Abstract
Background
Heart failure and atrial fibrillation (AF) often coexist in a deleterious cycle.
Objective
To evaluate the clinical and echocardiographic outcomes of patients with ventricular systolic dysfunction and AF treated with radiofrequency (RF) ablation.
Methods
Patients with ventricular systolic dysfunction [ejection fraction (EF) <50%] and AF refractory to drug therapy underwent stepwise RF ablation in the same session with pulmonary vein isolation, ablation of AF nests and of residual atrial tachycardia, named "background tachycardia". Clinical (NYHA functional class) and echocardiographic (EF, left atrial diameter) data were compared (McNemar test and t test) before and after ablation.
Results
31 patients (6 women, 25 men), aged 37 to 77 years (mean, 59.8±10.6), underwent RF ablation. The etiology was mainly idiopathic (19 p, 61%). During a mean follow-up of 20.3±17 months, 24 patients (77%) were in sinus rhythm, 11 (35%) being on amiodarone. Eight patients (26%) underwent more than one procedure (6 underwent 2 procedures, and 2 underwent 3 procedures). Significant NYHA functional class improvement was observed (pre-ablation: 2.23±0.56; postablation: 1.13±0.35; p<0.0001). The echocardiographic outcome also showed significant ventricular function improvement (EF pre: 44.68%±6.02%, post: 59%±13.2%, p=0.0005) and a significant left atrial diameter reduction (pre: 46.61±7.3 mm; post: 43.59±6.6 mm; p=0.026). No major complications occurred.
Conclusion
Our findings suggest that AF ablation in patients with ventricular systolic dysfunction is a safe and highly effective procedure. Arrhythmia control has a great impact on ventricular function recovery and functional class improvement.
Keywords: Catheter Ablation; Atrial Fibrillation; Ventricular Dsyfunction; Echocardiography; Arrhythmias, Cardiac
Introduction
The treatment of ventricular systolic dysfunction and of heart failure has advanced greatly in past decades. Primary therapy (angiotensin-converting-enzyme inhibitors, beta-blockers, diuretics, digitalis) combined with the approach of the multiple factors associated (electrical, endocrine, mechanical, valvular, ischemic) has shown to be of great clinical benefit. Electrical disorders frequently aggravate, or even cause, that syndrome. The cardiac resynchronization therapy success confirms that interaction. Atrial fibrillation (AF) is a chronic pathology, of difficult control, that has progressive characteristics, similarly to ventricular dysfunction. The coexistence of both conditions is a huge therapeutic challenge. Usually, the clinical presentation of AF varies from patients completely asymptomatic, diagnosed on routine exams, to individuals who feel each crisis, even non-sustained ones. Similarly, in the presence of ventricular dysfunction, there are adapted individuals and others to whom the arrhythmia causes significant clinical decompensation and hemodynamic deterioration, with progressive functional class worsening, and, eventually, acute pulmonary edema directly caused by the overlapping arrhythmia. In addition, regardless of the symptoms, arrhythmia can contribute to the progressive deterioration of cardiomyopathy, causing a highly deleterious refeeding. In a recently published meta-analysis, the presence of AF has determined an increase in morbidity and mortality of patients with ventricular dysfunction1. Despite the academic debate "rhythm control versus heart rate control"2, there is consistent evidence that the former may have a significant impact on ventricular function recovery and/or preservation3-5. However, the drug treatment of AF is restricted to amiodarone, with few satisfactory results. The past years have witnessed a large advance in the knowledge of the pathophysiology, and in AF ablation techniques and devices. Recently an increasing number of studies on the procedure in the presence of ventricular dysfunction has been published5-9, with very interesting and favorable results. Thus, we report the mid-term and long-term clinical and echocardiographic outcome of a group of patients with ventricular dysfunction and AF, submitted to rhythm control by use of a modified technique of radiofrequency (RF) ablation of AF at our service.
Methods
This is a retrospective study of our database with patients with AF and ventricular dysfunction submitted to rhythm control by use of stepwise arrhythmia ablation. This study assessed 31 patients (6 women and 25 men), aged between 37 and 77 years (mean age, 59.8 ± 10.6 years), with AF refractory to clinical treatment and ventricular dysfunction clinically and echocardiographically defined [ejection fraction (EF) ≤ 50%]. They underwent RF catheter ablation of AF between October 2006 and January 2013. The functional assessment of symptoms was performed according to the New York Heart Association (NYHA) classification. The follow-up protocol consisted of a return medical visit and electrocardiography at 1, 3, 6 and 12 months, in addition to every 6 months after the first year, or at any time if symptoms appeared. The patients were instructed to keep in touch with the health care team (extra visit, telephone, Internet) if symptoms reappeared between the regularly scheduled consultations. Holter was performed after the first month, at 6 and 12 months, and then annually after the first year, or at medical discretion. Echocardiography was performed after the third month, and then annually or based on clinical criteria. Patients on oral anticoagulation therapy were monitored to control prothrombin time (INR) or hospitalized to adjust or move into subcutaneous enoxaparin. Whenever possible, antiarrhythmic drugs were suspended prior to the procedure to prevent bradyarrhythmia and/or side effects from occurring. Right after the procedure, amiodarone was routinely administered to prevent typical arrhythmias of the early post-ablation phase (first 3 months) from happening, being gradually withdrawn or maintained based on clinical criteria. Anticoagulants were also maintained after ablation, with general instructions on their gradual withdrawal (after 6 months) in the case of satisfactory outcome, depending on CHADS2-VASC. The patients were followed up by the referring clinical cardiologist and the arrhythmia team. In general, decisions on the drugs were consensually taken during the follow-up.
Electrophysiological study and catheter ablation
The procedures were performed under general anesthesia. The technique for mapping and ablation, devices and protocol used by the team have already been published10-13; however, it can be summarized in three steps:
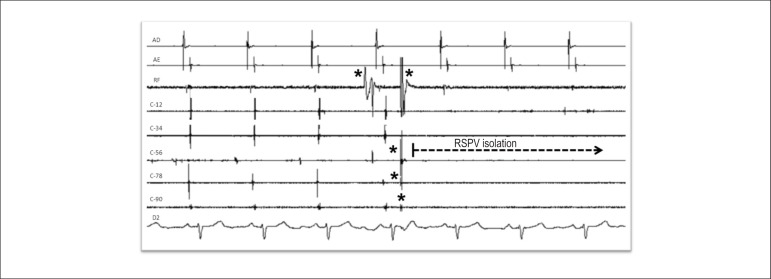
1. Isolation of the pulmonary veins (Figure 1)
Figure 1.
Right superior pulmonary vein isolation with radiofrequency application (arrow). AD: right atrium; AE: left atrium; C-12 to C-90: sequence of the poles of the circular catheter positioned in the right superior pulmonary vein antrum; RF: radiofrequency catheter recording; D2: D2 surface ECG lead; RSPV: right superior pulmonary vein; * Artifacts generated by the contact of radiofrequency catheter and circular catheter poles.
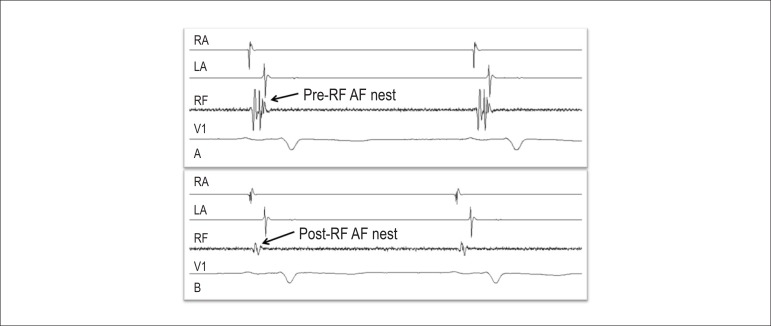
2. Ablation of AF nests (Figure 2)
Figure 2.
Endocavitary electrogram showing an AF nest before (A) and after radiofrequency ablation (B) in the left interatrial septum. AD: right atrium; AE: left atrium; RF: radiofrequency catheter recording; V1: V1 surface ECG lead.
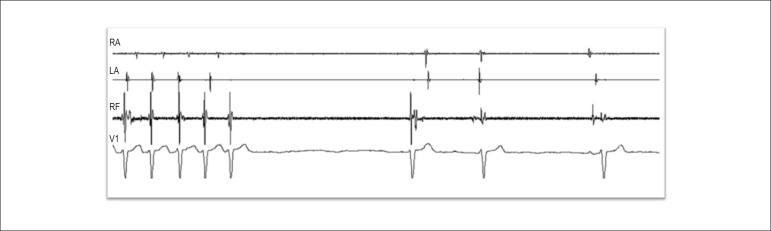
3. Search and ablation of background tachycardia, when induced (Figure 3).
Figure 3.
Background tachycardia reversal during radiofrequency application in the upper region of the left interatrial septum. RA: right atrium; LA left atrium; RF: radiofrequency catheter recording; V1: V1 surface ECG lead.
All patients underwent transesophageal echocardiography during the procedure with the following purposes:
At the beginning: to rule out the presence of occasional intracavitary thrombi (despite previous anticoagulation);
During the procedure: to help in the transseptal puncture (visualization of microbubbles with saline injection confirming the left atrial access) and in esophageal protection by using contralateral mechanical deviation during RF application in the left atrium;
At the end: to confirm the absence of thrombi and pericardial effusion.
Four punctures were performed in the right femoral vein. The patients were heparinized and their activated coagulation time values maintained between 300 and 400 seconds. A duo-decapolar catheter (Daig, Medtronic or Biotronik) was positioned in the coronary sinus to simultaneously map the right and left atrioventricular ring. That catheter was also used as a fixed reference for the EnSite/NavXTM electroanatomical mapping. In addition, a circular mapping catheter was used to obtain the anatomy (electroanatomical mapping) and to map the antrum of the pulmonary veins and AF nests in the right and left atria.
Irrigated ablation catheters were used in 22 (71%) patients (Johnson or Biotronik) and 8-mm non-irrigated tip catheters in 9 (29%) patients (Blazer EPT, Johnson or Biotronik). The Carto® System (Biosense Webster) or the EnSite/NavXTM System (St Jude Medical) was used to obtain the anatomy of the atrial chambers. Spectral mapping, as previously described10,12, was performed either with computerized spectrometer (Pachón®) for real-time spectral analysis (14 patients, 45.2%) or by using an equivalent resource with adjustment of the conventional polygraph filters (17 patients, 54.8%). After isolation of pulmonary veins and superior vena cava, ablation of the AF nests found in the right and left atria and in the interatrial septum was performed.
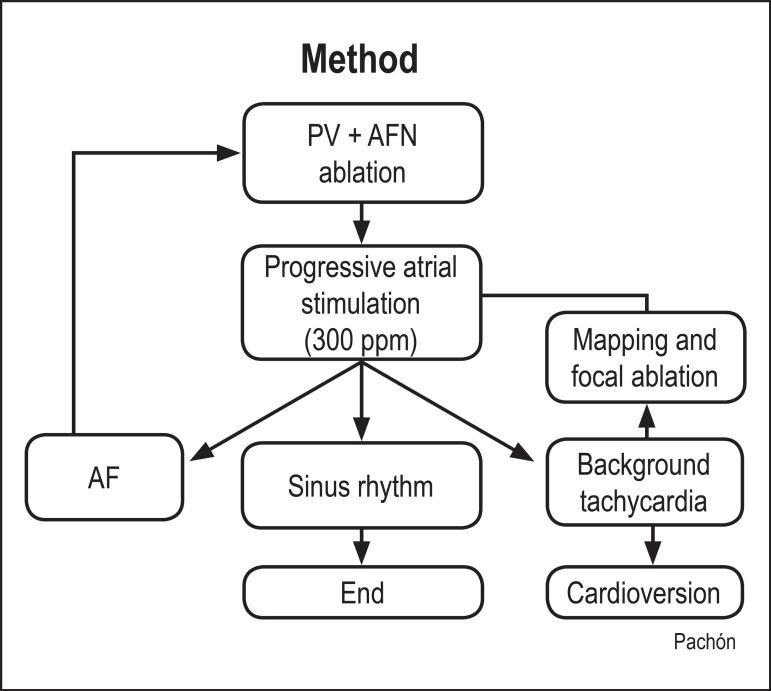
Then, induction of atrial tachyarrhythmias was attempted with progressive and rapid atrial stimulation (up to 300 ppm) or programed stimulation (Figure 4). If AF induction succeeded, additional ablation of AF nests and reconnection of pulmonary veins were performed. If regular tachycardia was induced, it would be studied and classified as atrial flutter, atrial tachycardia or background tachycardia11,12, and, then, according to the diagnosis, ablation with the appropriate technique would be performed. In addition, all patients underwent ablation of the typical atrial flutter through the blocking line of the cavotricuspid isthmus, regardless of previous recording of that arrhythmia. Vesical catheterization was performed to monitor diuresis and the possible use of diuretics, because of the fluid volume injected via the irrigated ablation catheter, based on clinical assessment and fluid balance.
Figure 4.
Methodology flowchart of this study ablations. PV: pulmonary veins; AF: atrial fibrillation; AFN: atrial fibrillation nests
The criteria to end ablation were as follows: 1) impossibility of re-inducing AF or tachycardia; 2) induction only of tachycardia or non-sustained AF shorter than 10 seconds; and 3) impossibility of residual tachycardia elimination or prolonged procedure (more than four hours), when external thoracic cardioversion was performed.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and qualitative variables (such as functional class), as absolute and relative frequencies. The pre- and post-ablation clinical (functional class) and echocardiographic data were compared by using, respectively, McNemar test and Student t test for paired data. All data had a normal distribution assessed by using Shapiro-Wilk test. The IBM SPSS Statistics software, version 19, was used for statistical analysis. A 5% significance level (p<0.05) was adopted, and two-sided p values were used.
Results
Table 1 shows the major demographic data. The mean EF assessed on echocardiography was 44.7% ± 6%. The etiologies of cardiomyopathy were as follows: idiopathic (19 patients, 61%); coronary artery disease (5, 16%); hypertension (4, 13%); and others [3 patients, 10%: myocardial sclerosis (1), post-myocarditis (1), and post valvular heart surgery (1)]. The clinical presentation of AF was as follows: paroxysmal in 2 patients (6.5%); persistent in 21 (68%); and long-standing persistent in 8 patients (25.5%). At the time of the procedure, most patients were on anticoagulation with warfarin (23 patients, 74%), amiodarone being the major antiarrhythmic drug used (17 patients, 55%), in addition to other cardiac drugs such as beta-blockers (17 patients, 55%), angiotensin-converting-enzyme inhibitors (8 patients, 26%) and diuretics (4 patients, 13%). The pre-ablation EF varied from 26% to 50% (mean of 44.7% ± 6%), and the left atrial diameter, from 34 to 64 mm (mean of 46.6 ± 7 mm). The symptoms reported were palpitation and mainly clinical findings of heart failure, and 3 patients had acute decompensation (acute pulmonary edema) at the time of the heart disease diagnosis. The mean pre-ablation NYHA functional class was 2.23 ± 0.56.
Table 1.
Study population
| N | 31 patients |
|---|---|
| Age (mean ± SD) | 59.8 ± 10.6 years |
| Sex | Male: 25 (81%) |
| Female: 6 (19%) | |
| Etiology | |
| - Idiopathic: 19 (61%) | |
| - CAD: 5 (16%) | |
| - Hypertensive: 4 (13%) | |
| - Others: 3 (10%) | |
| Type of AF | |
| - Paroxysmal: 2 (6.5%) | |
| - Persistent: 21 (68%) | |
| - Long-standing persistent: 8 (25.5%) | |
| Ejection fraction (mean ± SD) | 44.7 ± 6 % |
| Pre-ablation functional class (mean ± SD) | 2.23 ± 0.56 |
CAD: coronary artery disease; AF: atrial fibrillation; SD: standard deviation.
Procedure
At the beginning of the ablation, 22 patients (71%) had AF, 4 patients (13%) had atrial flutter, and only 5 patients (16%) were in sinus rhythm. After confirming the lack of intracavitary thrombi on intraoperative transesophageal echocardiography, patients with AF underwent external electrical cardioversion to proceed with mapping in sinus rhythm. In case of early relapse of the arrhythmia, the initial phase was performed with AF to assess the possibility of either reversal during RF applications or arrhythmia organization into a regular atrial tachycardia with entrance block (background tachycardia). In case of persistent AF, even after RF applications, new cardioversions were performed during the procedure. In the presence of atrial flutter, ablation would be performed until its reversion (in the absence of thrombi on echocardiography), followed by conventional stimulation maneuvers to ascertain bidirectional block of the cavotricuspid isthmus. During the procedure, by use of both progressive organization of the arrhythmia during ablation of the AF nests, and atrial stimulation protocols, background tachycardia12 could be identified in 8 patients (26%). That arrhythmia was mapped by use of precocity and/or entrainment techniques, ablation being performed whenever the focus was identified.
None of the following major complications of the procedure was observed: death; embolism/hemorrhages with sequelae or need for transfusion; tamponade or any complication requiring surgery; stenosis of the pulmonary veins; diaphragmatic paralysis; esophageal lesions and fistulae. Two patients had their hospital length of stay extended for up to 6 days: one due to early relapse with need for cardioversion and amiodarone bolus; another with transient change in kidney function to better compensate heart failure.
Outcome
The mean follow-up time was 20.3 ± 17 months. At the last assessment, 24 patients (77%) were in sinus rhythm, 11 (35%) being on amiodarone. Eight patients (26%) underwent more than one procedure as follows: six underwent two procedures, and two underwent three procedures. Of the seven patients (23%) in whom ablation failed, three progressed with refractory AF and tachycardiomyopathy associated with heart failure of difficult clinical control. Those patients underwent dual-chamber pacemaker implantation (two with biventricular resynchronization and one with bifocal resynchronization), followed by atrioventricular nodal ablation with significant improvement, being currently in functional class I or II. Two of those patients reverted to sinus rhythm, and according to the internal Holter of the generators, they maintain a very low AF burden (< 1%). The other four patients are being clinically followed up, on anticoagulation and heart rate control with drugs. Arrhythmia control provided significant clinical benefit, with improvement of the functional class as compared with that of the initial clinical assessment: mean pre-ablation and post-ablation functional classes of 2.23 ± 0.56 and 1.13 ± 0.35, respectively (p < 0.0001).
The assessment of the echocardiographic outcome also showed a significant improvement of the ventricular function measured by use of EF (pre-ablation, 44.68 ± 6.02%, post-ablation, 59 ± 13.2%; p = 0.0005) and a significant reduction in the left atrial diameter (pre-ablation, 46.61 ± 7.3mm, post-ablation, 43.59 ± 6.6mm; p = 0.026) (Table 2 and Figure 5).
Table 2.
Follow-up, procedures performed, clinical and echocardiographic outcomes of the patients of this study
| Follow-up: 20.3 ± 17 months | |||
|---|---|---|---|
| Number of procedures | |||
| 23 (74.2%) | 1 procedure | ||
| Ablations | 31 | 6 (19.3%) | 2 procedures |
| 2 (6.4%) | 3 procedures | ||
| Maintenance of sinus rhythm | |||
| Sinus rhythm | 24 (77%) | ||
| 4 clinical control | |||
| AF | 7 (23%) | 3 tachycardiomyopathy → | PM - good outcome |
| Clinical and echocardiographic findings | |||
| Pre-ablation | Post-ablation | p | |
| NYHA | 2.23 ± 0. | 1.13 ± 0.35 | < 0.0001 |
| EF | 44.68% ± 6 | 59% ± 13.2% | p = 0.0005 |
| LA | 46.61 ± 7.3 | 43.59 ± 6.6 mm | p = 0.026 |
AF: atrial fibrillation; PM: pacemaker; NYHA: New York Heart Association; EF: ejection fraction; LA: left atrium.
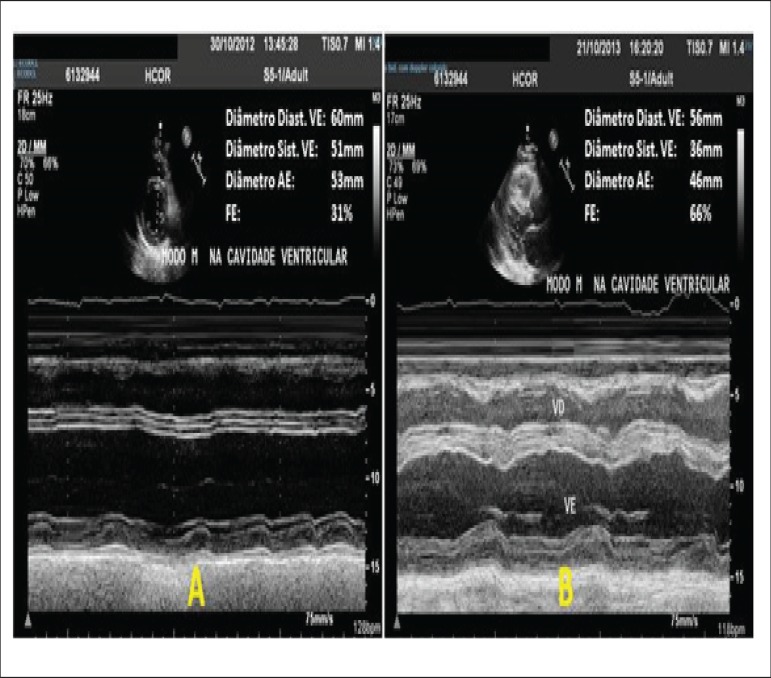
Figure 5.
M-mode echocardiography before (A) and 9 months after ablation (B), showing significant improvement in intracavitary diameters and ventricular function.
Discussion
Ventricular dysfunction and AF are the most common chronic pathologies in cardiology. When associated, the cause-effect relationship between them is often undefined. Ventricular dysfunction can lead to AF, which, on its turn, can lead to ventricular dysfunction, as in tachycardiomyopathy. Undoubtedly, the association of both conditions leads to a deleterious refeeding, with progressive worsening of the ventricular function and arrhythmia perpetuation. Observational and follow-up data of the Framingham Study14 have shown that the development of AF in patients with heart failure implies greater mortality, with a relative risk of 1.6 in men [confidence interval (CI): 1.2 - 2.1), and of 2.7 in women (CI: 2.0 - 3.6). Atrial fibrillation is believed to aggravate cardiac function and performance through four mechanisms: 1) change in heart rate with inadequate (elevated or low) chronotropic response to the innumerous metabolic demands, in addition to tachycardiomyopathy when the heart rate remains permanently elevated, which has deleterious effects on cardiac function; 2) irregular rhythm with abrupt variations of the cardiac cycle with extremely short filling times (diastole) alternated with long ones; 3) loss of the atrial systole necessary for optimal ventricular filling; 4) neuro-humoral activation and release of vasoconstrictors (angiotensin II and norepinephrine) that aggravate the heart failure syndrome. There is plenty of evidence that arrhythmia control contributes to cardiac function preservation and clinical improvement. The greatest difficulty in large intention-to-treat studies2,15 was that the group randomized to "heart rate control" reverted to sinus rhythm during follow-up, while the group randomized to "rhythm control" had high rates of AF; at the end, the proportions of sinus rhythm were similar in both groups, hindering the interpretation of the real benefit of sinus rhythm maintenance. A subsequent analysis of the AFFIRM study16 has suggested that sinus rhythm maintenance per se might have an impact on survival. Similarly, the CAFE II Study has also shown benefits of sinus rhythm to ventricular function and BNP levels17. The major obstacle to sinus rhythm maintenance resides in the low efficacy of antiarrhythmic drugs and their side effects. In that complex scenario, several publications have suggested that AF ablation is an additional resource to achieve higher efficacy in arrhythmia control. Jones et al9, in an interesting recently published study, have analyzed objective data of functional capacity, peak oxygen consumption (VO2), as the primary outcome. They have randomized two groups of patients with ventricular dysfunction and AF: one to rhythm control by use of ablation and another to heart rate control. Those authors have found that the group submitted to ablation (88% of success in 12 months) had a significant improvement in functional capacity measured by use of VO2, in addition to significant gains in the Minnesota score and BNP levels (secondary outcome). The most interesting is that those benefits were not seen at the first assessment three months after the procedure, although the patients were already in sinus rhythm, because those benefits only occur from the sixth month onward. Those authors have reported that the gains progress gradually after ablation, and, that the most important is sinus rhythm maintenance, which can provide progressive remodeling of ventricular function and functional capacity. Our study had a relatively long follow-up (20 months) with sinus rhythm maintenance (reablation, if necessary, and/or drugs), which might explain the significant gains in functional class and ventricular function. In addition, in our case series, the significant incidence of the idiopathic etiology (61%) is worthy of note. Considering the favorable outcome of those cases, one can speculate a direct effect of arrhythmia on ventricular dysfunction (tachycardiomyopathy) or, at least, AF as a significant aggravating factor of the cardiomyopathy severity. Another study by Packer et al4 is similar to those here analyzed. Those authors have observed a significant EF improvement, with 64% of the patients normalizing their ventricular function, and have concluded that AF ablation can substantially improve the quality of life (assessed by use of the "SF-36" score) and ventricular function. In that study, Packer et al4 have not detailed the etiology of the cardiopathy, reporting only that 13% had ischemic cardiopathy. One of the first studies on the subject, published in 2004 by Hsu et al7, showed more expressive results, with an EF gain of 21 percentage points 12 months after ablation. Those authors have reported that 92% of the patients had some ventricular function gain after restoring sinus rhythm, suggesting that ventricular dysfunction was mediated by tachyarrhythmia (tachycardiomyopathy) and that such phenomenon was likely underestimated in those patients. In fact, our data support that reasoning. The large number of patients classified as idiopathic suggests the presence of tachyarrhythmia-mediated cardiomyopathy. However, in patient selection, more important than, or even as important as, the etiology of cardiomyopathy might be the identification of the effect of AF installation on heart failure, because the functional class of many patients deteriorate along with the development of that arrhythmia, being possibly those who benefit most from ablation. The identification of that clinical correlation can be more or less evident, depending on the situation and temporal progression. Many patients show a clear correlation of functional class worsening with AF development, eventually progressing to acute pulmonary edema. Another group that can benefit from ablation and in which that correlation cannot be identified is that with AF and heart failure of difficult clinical control and functional class persistently high, despite the full and proper use of medication. In such cases, reversal to sinus rhythm (ablation or ablation + drugs) can significantly contribute to clinical compensation. The clinical cardiologist might intuitively establish that deleterious correlation between arrhythmia and heart disease when referring patients to the electrophysiologist, that is, the cases undergoing ablation are clinically selected. Another relevant aspect is the possible complications of the procedure, especially in patients with cardiomyopathy. Evidence shows that the complication rate in that population does not differ from that with a structurally normal heart8. In that group, there was no major complication, only prolonged hospital length of stay of two patients due to early relapse and heart failure decompensation.
It is worth considering that paroxysmal AF in the absence of heart disease responds well to pulmonary vein isolation. However, that is not true in the case of long-standing AF or in the presence of heart disease, mainly when there is heart failure. In such cases, an even greater ablation technique refinement is justified. Therefore, in addition to pulmonary vein isolation, those patients were treated with ablation of AF nests and of background tachycardia. The AF nests have already been incorporated as the AF substrate in the specialized literature11. In addition to increasing the rate of success in the isolation of pulmonary veins18, they are more reliable than the mapping of potential fractionated complexes during AF. On the other hand, in our experience, the search for background tachycardia and its ablation represent an additional advance in AF ablation, because, according to spectral analysis, that arrhythmia, due to its refractory forms, accounts for maintaining long-standing AF, being responsible for many relapses12.
Limitations
This study has the limitations of a retrospective study, such as different criteria for the drug treatment of heart failure. However, we tried to respect the clinical cardiologist's indication, maintaining similar pre- and post-ablation criteria. Our database is fed as an electronic medical record, in which the attending physician includes all information at the first medical visit (pre-ablation), and, later, follow-up data are constantly included at real time to minimize occasional flaws. Another limitation relates to the success criterion. Asymptomatic and undiagnosed AF is known to occur during follow-up. However, because of the particular characteristics of that group of patients with ventricular dysfunction, who are extremely symptomatic regarding arrhythmia, the occurrence of relapse without clinical findings is believed to be less likely. Another restriction is the variation in time of the post-ablation control echocardiography. The general instruction was to perform it three months after the intervention, providing time for remodeling to complete and avoiding overlapping with post-ablation protocol drugs. Another limitation relates to the use of the NYHA functional classification, which is a qualitative variable for clinical characterization and follow-up comparison; however, many studies use it due to its practicability.
It is worth noting that, at the time most ablations were performed, there were limitations regarding the materials, electrodes and learning curves of the professional team involved. That aspect became clear by the evident improvement of the results during this study, which enabled both technical and material refinement, mainly with the systematic use of irrigated electrodes and search for background tachycardia and its ablation. An important step was the addition of extensive elimination of AF nests, detected by use of the circular catheter, at the region of the crista terminalis and right atrial anterior wall, enabling the drastic reduction in post-ablation relapses.
Conclusion
The results of this study suggest that AF ablation of patients with ventricular dysfunction is a safe procedure of high medium-term efficacy. Controlling that arrhythmia has a great impact on ventricular function recovery and on clinical improvement assessed via functional class.
Footnotes
Author contributions
Conception and design of the research, Acquisition of data, Analysis and interpretation of the data, Statistical analysis, Writing of the manuscript, Critical revision of the manuscript for intellectual content: Lobo TJ, Pachon CT, Pachon JC, Pachon EI, Pachon MZ, Carlos Pachon J, Santillana TG, Zerpa JC, Albornoz RN, Jatene AD.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any thesis or dissertation work.
References
- 1.Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath-Ordoubadi F, Neyses L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11(7):676–683. doi: 10.1093/eurjhf/hfp085. [DOI] [PubMed] [Google Scholar]
- 2.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 3.Kieny JR, Sacrez A, Facello A, Arbogast R, Bareiss P, Roul G, et al. Increase in radionuclide left ventricular ejection fraction after cardioversion of chronic atrial fibrillation in idiopathic dilated cardiomyopathy. Eur Heart J. 1992;13(9):1292–1295. doi: 10.1093/oxfordjournals.eurheartj.a060351. [DOI] [PubMed] [Google Scholar]
- 4.Packer DL, Bardy GH, Worley SJ, Smith MS, Cobb FR, Coleman RE, et al. Tachycardia-induced cardiomyopathy: a reversible form of left ventricular dysfunction. Am J Cardiol. 1986;57(8):563–570. doi: 10.1016/0002-9149(86)90836-2. [DOI] [PubMed] [Google Scholar]
- 5.Khan MN, Jaïs P, Cummings J, Di Biase L, Sanders P, Martin DO, et al. PABA-CHF Investigators Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359(17):1778–1785. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 6.Chen MS, Marrouche NF, Khaykin Y, Gillinov AM, Wazni O, Martin DO, et al. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J Am Coll Cardiol. 2004;43(6):1004–1009. doi: 10.1016/j.jacc.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 7.Hsu LF, Jaïs P, Sanders P, Garrigue S, Hocini M, Sacher F, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351(23):2373–2383. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 8.Cha YM, Wokhlu A, Asirvatham SJ, Shen WK, Friedman PA, Munger TM, et al. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: a comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol. 2011;4(5):724–732. doi: 10.1161/CIRCEP.110.960690. [DOI] [PubMed] [Google Scholar]
- 9.Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61(18):1894–1903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 10.Pachón M JC, Pachón M EI, Pachón M JC, Lobo TJ, Pachón MZ, Vargas RN, et al. A new treatment for atrial fibrillation based on spectral analysis to guide the catheter RF-ablation. Europace. 2004;6(6):590–601. doi: 10.1016/j.eupc.2004.08.005. Erratum in: Europace. 2005;7(1):92-3. [DOI] [PubMed] [Google Scholar]
- 11.Lin JY, Chang SL, Lo LW, Chen SA. Shenasa M, Hindricks G, Borggrefe M, Breithardt G, Josephson ME, Zipe DP. Cardiac mapping. 4th ed. New Jersey: Wiley-Blackwell; 2012. Mapping of the atrial electrogram in sinus rhythm and different atrial fibrillation substrates; pp. 323–338. [Google Scholar]
- 12.Mateos JC, Mateos EI, Lobo TJ, Pachón MZ, Mateos JC, Pachón DQ, et al. Ablação da fibrilação atrial por cateter com radiofreqüência guiada por mapeamento espectral endocárdico dos "ninhos de FA" em ritmo sinusal. Arq Bras Cardiol. 2007;89(3):140–150. [Google Scholar]
- 13.Pachón JC, Pachón EI, Pachón JC, Lobo TJ, Pachón MZ, Jatene AD. "Cardioneuroablation" new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. 2005;7(1):1–13. doi: 10.1016/j.eupc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 15.Hagens VE, Crijns HJ, Van Veldhuisen DJ, Van Den Berg MP, Rienstra M, Ranchor AV, et al. Rate Control versus Electrical cardioversion for persistent atrial fibrillation study group Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the Rate control versus electrical cardioversion (RACE) study. Am Heart J. 2005;149(6):1106–1111. doi: 10.1016/j.ahj.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, et al. AFFIRM Investigators Relationships between sinus rhythm, treatment, and survival in the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. Circulation. 2004;109(12):1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 17.Shelton RJ, Clark AL, Goode K, Rigby AS, Houghton T, Kaye GC, et al. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II study) Heart. 2009;95(11):924–930. doi: 10.1136/hrt.2008.158931. [DOI] [PubMed] [Google Scholar]
- 18.Arruda M, Natale A. Ablation of permanent AF: adjunctive strategies to pulmonary veins isolation: targeting AF NEST in sinus rhythm and CFAE in AF. J Interv Card Electrophysiol. 2008;23(1):51–57. doi: 10.1007/s10840-008-9252-z. [DOI] [PubMed] [Google Scholar]