 per QALY in all scenarios.
Conclusions
Considering a reasonable WTP threshold, intermediate dose statin therapy is economically attractive, and should be a priority intervention in prevention of cardiovascular events in Brazil.
Keywords: Hydroxymethylglutaryl-CoA Reductase Inhibitors, Cardiovascular Diseases, Prevention, Cost-Benefit Analysis, Unified Health System
Introduction
The efficacy of statins has been studied in several large randomized clinical trials (RCTs), and the pooled results of these trials showed reduction of cardiovascular events (CVEs) in various scenarios1-3. Of utmost importance is the expected large proportion of adults who would fulfill criteria for prevention of cardiovascular events and require statin therapy. Current annual expenditures with statins in the Brazilian Unified National Health System (SUS) is approximately 65,000,000 international dollars (Int$), of which the largest market share belongs to atorvastatin4.
The cost-effectiveness of statins in CVE prevention has been appraised in numerous studies in different countries5, with incremental cost-effectiveness ratios (ICERs) showing considerable variation. Compared with placebo, statins generally have acceptable ICERs according to the willingness-to-pay (WTP) thresholds of most countries, especially in secondary CV prevention6,7, with more conflicting results in primary CV prevention1,8,9. In studies comparing high- versus low-intensity schemes, the conclusions show great variation10,11. These analyses, however, were conducted in high-income countries, with limited transferability to Brazil, given the different cost parameters and willingness-to-pay thresholds12.
In Brazil, national treatment guidelines recommend statins for secondary CV prevention or for individuals with high low-density lipoprotein (LDL) cholesterol levels13. Statins were introduced in the Brazilian healthcare system in 2002. Although access to these drugs has been progressively facilitated with inclusion of simvastatin in the primary care pharmacy, their availability to the population is neither universal nor available on a regular basis.
There is no consensus among distinct healthcare systems on whether to broadly offer statins for cardiovascular prevention. Considering recently revised international guidelines14, current aspects to be addressed are: 1) what the optimal intensity of therapy is, and 2) what should be the 10-year cardiovascular risk threshold to initiate statin therapy. These definitions are of particular importance for Brazil, considering the financial and healthcare impact of such choices. Therefore, the purpose of this study was to conduct a cost-utility analysis from the Brazilian Unified National Health System (SUS) perspective of three different regimens of statins (high, moderate and low intensity) in both primary and secondary prevention of CV events.
Methods
Target Population
There were two target populations in this study. The first target population was comprised of male and female patients from 45 to 85 years old in secondary prevention of CV events, who recently suffered a first qualifying event: stable angina (SA), myocardial infarction (MI) or stroke. The second target population included men and women in primary prevention, who had a 10-year risk of hard CV events varying from 5% to 20%. Some examples (using the Framingham risk prediction equations15)) of the risk profile of primary prevention patients are given below:
A person with a 5% risk could be a 45-49 years old male, with total cholesterol (TC) of 160-199 mg/dL and high-density lipoprotein cholesterol (HDL-C) of 35-44 mg/dL, with a systolic blood pressure (SBP) of 120-129 mmHg, non-smoker and non-diabetic.
A 10% risk in ten years is depicted by a 50-54 years old female, with TC of 160-199 mg/dL and HDL-C of 35-44 mg/dL, with a SBP of 140-149 mmHg, non-smoker and non-diabetic.
A 15% risk is illustrated by a 60-64 years old male, with TC of 160-199 mg/dL and HDL-C of 45-49 mg/dL, with a SBP higher than 150 mmHg, non-smoker and non-diabetic.
Finally, a 20% risk could be represented by a 50-54 years old female, with TC of 160-199 mg/dL and HDL-C of 45-49 mg/dL, with a SBP of 140-149 mmHg, smoker and diabetic.
Interventions evaluations and effectiveness estimation
Three statin strategies were evaluated: high-, moderate- and low-intensity regimens. The benefit of statins was modeled through reduction of non-fatal MIs, strokes and CV deaths. To determine clinical effectiveness for each alternative strategy, a systematic review in MEDLINE and Cochrane CENTRAL was conducted, in which we searched for clinical trials comparing any statin against placebo, usual care or other statin, in which at least one of the aforementioned outcomes was assessed. The details of this systematic review have been previously described16. Briefly, treatments were categorized according to the expected LDL cholesterol reduction. Regimens with an expected LDL cholesterol reduction of up to 30% (such as simvastatin 10 mg and pravastatin 20-40 mg) were considered low-dose statins; between 30% and 40% (atorvastatin 10 mg, fluvastatin 60-80 mg, lovastatin 30-40 mg, simvastatin 20-40 mg), moderate dose; and over 40% (atorvastatin 20-80 mg and rosuvastatin 20 mg), high dose17.
Only studies with duration of 6 months or more and a total number of patients greater than 100 were included. We excluded trials that focused on distinct clinical settings, namely, patients with advanced renal disease, heart failure, and studies that included exclusively patients of Asian origin (for whom the response to statins is markedly heightened as compared to Caucasians)18.
Studies were then divided into primary or secondary CV prevention according to the characteristics of included patients; trials with mixed sets of populations (more than 15% primary prevention patients in studies with a predominant secondary prevention subjects, or vice versa) were excluded. The systematic review yielded 26 secondary prevention studies (73,634 patients) and 14 primary prevention studies (62,905 patients), which are displayed in Table 1.
Table 1.
Characteristics of studies included in the systematic review
| Study | Year | Patients randomized | Interventions (mg/day) | Type of comparison | Mean age | Follow-up (yrs) | |
|---|---|---|---|---|---|---|---|
| Primary prevention | |||||||
| ACAPS | 1994 | 919 | L30 vs placebo | Moderate dose vs. placebo/no treatment | 61.7 | 2.8 | |
| AFCAPS/TexCAPS | 1998 | 6,605 | L30 vs placebo | Moderate dose vs. placebo/no treatment | 58.7 | 5.2 | |
| ALLHAT-LLT | 2002 | 10,355 | P40 vs UC | Low dose vs. placebo/no treatment | 66.4 | 4.8 | |
| ASCOT-LLA | 2003 | 10,305 | A10 vs placebo | Moderate dose vs. placebo/no treatment | 63.2 | 3.3 | |
| CAIUS | 1996 | 305 | P40 vs placebo | Low dose vs. placebo/no treatment | 55.0 | 3 | |
| CARDS | 2004 | 2,838 | A10 vs placebo | Moderate dose vs. placebo/no treatment | 62.0 | 4 | |
| DALI | 2001 | 145 | A80 vs A10 vs placebo | High vs. moderate dose vs. placebo/no treatment | 59.4 | 0.6 | |
| HYRIM | 2005 | 568 | F40 vs placebo | Low dose vs. placebo/no treatment | 57.1 | 4 | |
| JUPITER | 2008 | 17,802 | R20 vs placebo | High dose vs. placebo/no treatment | 66.0 | 1.9 | |
| KAPS | 1995 | 447 | P40 vs placebo | Low dose vs. placebo/no treatment | 57.4 | 3 | |
| Mohler | 2003 | 240 | A80 vs A10 vs placebo | High vs. moderate dose vs. placebo/no treatment | 68.0 | 1 | |
| PREVEND IT | 2004 | 864 | P40 vs placebo | Low dose vs. placebo/no treatment | 51.3 | 3.8 | |
| WOSCOPS | 1995 | 6,595 | P40 vs placebo | Low dose vs. placebo/no treatment | 55.2 | 4.9 | |
| Secondary prevention | |||||||
| 3T | 2003 | 1,093 | A30 vs S35 | High vs. moderate dose | 62.8 | 1 | |
| 4S | 1994 | 4,444 | S30 vs placebo | Moderate dose vs. placebo/no treatment | 58.0 | 5.4 | |
| ALLIANCE | 2004 | 2,442 | A40 vs UC | High dose vs. placebo/no treatment | 61.2 | 4.3 | |
| A-to-Z | 2004 | 4,497 | S80 vs S20 | High vs. moderate dose | 61.0 | 2 | |
| CARE | 1996 | 4,159 | P40 vs placebo | Low dose vs. placebo/no treatment | 59.0 | 5 | |
| CCAIT | 1994 | 331 | L40 vs placebo | Moderate dose vs. placebo/no treatment | 53.8 | 2 | |
| CIS | 1997 | 254 | S40 vs placebo | Moderate dose vs. placebo/no treatment | 49.3 | 2.3 | |
| CLAPT | 1999 | 226 | L40 vs UC | Moderate dose vs. placebo/no treatment | 53.9 | 2 | |
| FLARE | 1999 | 834 | F80 vs placebo | Moderate dose vs. placebo/no treatment | 60.5 | 0.8 | |
| FLORIDA | 2002 | 540 | F80 vs placebo | Moderate dose vs. placebo/no treatment | 60.5 | 1 | |
| GISSI-P | 2000 | 4,271 | P30 vs UC | Low dose vs. placebo/no treatment | 59.9 | 2 | |
| IDEAL | 2005 | 8,888 | A80 vs S20 | High vs. moderate dose | 61.7 | 4.8 | |
| LIPID | 1998 | 9,014 | P40 vs placebo | Low dose vs. placebo/no treatment | 62.0 | 6.1 | |
| LIPS | 2002 | 1,677 | F80 vs placebo | Moderate dose vs. placebo/no treatment | 60.0 | 3.9 | |
| LISA | 1999 | 365 | F60 vs placebo | Moderate dose vs. placebo/no treatment | 59.8 | 1 | |
| MAAS | 1994 | 381 | S20 vs placebo | Moderate dose vs. placebo/no treatment | 55.3 | 4 | |
| PLAC I | 1995 | 408 | P40 vs placebo | Low dose vs. placebo/no treatment | 57.0 | 3 | |
| PLAC II | 1995 | 151 | P30 vs placebo | Low dose vs. placebo/no treatment | 62.0 | 3 | |
| PREDICT | 1997 | 695 | P40 vs placebo | Low dose vs. placebo/no treatment | 58.4 | 0.5 | |
| PROVE IT - TIMI | 2004 | 4,162 | A80 vs P40 | High vs. low dose | 58.2 | 2 | |
| REGRESS | 1995 | 884 | P40 vs placebo | Low dose vs. placebo/no treatment | 56.2 | 2 | |
| REVERSAL | 2004 | 502 | A80 vs P40 | High vs. low dose | 56.2 | 1.5 | |
| SAGE | 2007 | 891 | A80 vs P40 | High vs. low dose | 72.5 | 1 | |
| SCAT | 2000 | 460 | S30 vs placebo | Moderate dose vs. placebo/no treatment | 61.0 | 4 | |
| Schmermund | 2006 | 366 | A80vs A10 | High vs. moderate dose | 61.5 | 1 | |
| SEARCH | 2010 | 12,064 | S80 vs S20 | High vs. moderate dose | 64.2 | 6.7 | |
| SPARCL | 2006 | 4,731 | A80 vs placebo | High dose vs. placebo/no treatment | 62.8 | 4.9 | |
| TNT | 2005 | 10,001 | A80vs A10 | High vs. moderate dose | 60.5 | 4.9 | |
A: atorvastatin; F: fluvastatin; L: lovastatin; P: pravastatin; R: rosuvastatin; S: simvastatin; UC: usual care.
The meta-analysis model chosen was the Bayesian mixed treatment comparison (MTC) approach, in order to include both direct and indirect evidence19, except in cases where only direct evidence was available, for which a conventional random-effects meta-analysis was carried out. The relative risks (RR) for events of high-, moderate- and low-dose statins against no statin are displayed in Table 2. We assumed that the effectiveness of statins was maintained constant throughout life, similar to other statin cost-effectiveness models20-22.
Table 2.
Base case estimates and ranges used in sensitivity analyses
| Input variable | Base case value | Range | Distribution | Source | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Statin effectiveness: relative risks vs no statin | |||||||||||
| Myocardial infarction | |||||||||||
| Primary prevention | |||||||||||
| Low dose | 0.76 | 0.57 - 0.97 | Log normal | 40 | |||||||
| Intermediate dose | 0.65 | 0.50 - 0.85 | Log normal | 40 | |||||||
| High dose | 0.39 | 0.22 - 0.64 | Log normal | 40 | |||||||
| Secondary prevention | |||||||||||
| Low dose | 0.74 | 0.65 - 0.84 | Log normal | 40 | |||||||
| Intermediate dose | 0.68 | 0.59 - 0.78 | Log normal | 40 | |||||||
| High dose | 0.58 | 0.50 - 0.67 | Log normal | 40 | |||||||
| Cardiovascular death | |||||||||||
| Primary prevention | |||||||||||
| Low dose | 0.85 | 0.72 - 1.01 | Log normal | 40 | |||||||
| Intermediate dose | 0.85 | 0.72 - 1.01 | Log normal | 40 | |||||||
| High dose | 0.81 | 0.70 - 0.93 | Log normal | 40 | |||||||
| Secondary prevention | |||||||||||
| Low dose | 0.83 | 0.69- 1.03 | Log normal | 40 | |||||||
| Intermediate dose | 0.72 | 0.58 - 0.89 | Log normal | 40 | |||||||
| High dose | 0.68 | 0.53 - 0.85 | Log normal | 40 | |||||||
| Stroke | |||||||||||
| Primary prevention | |||||||||||
| Low dose | 0.94 | 0.63 - 1.36 | Log normal | 40 | |||||||
| Intermediate dose | 0.70 | 0.47 - 1.00 | Log normal | 40 | |||||||
| High dose | 0.56 | 0.29 - 1.00 | Log normal | 40 | |||||||
| Secondary prevention | |||||||||||
| Low dose | 0.85 | 0.72 - 0.98 | Log normal | 40 | |||||||
| Intermediate dose | 0.85 | 0.72 - 0.98 | Log normal | 40 | |||||||
| High dose | 0.77 | 0.64 - 0.90 | Log normal | 40 | |||||||
| Costs (Int$) | |||||||||||
| Annual cost of care | |||||||||||
| Previous MI or SA | 1,699 | 849 - 2,548 | Triangular | 28 | |||||||
| Stroke | 571 | 286 - 857 | Triangular | Own estimate | |||||||
| Stroke - additional first year a | 268 | 134 - 403 | Triangular | Own estimate | |||||||
| Primary prevention, 5% risk in 10 years | 18 | 9 - 27 | Triangular | Own estimate | |||||||
| Primary prevention, 10% risk in 10 years | 56 | 28 - 84 | Triangular | Own estimate | |||||||
| Primary prevention, 15% risk in 10 years | 475 | 238 - 713 | Triangular | Own estimate | |||||||
| Primary prevention, 20% risk in 10 years | 575 | 288 - 862 | Triangular | Own estimate | |||||||
| Acute MI hospitalization | 1,501 | 751 - 2,253 | Triangular | 27 | |||||||
| Acute stroke hospitalization | 680 | 341 - 1,021 | Triangular | 27 | |||||||
| Low dose statin - annual cost | 26 | 13 - 39 | Triangular | Information from MOH | |||||||
| Alternative scenario | 65 | - | - | 41 | |||||||
| Intermediate dose statin - annual cost | 45 | 22 - 67 | Triangular | Information from MOH | |||||||
| Alternative scenario | 231 | - | - | 41 | |||||||
| High dose statin - annual cost | 224 | 112 - 335 | Triangular | Information from MOH | |||||||
| Alternative scenario | 410 | - | - | 41 | |||||||
| Utilities | |||||||||||
| General population | 0.80 | 0.63 - 0.93 | Beta | 26 | |||||||
| Previous MI or SA | 0.74 | 0.61 - 0.86 | Beta | 25 | |||||||
| Stroke | 0.60 | 0.49 - 0.69 | Beta | Own estimate | |||||||
| Discount rate(cost and effectiveness) | 5% | 0% - 10% | Uniform | 42 | |||||||
Speech and physical therapy; MI: myocardial infarction; MOH: Ministry of Health; SA: stable angina. All effectiveness data are based on our previous meta-analysis, with the stratification of the data according to type of prevention (primary or secondary) in the clinical trials.
Economic Models
Five microsimulation Markov models were constructed: one for secondary and the remainder for primary prevention patients (with 10-year risks for CV events ranging from 5% to 20%). All models contained three statin strategies (low, moderate and high dose) and a no-statin arm.
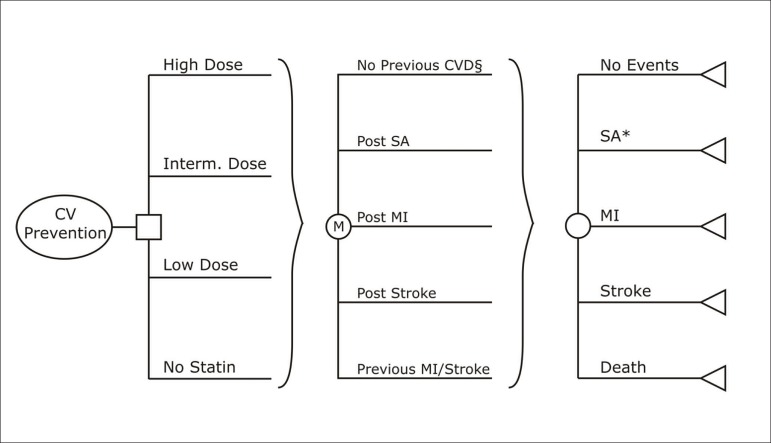
The secondary prevention model included the following initial health states: post-MI, post-stable angina, and post-stroke. The primary prevention models also included an event-free state, where all patients started in the simulations. A simplified schematic representation of the models is displayed in Figure 1.
Figure 1.
Schematic representation of the cost-effectiveness models.
* If a patient in the post-stroke state had a diagnosis of stable angina, he would remain in the same state, but with a tracker variable signaling the angina diagnosis.
§ The structure of the secondary prevention model was similar, with the exception of the "No previous CVD” Markov state, which was omitted. CV: cardiovascular; CVD: cardiovascular disease; MI: myocardial infarction; SA: stable angina.
Each model simulated cohorts of patients with distributions of gender and starting age according to the Brazilian general population between 45 and 85 years old23. Age-dependent non-cardiovascular mortality rates were estimated from the National Brazilian Vital Statistics life tables24. Baseline risks for initial CV events in the primary prevention models were pre-determined, with ten-year risks ranging from 5% to 20%. National data was missing for some the estimation of some parameters in the model, and population-based estimates from the United Kingdom were applied for: (1) proportions of angina, MI, stroke or CV death as a first CV event for the primary prevention models; (2) proportions of angina, MI and stroke as initial health states in the secondary prevention model; (3) transition probabilities between the angina, MI, stroke and death states in all models, stratified by age and gender1.
Costs were expressed in International Dollars (Int$), using the 2011 conversion rate reported by the World Bank, in which 1 Int$ = 1.81 R$. Effectiveness was measured in quality-adjusted life years (QALYs). The discount rate for costs and effects used throughout the model was 5% per year. We conducted our analysis from the perspective of a public third-party payer (SUS) with a lifetime horizon. Each microsimulation was run with 200,000 trials. The software used for the models was TreeAge Pro 2009 (TreeAge Software, Inc., Williamstown, Massachusetts).
Utilities
Two studies from Brazilian patients were used to derive utility data for the ischemic heart disease25 and event-free states26. No prior national study has estimated the effect of stroke on utility values. In the Ward et al. paper, the utility of stroke was equivalent to 80% of the MI utility, and we applied this proportion in our MI utility to estimate the stroke utility in the model (Table 1).
Costs
Direct medical costs were calculated based on both initial treatment costs and future medical procedures. Hospitalization costs for acute MI and acute stroke were extracted from Brazilian SUS data, which included the average national cost for these admissions for the year 201127. The annual cost of care for patients with previous MI or stable angina (including all medications except statins, laboratory tests, revascularization procedures and medical visits) were derived from a study previously published by our group, adjusted for inflation28. The annual cost of care for patients with previous stroke was calculated based on expert neurologist opinion that considered cost data, including medications, outpatient visits, rehabilitation therapies and exams from a post-stroke clinic. The annual cost of care of primary prevention was estimated from expected resources consumption of 5% to 20% 10-year risk patients, including medications, laboratory and cardiology (EKG, echocardiogram, treadmill test) exams, and outpatient visits. All costs represent public reimbursement values.
Calculated statin costs assumed group representatives: atorvastatin 20 mg, simvastatin 40 mg and simvastatin 10 mg for high-, moderate- and low-dose strategies. These drugs were selected according to the prescription patterns observed in the Brazilian SUS. The price per pill paid by the Brazilian government for these drugs was Int$0.58, Int$0.09 and Int$0.04, respectively. The final drug prices used in the models also included its distribution cost. According to an unpublished study conducted in the Brazilian SUS, the general distribution cost for each pill would be equivalent to Int$0.035 (Mengue S, personal communication).
Sensitivity Analyses
We performed sensitivity analyses on most model parameters and also a second-order Monte Carlo simulation (probabilistic sensitivity analysis or PSA) with 1,000 samples. The treatment effect parameters were the RRs for MI, stroke and CV death. These were evaluated in conjunction in the sensitivity analysis. For example, the evaluation of the lowest estimate of each dose-effectiveness was obtained simultaneously employing the upper limits of the RRs of MI, stroke and CV death found for treatment with that dose. RRs were varied between the boundaries of the meta-analyses' credible intervals; the distribution used for these parameters was log-normal.
Costs were varied between ±50% of their original values and were modeled through a triangular distribution in the probabilistic sensitivity analysis. We used a beta distribution for utilities and its 2.5% and 97.5% percentiles set the boundaries for one-way sensitivity analyses. Discounts for costs and utilities varied between 0% and 10% and followed a uniform distribution.
We also created an alternative scenario of statin costs using the lowest sale price of generic statins (Table 1).
To appraise the influence of parameter variation in the model, we performed the following:
In each of the five scenarios, we determined which statin would be used, according to a pre-defined WTP threshold of Int$11,770, equal to the Brazilian gross domestic product (GDP) per capita in 201129. In each scenario, only one strategy would be chosen: for example, if (1) the low dose dominated no statin, (2) the moderate dose had an ICER of Int$6,000 versus low dose, and (3) the high dose had an ICER of Int$30,000 versus moderate dose, than the moderate dose would be the statin of choice.
These analyses were performed at lower and higher estimates of selected parameters, to evaluate if the statin of choice was altered by the uncertainties in these parameters.
The WTP equal to the country's GDP per capita was chosen according to the definition of the World Health Organization (WHO) of ICER values that should be regarded as highly cost-effective30.
Results
Base case results
Discounted lifetime costs and utilities of the four strategies in the five scenarios are displayed in Table 3. Within the time frame provided by the study's horizon, estimated QALYs ranged from 8.11 to 10.57 and cumulative costs ranged from Int$1,006 to Int$16,825 in the no-statin group. In a ten-year period, the proportion of patients suffering events (SA, MI, stroke and CV death) in the primary prevention models in this group were as follows: 4.63% in the 5% ten-year risk group, 9.46% in the 10% ten-year risk, 14.16% in the 15% ten-year risk, and 18.93% in the 20% ten-year risk. The reason that the event rate was lower than the defined event rate in the model's equations is that the population susceptible to these events diminished throughout time due to non-CV deaths.
Table 3.
Base case analysis. Costs, effectiveness and incremental cost-effectiveness ratios for alternative treatment strategies in secondary and primary prevention
| Secondary prevention | Primary prevention, 20% risk | Primary prevention, 15% risk | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Costs (Int$) | QALYs | ICER | Costs (Int$) | QALYs | ICER | Costs (Int$) | QALYs | ICER | |
| No statin | Int$ 16,825 | 8.11 | - | Int$ 9,056 | 9.99 | - | Int$ 7,627 | 10.19 | - | |
| Low dose | Int$ 17,430 | 8.32 | Int$ 2,827 a | Int$ 9,224 | 10.06 | Dominated d | Int$ 7,817 | 10.25 | Dominated d | |
| Intermediate dose | Int$ 17,892 | 8.46 | Int$ 3,526b | Int$ 9,364 | 10.14 | Int$ 2,081 a | Int$ 7,954 | 10.31 | Int$ 2,819 a | |
| High dose | Int$ 20,115 | 8.51 | Int$ 40,418 c | Int$ 11,524 | 10.22 | Int$ 26,667 c | Int$ 10,148 | 10.37 | Int$ 33,754 c | |
| Primary prevention, 10% risk | Primary prevention, 5% risk | |||||||||
| Treatment | Costs (Int$) | QALYs | ICER | Costs (Int$) | QALYs | ICER | ||||
| No statin | Int$ 2,175 | 10.36 | - | Int$ 1,006 | 10.57 | - | ||||
| Low dose | Int$ 2,356 | 10.40 | Dominated d | Int$ 1,267 | 10.59 | Dominated d | ||||
| Intermediate dose | Int$ 2,470 | 10.44 | Int$ 3,554a | Int$ 1,440 | 10.62 | Int$ 9,644 a | ||||
| High dose | Int$ 4,661 | 10.49 | Int$ 47,630 c | Int$ 3,727 | 10.64 | nt$ 95,292 c | ||||
against no statin
against low dose
against intermediate dose
dominated by extension
QALY: Quality-adjusted life year; ICER: Incremental cost-effectiveness ratio.
The ICERs in secondary prevention were as follows: Int$2,827 per QALY in the low dose versus no-statin comparison, Int$3,526 per QALY in the moderate versus low-dose analysis, and Int$40,418 per QALY in the high versus moderate-dose assessment (Table 2).
The primary prevention models were modified based on the results found with the secondary prevention model. Considering the very favorable ICER of the moderate dose in secondary prevention, we assumed that patients who started on low dose or received no statin in the primary prevention models were prescribed moderate doses after a CV event. Assessing the simulation span, the proportion of patients migrating to secondary prevention in the no-statin strategy throughout the models was 10.6% in the 5% ten-year risk model and 35.8% in the 20% ten-year risk model.
In all primary prevention scenarios, the low dose was dominated by extension. Across the scenarios, the moderate dose versus no-statin comparison showed base case ICERs between Int$2,081 and Int$9,644 per QALY, and the high versus moderate dose showed base case ICERs between Int$26,667 and Int$95,292 per QALY (Table 2). Therefore, in all five scenarios, the preferred regimen would be the moderate-dose statin in the base case, considering the WTP of Int$11,770 per QALY.
Sensitivity analyses
In the secondary prevention model, the only parameters that influenced overall results were the effectiveness of the various statin regimens; other parameters had no significant impact. When the effectiveness of the low-dose regimen was set to its maximum or the effectiveness of moderate dose was set to its minimum, the low-dose statin would be preferred. On the other hand, when the effects of high dose were maximized, this would be the preferred regimen.
In the primary prevention models, the maximization of low-dose effectiveness or minimization of moderate-dose effectiveness also resulted in the low-dose regimen being the most cost-effective. In the 5% ten-year risk scenario, the low dose would also be preferred if its cost was set to its minimum or if the moderate-dose cost was maximal. The no-statin regimen would be the preferred strategy only in three situations, all in the 5% ten-year risk scenario: minimum general population utility, minimal moderate-dose effectiveness and higher discount rate. The high-dose regimen would be considered the most cost-effective regimen in only two conditions: maximum high-dose effectiveness (20% ten-year risk scenario) or minimum high-dose costs (in both 20% and 15% ten-year risk scenario). Under several uncertainties considered in the sensitivity analyses, for the majority of scenarios the moderate dose was the most cost-effective strategy and was not affected by variation of other model parameters.
In the alternative setting using retail sales prices for statins, a major impact throughout all risk groups was observed. If the retail sales price was applied rather than the lower government cost, the low-dose regimen would be the preferred choice in secondary prevention, and in the 20% and 15% ten-year risk setting, the most cost-effective option would be no statin in the other two primary prevention scenarios.
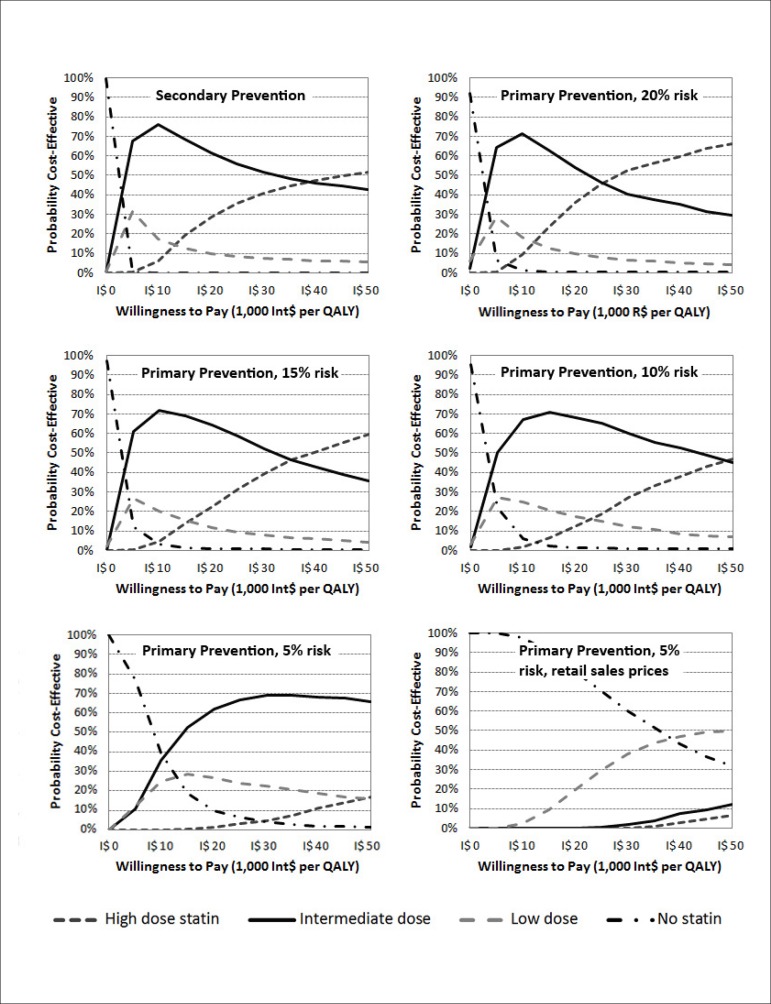
Cost-effectiveness acceptability curves
The cost-effectiveness acceptability curves generated with the 1,000 samples from each model's second-order Monte Carlo simulation are displayed in Figure 2. The moderate-dose strategy showed to be the leading probability of being cost-effective, compared to all other strategies, from thresholds as low as Int$4,000 per QALY (except for the 5% ten-year risk scenario, where this occurred above the threshold of circa Int$10,000). Considering thresholds between one and two times the Brazilian GDP per capita, the moderate-dose strategy was the most cost-effective option across all scenarios, with probabilities ranging from 50% to 70% on average.
Figure 2.
Cost-effectiveness acceptability curves of the five base-case scenarios (secondary and primary prevention, with ten-year risks ranging between 5% and 20% in the latter) and of the 5% ten-year risk primary prevention alternative scenario with statin prices fixed at the retail sales prices of the drugs.
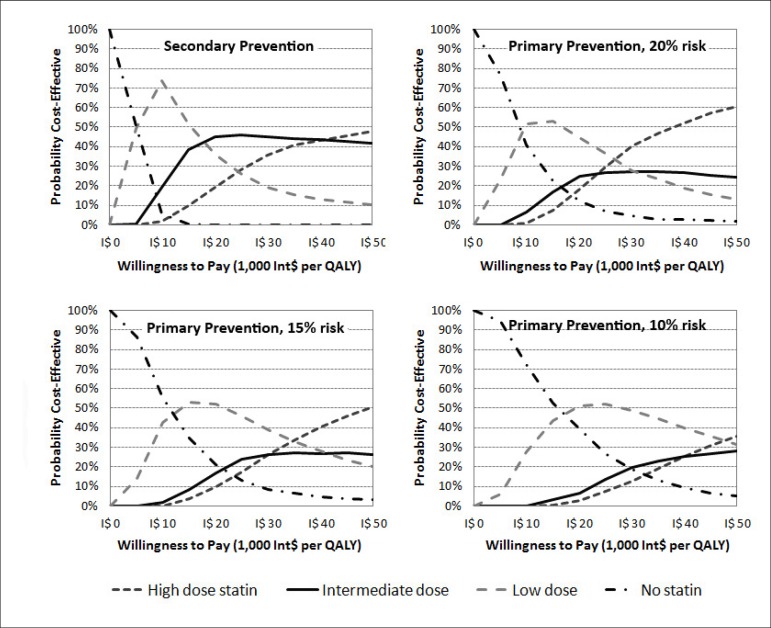
We performed alternative sets of second-order Monte Carlo simulations, where statins costs were fixed at the retail sales prices. In the 5% scenario, the no-statin strategy was the most cost-effective option considering a WTP threshold as high as Int$40,000 per QALY (Figure 2). In the other primary prevention scenarios, the probability of the low dose being more cost-effective than the no-statin strategy increased as a result of escalating 10-year event risk (Figure 3). As opposed to the base case scenarios, the high dose, rather than the moderate dose, was the one that surpassed the low dose as the more cost-effective strategy at higher WTP values. In the secondary prevention scenario, the low dose had a higher probability of being cost-effective from thresholds between Int$5,000 and Int$18,000 per QALY.
Figure 3.
Cost-effectiveness acceptability curves of alternative scenarios (secondary prevention and 10% to 20% ten-year risk primary prevention), where statin prices were fixed at the retail sales prices of the drugs. The curves show the probabilities that the various statin doses would be cost-effective at varying threshold costeffectiveness values.
Discussion
In this cost-effectiveness study, designed to evaluate the relative economic value of different intensity statin therapies from the perspective of the Brazilian SUS, the moderate-dose scheme was the most attractive option. All moderate-dose ICERs were lower than the threshold suggested by the WHO to consider a technology as very attractive from the cost-effectiveness viewpoint, a threshold equal to the GDP per capita30, (Int$11,770) for Brazil in 201131. These results were consistent in the second-order Monte Carlo simulations, in which the moderate-dose strategy was the most cost-effective option across all scenarios with WTP values as low as Int$10,000 per QALY, with a probability higher than 50% in all scenarios at the Int$11,770 threshold.
One could argue that the WHO Choice initiative also suggests using 3 times GDP per capita as cost-effective threshold for middle income countries. Under such WTP limit, still intermediate dose statin would be the most attractive option for most patients. Nonetheless, a more conservative approach was applied considering that this technology is being used for primary prevention, in very prevalent settings for a large number of patients, and for long periods of time.
The most important factor in our analyses was the very low cost of the generic simvastatin 40 mg purchased directly by the Brazilian government compared to atorvastatin 20 mg. As with other middle-income countries, Brazil imports some generic drugs (including simvastatin) from the Asian market at very low costs. In the alternative scenario, in which we used the retail sales price for generic statins in Brazil, the results were quite different. In the very low risk patients (5% ten-year risk), the no-statin strategy predominated in WTP as high as Int$40,000 per QALY. Based on this analysis, statins would be used only in the 20% ten-year risk scenario and in secondary prevention, but with a low- rather than a moderate-dose regimen. Another interesting aspect is that, accepting higher WTP thresholds, the high dose (and not the moderate) surpassed the low dose as being the most cost effective (Figure 3). Such disparity in comparison to the base case scenarios was caused by two factors: first, the relative difference between statin prices in the alternative scenarios is not similar to the one observed in the base case, where the difference between low and moderate dose is minimal and between these two and high dose is significant.
Second, high-dose effectiveness in primary prevention is much greater when compared to the other two statin strategies, especially considering MI incidence reduction. Although the relative risks used in both base case and retail sales price models are the same, the very large difference in statin costs in the base case blunts the effectiveness of the high-dose strategy, with a consequent high ICER. It should be kept in mind, however, that the meta-analysis results used to estimate the high dose effectiveness was significantly determined by the JUPITER study32, of which methods have been questioned in the literature33. Conversely, the secondary prevention alternative scenario had results more consistent with the base case: in the range of Int$ 24,000 - 47,000 per QALY, which is equivalent to 2-4 times the Brazilian GPD per capita, the intermediate dose had the highest probability of being the most cost-effective option.
At first glance, the lower ICERs observed in the higher risk primary prevention scenarios, when compared to secondary prevention, seems contradictory. The explanation for this phenomenon lies in the large annual cost of care of patients with previous vascular events in Brazil, especially when compared with the expenditures due to acute events, proportionally lower than that observed in high-income countries. Consequently, although statins prevent a larger absolute number of events in secondary prevention, the proportional cost of events in the primary prevention is much larger, because this value is the sum of the acute event expenditure plus the difference in costs between secondary and primary prevention (equal to Int$1,124 in the 20% ten-year risk scenario, for example) multiplied by the number of years that the patient lives in secondary prevention after his first event.
Several cost-effectiveness studies evaluating statins in other countries generally showed acceptable cost-effectiveness ratios for patients in secondary prevention or high risk primary prevention populations. Nonetheless, the methods and assumptions used in these investigations were different from those of our study. Firstly, the majority of these studies were either economic evaluations piggybacked on RCTs6,9,20,21 or modeling approaches in which the effectiveness parameters were based on only a few RCTs7,34,35. By contrast, in our study, the effectiveness data were based on more than 136,000 patients from 40 RCTs. Although the study by Ward et al. performed a comprehensive meta-analysis on statin effectiveness, their comparison was between statin and no treatment, combining all statins and intensity regimes1. Secondly, many of these studies evaluated cost-effectiveness using the cost of brand name statins, having been performed before the availability of lower cost generic formulations. Furthermore, our study was conducted in a middle-income country, where costs of drugs, ambulatory care of patients and hospital care of vascular events are significantly less than in other countries1,11,36. Finally, our study was the first to our knowledge to include three statin intensity regimes and a control group, allowing a broader view of these drugs.
So far, only one economic evaluation of statins has been performed in Brazil, where the cost-effectiveness of atorvastatin 10 mg and simvastatin 40 mg in secondary prevention were appraised37. The authors described dominance of simvastatin over both no treatment and atorvastatin, but the lack of a description of the details of the systematic review employed to determine the effectiveness of the treatments compromises the understanding of these results.
The main strengths of our study were the systematic review performed, the comprehensive model employed and the division of statins into three groups. Our microsimulation modeling was built to track previous events and to use this information to interfere in future events, which is the cornerstone for the adequate representation of complex disease processes involved in cardiovascular disease prevention. The systematic review conducted to obtain the parameters for statin effectiveness was thorough and aggregated both direct and indirect evidence, combining all relevant randomized studies on statins. Finally, the majority of economic models thus far have compared strategies with a selected statin versus no treatment or high versus low dose. The availability of statins is currently vast, and the division of statins into three groups of potency therefore seems more reasonable.
Some limitations of our research should be mentioned. The lack of parameters for transitions between health states and for distribution of patients among initial health states (in the secondary prevention model) based on Brazilian data led us to apply parameters based on international data. For instance, the relative rates of mortality for stroke and cardiovascular disease are different in Brazil when compared to most high-income countries, although it is difficult to predict the impact that this would have on final results38. Another possible source of bias for our results is our estimation of statin effectiveness in primary prevention, which was based on studies in which the patients were generally of moderate or high risk. Therefore, the benefit of treatment in the 5% (and possibly in the 10%) ten-year risk scenario(s) could have been overestimated. Finally, the effectiveness estimates were based on clinical trial data, where patients usually have higher compliance to treatment, and therefore the treatment effects might have been overestimated.
Conclusions
Statins have been available in the Brazilian SUS for ten years, with no formal economic evaluation performed to guide this decision process. Today, although prescriptions are centered on atorvastatin and simvastatin, all statins except rosuvastatin are offered in the pharmacy assistance program. The results of our study suggest that if the current prices of statin acquisition are maintained, the prescription of 40 mg of simvastatin for both primary and secondary prevention is highly cost-effective and therefore should be pursued, especially in secondary and high-risk primary prevention patients, where the magnitude of the benefit is greater and a national public health policy already endorses the use of statins.
Nonetheless, other criteria beyond clinical benefit and cost-effectiveness ratios must be considered in the evaluation of the adoption of a technology, such as equity and accessibility, as well as the budget impact of such an implementation. Considering the enormous proportion of the population that have low to moderate CV risk (5% to 10% ten-year risks)39, the economic impact of widespread CV prevention with statins must also be evaluated, especially for prescription to those in the lower risk stratum, where the benefit of statins is limited. Conversely, our data demonstrate that the higher dose of atorvastatin at its current price is not justifiable, even for high-risk patients.
Acknowledgments
Dr. Polanczyk and Dr. Duncan received research sponsorship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. Dr. Ribeiro also received research sponsorship from CNPq for this study. This study was supported by research grant #37/2008 from the MCT/CNPq and the Brazilian Ministry of Health. The authors disclose no conflict of interest regarding this manuscript. The funding sources had no role in designing, analyzing or writing the manuscript.
Footnotes
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conception and design of the research: Ribeiro RA, Polanczyk CA; Acquisition of data: Ribeiro RA, Ziegelmann PK, Stella SF, Vieira JLC, Restelatto LMF; Analysis and interpretation of the data: Ribeiro RA, Duncan BB, Ziegelmann PK, Stella SF, Vieira JLC, Restelatto LMF, Polanczyk CA; Statistical analysis: Ribeiro RA; Obtaining financing: Polanczyk CA; Writing of the manuscript: Ribeiro RA; Critical revision of the manuscript for intellectual content: Duncan BB, Polanczyk CA.
Sources of Funding
This study was funded by CNPq and Ministério da Saúde.
Study Association
This article is part of the thesis of Doctoral submitted by Rodrigo Antonini Ribeiro, from Universidade Federal do Rio Grande do Sul.
References
- 1.Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11(14):1–160. doi: 10.3310/hta11140. [DOI] [PubMed] [Google Scholar]
- 2.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376–b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills EJ, Wu P, Chong G, Ghement I, Singh S, Akl EA, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170 255 patients from 76 randomized trials. QJM. 2011;104(2):109–124. doi: 10.1093/qjmed/hcq165. [DOI] [PubMed] [Google Scholar]
- 4.Estatinas na prevenção primária de eventos cardiovasculares. Boletim Brasileiro de Avaliação de Tecnologias em Saúde (BRATS) 2009;4(9):1–13. [Google Scholar]
- 5.Franco OH, Peeters A, Looman CW, Bonneux L. Cost effectiveness of statins in coronary heart disease. J Epidemiol Community Health. 2005;59(11):927–933. doi: 10.1136/jech.2005.034900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsevat J, Kuntz KM, Orav EJ, Weinstein MC, Sacks FM, Goldman L. Cost-effectiveness of pravastatin therapy for survivors of myocardial infarction with average cholesterol levels. Am Heart J. 2001;141(5):727–734. doi: 10.1067/mhj.2001.114805. [DOI] [PubMed] [Google Scholar]
- 7.Ganz DA, Kuntz KM, Jacobson GA, Avorn J. Cost-effectiveness of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor therapy in older patients with myocardial infarction. Ann Intern Med. 2000;132(10):780–787. doi: 10.7326/0003-4819-132-10-200005160-00003. Erratum in: Ann Intern Med. 2002;136(8):635. [DOI] [PubMed] [Google Scholar]
- 8.Caro J, Klittich W, McGuire A, Ford I, Pettitt D, Norrie J, et al. International economic analysis of primary prevention of cardiovascular disease with pravastatin in WOSCOPS. West of Scotland Coronary Prevention Study. Eur Heart J. 1999;20(4):263–268. doi: 10.1053/euhj.1999.1193. [DOI] [PubMed] [Google Scholar]
- 9.Raikou M, McGuire A, Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, et al. Cost-effectiveness of primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes: results from the Collaborative Atorvastatin Diabetes Study (CARDS) Diabetologia. 2007;50(4):733–740. doi: 10.1007/s00125-006-0561-4. [DOI] [PubMed] [Google Scholar]
- 10.Taylor DC, Pandya A, Thompson D, Chu P, Graff J, Shepherd J, et al. Cost-effectiveness of intensive atorvastatin therapy in secondary cardiovascular prevention in the United Kingdom, Spain, and Germany, based on the Treating to New Targets study. Eur J Health Econ. 2009;10(3):255–265. doi: 10.1007/s10198-008-0126-1. [DOI] [PubMed] [Google Scholar]
- 11.Lindgren P, Graff J, Olsson AG, Pedersen TJ, Jonsson B, IDEAL Trial Investigators Cost-effectiveness of high-dose atorvastatin compared with regular dose simvastatin. Eur Heart J. 2007;28(12):1448–1453. doi: 10.1093/eurheartj/ehm020. [DOI] [PubMed] [Google Scholar]
- 12.Drummond M, Barbieri M, Cook J, Glick HA, Lis J, Malik F, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health. 2009;12(4):409–418. doi: 10.1111/j.1524-4733.2008.00489.x. [DOI] [PubMed] [Google Scholar]
- 13.Ministério da Saúde . Dislipidemias em pacientes de alto risco de desenvolver eventos cardiovasculares. Brasília: 2002. [Google Scholar]
- 14.Stone NJ, Robinson JG, Lichtenstein AH, BaireyMerz CN, Lloyd-Jones DM, Blum CB, et al. American College of Cardiology. American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Pt BJ Am CollCardiol. 2014;63(25):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro RA, Ziegelmann PK, Duncan BB, Stella SF, da Costa Vieira JL, Restelatto LM, et al. Impact of statin dose on major cardiovascular events: a mixed treatment comparison meta-analysis involving more than 175,000 patients. Int J Cardiol. 2013;166(2):431–439. doi: 10.1016/j.ijcard.2011.10.128. [DOI] [PubMed] [Google Scholar]
- 17.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326(7404):1423–1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99(3):410–414. doi: 10.1016/j.amjcard.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 20.Caro J, Klittich W, McGuire A, Ford I, Norrie J, Pettitt D, et al. The West of Scotland coronary prevention study: economic benefit analysis of primary prevention with pravastatin. BMJ. 1997;315(7122):1577–1582. doi: 10.1136/bmj.315.7122.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihaylova B, Briggs A, Armitage J, Parish S, Gray A, Collins R, Heart Protection Study Collaborative Lifetime cost effectiveness of simvastatin in a range of risk groups and age groups derived from a randomised trial of 20,536 people. BMJ. 2006;333(7579):1145–1145. doi: 10.1136/bmj.38993.731725.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan PS, Nallamothu BK, Gurm HS, Hayward RA, Vijan S. Incremental benefit and cost-effectiveness of high-dose statin therapy in high-risk patients with coronary artery disease. Circulation. 2007;115(18):2398–2409. doi: 10.1161/CIRCULATIONAHA.106.667683. [DOI] [PubMed] [Google Scholar]
- 23.Ministério da Saúde População residente - Brasil. [Citado em 2010 Set 14]. Disponível em: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?ibge/cnv/popbr.def.
- 24.Instituto Brasileiro de Geografia e Estatística Tábuas completas de mortalidade. 2007. [Citado em 2010 Set 14]. Disponível em: http://www.ibge.gov.br/home/estatistica/populacao/tabuadevida/2007/default.shtm.
- 25.Cruz LN, Fleck MP, Polanczyk CA. Depression as a determinant of quality of life in patients with chronic disease: data from Brazil. Soc Psychiatry Psychiatr Epidemiol. 2010;45(10):953–961. doi: 10.1007/s00127-009-0141-2. [DOI] [PubMed] [Google Scholar]
- 26.Cruz LN, Polanczyk CA, Camey SA, Hoffmann JF, Fleck MP. Quality of life in Brazilnormative values for the WHOQOL-bref in a southern general population sample. Qual Life Res. 2011;20(7):1123–1129. doi: 10.1007/s11136-011-9845-3. [DOI] [PubMed] [Google Scholar]
- 27.Ministério da Saúde. DATASUS Informações de saúde: epidemiológicas e morbidade. [Citado em 2010 Set 14]. Disponível em: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sim/cnv/obtuf.def.
- 28.Ribeiro RA, Mello RG, Melchior R, Dill JC, Hohmann CB, Lucchese AM, et al. Annual cost of ischemic heart disease in Brazil. Public and private perspective. Arq Bras Cardiol. 2005;85(1):3–8. doi: 10.1590/s0066-782x2005001400002. [DOI] [PubMed] [Google Scholar]
- 29.International Monetary Fund Gross domestic product based on purchasing-power-parity (PPP) per capita GDP. 2012. [Cited on 2012 Nov 7]. Available from: http://www.imf.org/external/pubs/ft/weo/2012/01/weodata/weorept.aspx?pr.x=48&pr.y=11&sy=2009&ey=2012&scsm=1&ssd=1&sort=country&ds=.&br=1&c=223&s=NGDPD%2CNGDPDPC%2CPPPGDP%2CPPPPC%2CLP&grp=0&a.
- 30.World Health Organization. Commission on macroeconomics and health . Macroeconomics and health: investing in health for economic development. Washington: Commission on Macroeconomics and Health; 2011. [Google Scholar]
- 31.Index Mundi Brazil GDP - per capita (PPP) [Cited in 2011 May 21]. Available from: http://www.indexmundi.com/brazil/gdp_per_capita_%28ppp%29.html.
- 32.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 33.de Lorgeril M, Salen P, Abramson J, Dodin S, Hamazaki T, Kostucki W, et al. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. Arch Intern Med. 2010;170(12):1032–1036. doi: 10.1001/archinternmed.2010.184. [DOI] [PubMed] [Google Scholar]
- 34.Pickin DM, McCabe CJ, Ramsay LE, Payne N, Haq IU, Yeo WW, et al. Cost effectiveness of HMG-CoA reductase inhibitor (statin) treatment related to the risk of coronary heart disease and cost of drug treatment. Heart. 1999;82(3):325–332. doi: 10.1136/hrt.82.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto CG, Carrageta MO, Miguel LS. Cost-effectiveness of rosuvastatin in the prevention of ischemic heart disease in Portugal. Value Health. 2008;11(2):154–159. doi: 10.1111/j.1524-4733.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 36.Nagata-Kobayashi S, Shimbo T, Matsui K, Fukui T. Cost-effectiveness of pravastatin for primary prevention of coronary artery disease in Japan. Int J Cardiol. 2005;104(2):213–223. doi: 10.1016/j.ijcard.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Araujo DV, Ribeiro de Souza CP, Bahia LR, Rey HC, Dos B, Santos Junior, Tura BR, et al. Analysis of cost-effectiveness of simvastatin versus atorvastatin in the secondary prevention of cardiovascular events within the Brazilian public healthcare system. Value Health. 2011;14(5) Suppl 1:S29–S32. doi: 10.1016/j.jval.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt MI, Duncan BB, Azevedo e Silva G, Menezes AM, Monteiro CA, Barreto SM, et al. Chronic non-communicable diseases in Brazil: burden and current challenges. Lancet. 2011;377(9781):1949–1961. doi: 10.1016/S0140-6736(11)60135-9. [DOI] [PubMed] [Google Scholar]
- 39.Nascimento RM, Neto, Krieger JE, Machado-Coelho GL, Pereira Ada C. Hearts of Brazil Project. Arq Bras Cardiol. 2005;85(3):218–221. doi: 10.1590/s0066-782x2005001600015. [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro RA, Ziegelmann PK, Duncan BB, Stella SF, da Costa Vieira JL, Restelatto LM, et al. Impact of statin dose on major cardiovascular events: A mixed treatment comparison meta-analysis involving more than 175,000 patients. Int J Cardiol. 2013;166(2):431–439. doi: 10.1016/j.ijcard.2011.10.128. [DOI] [PubMed] [Google Scholar]
- 41.Consulta Remédios. 2011. [Citado em 2012 Abr 20]. Disponível em: http://consultaremedios.com.br/
- 42.Ministério da Saúde . Methodological guidelines: economic evaluation of health technologies. Brasília: 2012. [Citado em 2012 Abr 20]. Disponível em: http://www.ispor.org/peguidelines/source/Economic-Evaluation-Guidelines-in-Brazil-Final-Version-2009.pdf. [Google Scholar]