Abstract
Background:
Apical leakage assessment is a way to compare the efficiency of a filling material to seal the apical region of the tooth. Many microleakage testing techniques have been introduced through the years, but there has been no agreement as to which technique gives the most accurate results. The aim of this study was to compare the accuracy of fluid filtration and bacterial leakage techniques in the assessment of the apical sealing ability of mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM).
Materials and Methods:
A sample of 34 extracted single-rooted human teeth were selected and prepared. The samples were divided in to 2 experimental groups. The apical 3 mm of each root was resected at 90° to its long axis and root end preparation was done with ultrasonic tips to a depth of 3 mm and filled with MTA and CEM, respectively. Assessment of apical sealing ability was done with fluid filtration technique and bacterial leakage technique along 90 days with Enterococcus faecalis bacteria. Mann-Whitney U-test and Chi-square test were used to analyze the data using SPSS (SPSS Inc., Chicago, IL, USA). P less than 0.05 was considered as significant.
Results:
There was no significant difference in apical sealing ability between MTA and CEM in bacterial leakage and fluid filtration techniques. Samples which had bacterial leakage showed higher leakage values by fluid filtration technique.
Conclusion:
Both techniques showed same results and there was no significant difference between fluid filtration and bacterial leakage techniques in assessment of apical microleakage.
Keywords: Bacterial leakage, fluid filtration, microleakage, root-end filling
INTRODUCTION
Microleakage is defined as seepage of fluids, debris, microorganisms or ions along the interface between a restorative or a filling material and the wall of the tooth.[1] Clinical studies have revealed that apical leakage of root filling materials is one of the main causes of endodontic therapy failures.[2] Through the years a variety of different apical leakage assessment methods have been introduced and developed.[3] They estimate the sealing capacity of a filling material or a filling method, generally by measuring the extent and path of penetration of a tracing agent into the filled canal. The most frequent tracers that have been used are dyes, radioactive isotopes and bacteria and its products.[2,3]
The fluid filtration method was developed by Derkson et al.[4] and modified by Wu et al.[5] for use in endodontics. In the fluid filtration technique, the coronal or apical tip of the sample is attached to a micropipette which has been filled with liquid. A small air bubble is inserted into the pipette. The entire system has been kept under a constant pressure. The amount of bubble displacement during a specified period of time is measured, which is a sign of the amount of liquid passage through the sample and present the microleakage of that sample and is calculated in μl/min.[6]
The bacterial microleakage technique was first used by Fraser in order to evaluate the leakage of glass tubes which were filled with amalgam.[1] In the bacterial microleakage technique, bacteria are used as markers. Bacterial culture is settled in contact with the coronal part of the tooth and the apical tip of the sample is in contact with the sterile culture medium, as the only path between the bacterial culture (upper chamber) and sterile culture medium (lower chamber) is the root canal filling. This assembly is incubated in 37°C and the samples are controlled every day to assess turbidity in the lower chamber. The period of time that takes the culture medium to become turbid is a marker of root canal contamination. This method was first introduced as Dual Chamber technique by Torabinejad et al.[7] In this technique, it is important to make sure about the vitality and activity of the bacteria, so the bacterial suspension in the upper chamber is refreshed every other day. Moreover, in order to verify the authenticity of the experiment, it is necessary to define the bacteria which caused the culture medium (lower chamber) turbidity.[8]
Mineral trioxide aggregate (MTA) was introduced specifically as a retrograde filling material.[9] Various aspects of its properties were compared to other materials in several in vitro and in vivo studies. Biocompatibility and sealing ability of MTA was better than others materials; in addition, it has cementum sedimentation quality.[10,11,12] Recently, calcium enriched mixture (CEM) cement was introduced in endodontics by Asgary et al.[13] The clinical application of CEM is the same as MTA.[14] The properties of CEM are high alkalinity, release of calcium hydroxide and adequate antimicrobial quality.[15]
There is an expectation that different microleakage methodologies give similar results when assessing the same material or technique, but since these methodologies are not standardized, different methodologies would lead to conflicting results.[16,17] Therefore, it seems necessary to compare the accuracy of various techniques and suggest a standard method for determination of the sealing ability of restorative or filling materials and techniques in endodontic therapies. The aim of this study was to compare the results of fluid filtration and bacterial microleakage techniques in the evaluation of the sealing ability of retrograde MTA and CEM.
MATERIALS AND METHODS
Sample preparation
After approval by the Research Ethics Committee of the Mashhad University of Medical Sciences (protocol number #88160), 34 teeth were selected from a large series of single-rooted anterior teeth which had recently been extracted. The teeth had mature apices, no caries or internal or external resorption, no cracks on root surfaces and no curves (curves less than 20° were included). After sample collection the root surfaces were cleaned with a periodontal curette to remove all calculi and soft-tissue. Then, the samples were disinfected by 5.25% sodium hypochlorite (Naocl) for an hour and were kept in distill water.[18] The coronal part of the samples was cut in order to standardize 15 mm of each root. Apical foramen diameter of all samples were checked by inserting a #15 K-file (Dentsply, Maillefer, Ballaigues, Switzerland) into the canal. Using this method, the apical foramen of all teeth was standardized. Canal preparation was carried out using K-file #40 (Dentsply, Maillefer, Ballaigues, Switzerland) with step-back technique. Coronal portion of canals were flared by Gates-Glidden drills #2, 3 and 4 (Dentsply, Maillefer, Switzerland). After using each instrument, canals were rinsed by 1 ml of 5.25% Naocl.[16] Then, samples were randomly divided into two experimental groups, each group containing 15 samples; positive and negative control groups had four samples.
In experimental groups, the apical 3 mm of each root was resected at 90° to its long axis and root end preparation was done with ultrasonic tips (piezo-electric [Spartan, Fenton, MO, USA]) to a depth of 3 mm. In order to provide an intracanal matrix to support the condensation of retrograde filling material, high convergence gutta-percha was adapted into the coronal part of the apical cavity [Figure 1a]. Then, the 3 mm apical cavity was filled with MTA (Dentsply, Tulsa Dental, Tulsa, OK, USA) or CEM (BioniqueDent, Tehran, Iran) using an MTA carrier and filling density was verified by radiographs [Figure 1b]. Samples were kept in 100% humidity and 37°C for retrograde material setting.
Figure 1.
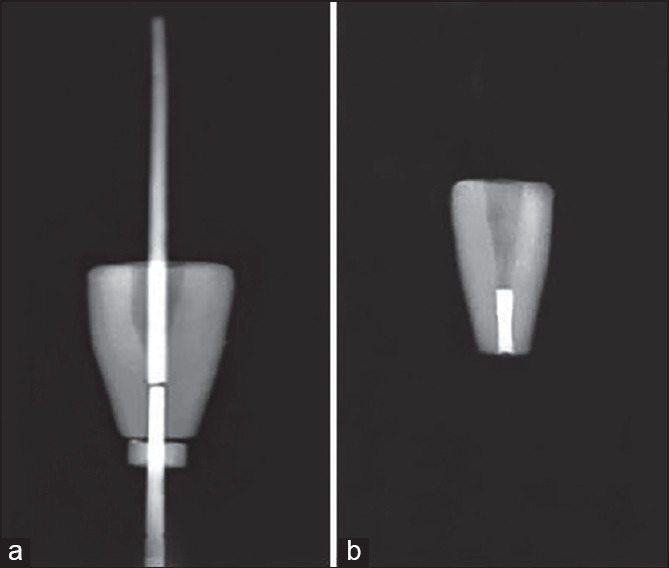
(a) Root end preparation, (b) retrograde filling
External surfaces of the experimental samples, except the apical region, were sealed with two layers of nail polish and sticky wax. Two samples of positive control group were prepared, but not filled and external surfaces were sealed similar to experimental samples. The outer surface and apical region of 2 samples of negative control group, which were prepared and filled according to the experimental groups, were sealed with two layers of nail polish and sticky wax.
Fluid filtration technique
This system evaluates the passage of liquid through the samples in order to assess the sealing ability of that case. This is done by measuring the bubble displacement which is produced on the path of liquid movement. In order to make the liquid move and assessing the leakage, there was an oxygen gas pressure behind the liquid, which was kept constant throughout the experiment on 0.5 atm using a manometer. A small air bubble was inserted into a 0.1 mL pipette with a syringe. The bubble must include the whole internal diameter of pipette in order to be sure that the bubble displacement is a trustful sign of liquid passage through the tube.
First, the positive control group's samples were attached to the system and rapid movement of the bubble was noticed from beginning to the end of the pipette. Then, negative control group's samples were attached and no bubble movement was observed in 8 min. The system was ready for use after passing these 2 tests. A 10 megapixel digital Camera (Canon powershot G 11, Japan) [Figure 2a] and Adobe Photoshop 7.0 software (Adobe Sys-tems Inc., San Jose, USA) were used to record and analyze the bubble movement [Figure 2b]. The system was allowed to equilibrate for 30 s before measuring the bubble movement. Afterward, the first picture was taken from the initial position of the bubble in pipette. The following 4 pictures were taken at 2 min intervals. After the computation, the amount of the fluid passing through the samples was calculated in μl/min.
Figure 2.
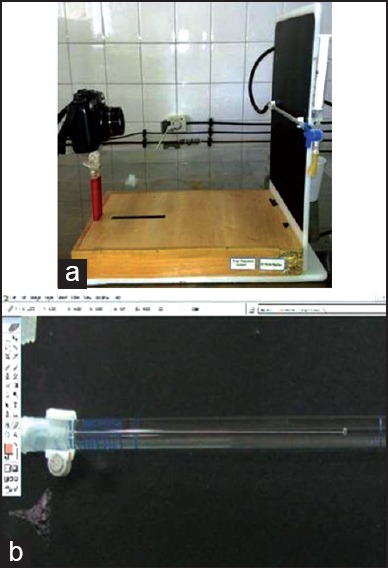
(a) Camera system, (b) small air bubble in 0.1 cc pipette of fluid filtration system
Bacterial microleakage technique
First, the bottom of an Eppendorf test micro tubes was cut with the blade. The teeth, which all had a layer of hot sticky wax on their coronal half of the root surfaces, were inserted into the cut micro tubes. The coronal part of the roots and the access cavities were in the tubes and the 2-3 mm of apical part was out. The junction between tubes and teeth were sealed internally again by sticky wax. The assemblies [Figure 3a] were sterilized in ethylene oxide gas for 8 h by Anprolene machine (Anderson products Inc., Haw River, NC, USA).
Figure 3.
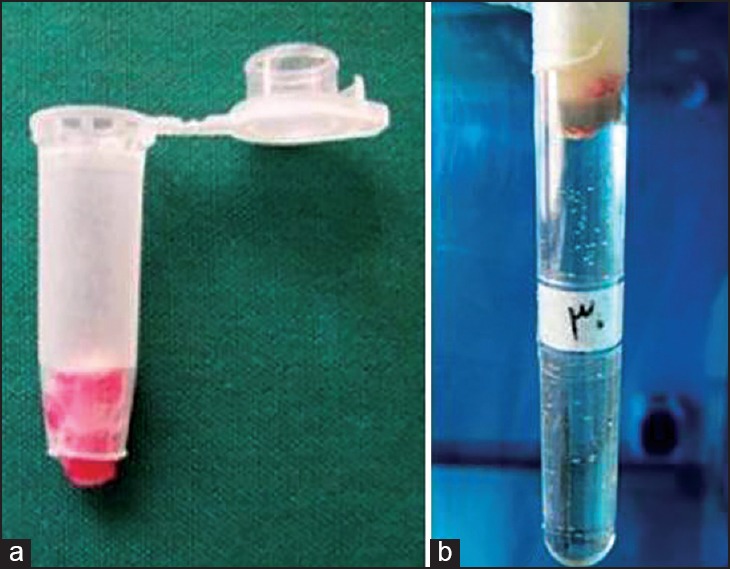
(a) The root-micro tubes assembly, (b) the root-micro tubes assembly and test tube
Muller-Hinton Broth culture medium (Merck, Darmstadt, Germany) was added to test tubes#12 (Pouyanteb, Tehran, Iran), using a syringe and sterile needle (22-gauge), in a way that the 10-15 mm of the tubes remained empty for placing the assemblies. Afterwards, tubes containing culture mediums were sterilized in 116-121°C for 10-15 min by autoclave (Iran Abzarteb company, Tehran, Iran). The root-micro tubes assemblies were settled inside test tubes as the root tips were contacting the culture medium. The junction region between micro tubes and test tubes were sealed using sticky wax and parafilm straps [Figure 3b].
A culture containing 9 × 108 CFU/ml of Enterococcus faecalis in 1 ml of Muller-Hinton Broth solution was placed into the micro tubes in contact with the coronal access opening of the roots. The bacterial suspension was refreshed every 3 days. The culture medium in the lower chamber was monitored daily for 3 months for turbidity. In order to be sure about E. faecalis presence in turbid mediums, samples were picked from the lower chambers and cultured in bile esculinagar (Merck, Darmstadt, Germany). The black discoloration of the medium, which was amber at first, was a sign of E. faecalis presence.
Statistical analysis
Mann-Whitney U-test and Chi-square test were used to analyze the data using SPSS 12 (SPSS Inc., Chicago, IL, USA). P less than 0.05 was considered as significant.
RESULTS
The MTA group showed no statistically significance difference with CEM group in the fluid filtration technique [Table 1]. There was no significant difference in apical sealing ability between MTA and CEM in bacterial leakage technique [Table 2].
Table 1.
Comparison of bacterial microleakage in CEM and MTA groups
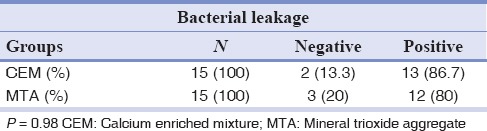
Table 2.
Comparison of mean fluid filtration values in CEM and MTA groups
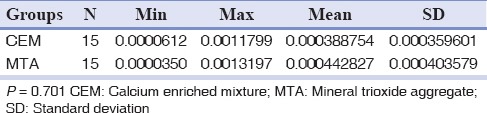
In both groups mean fluid filtration values in positive bacterial leakage cases were higher than the negative cases. All negative and positive control groups showed results as expected.
DISCUSSION
In the present study, fluid filtration and bacterial microleakage techniques were compared for assessment of sealing ability of retro-filing materials (MTA and CEM) and consistent results were obtained. MTA was developed in 1995 and its application for the treatments of radicular perforation, root-end retrofilling, apexiflcation and conservative pulp therapy is expected. CEM cement is another retrofilling material with clinical applications the same as MTA.[9,13] Although the main purpose of this study was to compare the fluid filtration and bacterial microleakage as techniques for evaluation of the sealing ability of materials, another finding of this study was that MTA and CEM have similar sealing abilities. This finding was comparable with the results of studies of Milani et al.[9] and Asgary et al.[13] MTA and CEM are hydrophilic endodontic cements and allow access of cement within gaps and help the entrance of small cement particles into dentinal tubules. Furthermore, MTA and CEM exhibit slight expansion after setting[14] and provide enhanced adaptation of the biomaterials to the walls. In addition, MTA and CEM form hydroxyapatite and provide an improved seal at the interface of biomaterials and dentin walls.[13,19,20]
Several articles in literature could be found that investigate the sealing capacity of different materials.[1,2,3,4,5,6,7,8] The main topics covered by the top-cited articles were microleakage and endodontic microbiology.[21] However, there are few studies that compared the effectiveness of the techniques for measuring the sealing ability.[3,16,17] Veríssimo and do Vale concluded that there was a real lack of technique standardization even when the same method is used, which may lead to variable results.[3]
Dye penetration is a common microleakage measuring technique and in spite of the disadvantages, the popularity of this technique has not diminished due to ease of use and low cost.[22] One of the major drawbacks of this technique is the small size of the dye molecules and probably false positive results and overestimation of leakage. The reliability, reproducibility and clinical relevance of dye penetration are questionable.[23] Among microleakage measuring techniques, the bacterial leakage model is considered to be the most clinically and biologically relevant.[18]
Fluid filtration technique presents many advantages in comparison to dye penetration technique, as the samples are not destroyed[24] and the results are precise (small volumes can be recorded) and quantitative.[25] Another reason for selecting fluid filtration was that most other techniques are not suitable under some experimental conditions, such as chemical reactions. In fact, some staining agents used in conventional testing techniques react with dentin, which may lead to unfavorable results. The fluid filtration technique avoids these disadvantages because it does not require any chemical agent for testing.[26] Pommel and Camps declared that some procedures in this technique were not standardized, as the pressure might range between 1 and 20 psi and the experiment time from 1 min to 3 h.[27] This would affect the results, as the longer measuring time led to lower filtration values and higher pressure was associated with higher leakage values. They suggested 15 cm H2O would be efficient, as it was close to physiologic pressure. According to their study, the pressure, bubble size, or micropipette diameter could affect the system sensitivity.[27]
In the current study, we compared fluid filtration and bacterial microleakage techniques. For bacterial microleakage technique, a single subtype of E. faecalis was used. It is one of the most common bacterial causes of endodontic therapy failures, can penetrate through the tubules and it is resistant against irrigation and intracanal medication.[28]
In their study, Mortensen et al.[29] and Krakow et al.[30] revealed that the bacterial microleakage technique was more accurate in leakage assessment, in comparison to dye penetration or radioisotopes techniques. In contrast to the present study, Karagenç et al.[31] compared 4 leakage testing techniques in assessing the leakage of two root canal filling techniques. They showed there was a lack of correlation between bacterial leakage and fluid filtration techniques. Different methodologic aspects between the present study and Karagenç et al. study may be the reason for different results. For example, in the present study, we used an oxygen gas pressure behind the liquid, which was kept constant throughout the experiment on 0.5 atm, but in Karagenç et al.[31] study fluid pressure was applied with helium gas using a pressure of 250 mm Hg. The lack of standardization in microleakage techniques hinder comparisons of the studies and could explain the divergent results.[32]
However, according to the results of the present study, there was no statistically significant difference between the sealing ability of MTA and CEM cement using the fluid filtration and bacterial leakage techniques. Samples which had bacterial leakage showed higher leakage values by fluid filtration technique. This study concluded high correlation between the two techniques.
CONCLUSION
Based on the results of this study, there was no significant difference between two techniques. The bacterial leakage technique can be replaced with the fluid filtration technique, because the bacterial leakage method takes more time, the procedure is more complex and it requires a skilled microbiologist.
ACKNOWLEDGMENTS
This study was supported by a grant from the Vice Chancellor of Research Council of Mashhad University of Medical Sciences, Iran. The results presented in this study have been taken from a student thesis (no. 439) in Mashhad University of Medical Sciences (MUMS). We would like to thank Mr. Abdollah Javan for his advice as a statistician in this study. The authors deny any conflicts of interest related to this study.
Footnotes
Source of Support: This study was supported by a grant from the Vice Chancellor of Research Council of Mashhad University of Medical Sciences, Iran.
Conflict of Interest: None declared.
REFERENCES
- 1.Ciftçi A, Vardarli DA, Sönmez IS. Coronal microleakage of four endodontic temporary restorative materials: An in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e67–70. doi: 10.1016/j.tripleo.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Vasconcelos BC, Bernardes RA, Duarte MA, Bramante CM, Moraes IG. Apical sealing of root canal fillings performed with five different endodontic sealers: Analysis by fluid filtration. J Appl Oral Sci. 2011;19:324–8. doi: 10.1590/S1678-77572011005000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veríssimo DM, do Vale MS. Methodologies for assessment of apical and coronal leakage of endodontic filling materials: A critical review. J Oral Sci. 2006;48:93–8. doi: 10.2334/josnusd.48.93. [DOI] [PubMed] [Google Scholar]
- 4.Derkson GD, Pashley DH, Derkson ME. Microleakage measurement of selected restorative materials: A new in vitro method. J Prosthet Dent. 1986;56:435–40. doi: 10.1016/0022-3913(86)90384-7. [DOI] [PubMed] [Google Scholar]
- 5.Wu MK, De Gee AJ, Wesselink PR, Moorer WR. Fluid transport and bacterial penetration along root canal fillings. Int Endod J. 1993;26:203–8. doi: 10.1111/j.1365-2591.1993.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 6.Moradi S, Naghavi N, Rohani E, Javidi M. Evaluation of microleakage following application of a dentin bonding agent as root canal sealer in the presence or absence of smear layer. J Oral Sci. 2009;51:207–13. doi: 10.2334/josnusd.51.207. [DOI] [PubMed] [Google Scholar]
- 7.Torabinejad M, Ung B, Kettering JD. In vitro bacterial penetration of coronally unsealed endodontically treated teeth. J Endod. 1990;16:566–9. doi: 10.1016/S0099-2399(07)80198-1. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz M, Baca P, Pardo-Ridao MD, Arias-Moliz MT, Ferrer-Luque CM. Ex vivo study of bacterial coronal leakage in indirect pulp treatment. Med Oral Patol Oral Cir Bucal. 2013;18:e319–24. doi: 10.4317/medoral.18425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milani AS, Shakouie S, Borna Z, Sighari Deljavan A, Asghari Jafarabadi M, Pournaghi Azar F. Evaluating the effect of resection on the sealing ability of MTA and CEM cement. Iran Endod J. 2012;7:134–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Zairi A, Lambrianidis T, Pantelidou O, Papadimitriou S, Tziafas D. Periradicular tissue responses to biologically active molecules or MTA when applied in furcal perforation of dogs’ teeth. Int J Dent 2012. 2012 doi: 10.1155/2012/257832. 257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda H, Nakano T, Tomokiyo A, Fujii S, Wada N, Monnouchi S, et al. Mineral trioxide aggregate induces bone morphogenetic protein-2 expression and calcification in human periodontal ligament cells. J Endod. 2010;36:647–52. doi: 10.1016/j.joen.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Holland R, Bisco Ferreira L, de Souza V, Otoboni Filho JA, Murata SS, Dezan E., Jr Reaction of the lateral periodontium of dogs’ teeth to contaminated and noncontaminated perforations filled with mineral trioxide aggregate. J Endod. 2007;33:1192–7. doi: 10.1016/j.joen.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Asgary S, Eghbal MJ, Parirokh M. Sealing ability of a novel endodontic cement as a root-end filling material. J Biomed Mater Res A. 2008;87:706–9. doi: 10.1002/jbm.a.31678. [DOI] [PubMed] [Google Scholar]
- 14.Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The properties of a new endodontic material. J Endod. 2008;34:990–3. doi: 10.1016/j.joen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Asgary S, Kamrani FA. Antibacterial effects of five different root canal sealing materials. J Oral Sci. 2008;50:469–74. doi: 10.2334/josnusd.50.469. [DOI] [PubMed] [Google Scholar]
- 16.Wedding JR, Brown CE, Legan JJ, Moore BK, Vail MM. An in vitro comparison of microleakage between Resilon and gutta-percha with a fluid filtration model. J Endod. 2007;33:1447–9. doi: 10.1016/j.joen.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Shahi S, Rahimi S, Yavari HR, Shakouie S, Nezafati S, Abdolrahimi M. Sealing ability of white and gray mineral trioxide aggregate mixed with distilled water and 0.12% chlorhexidine gluconate when used as root-end filling materials. J Endod. 2007;33:1429–32. doi: 10.1016/j.joen.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Moradi S, Disfani R, Baziar H, Daneshvar F, Jafarzadeh H. Use of fluid filtration method to evaluate the effect of master cone size on the apical seal of severely curved root canals. J Oral Sci. 2013;55:93–8. doi: 10.2334/josnusd.55.93. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 20.Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J. Effect of two storage solutions on surface topography of two root-end fillings. Aust Endod J. 2009;35:147–52. doi: 10.1111/j.1747-4477.2008.00137.x. [DOI] [PubMed] [Google Scholar]
- 21.Fardi A, Kodonas K, Gogos C, Economides N. Top-cited articles in endodontic journals. J Endod. 2011;37:1183–90. doi: 10.1016/j.joen.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Pusinanti L, Rubini R, Pellati A, Zerman N. A simplified post preparation technique after Thermafil obturation: Evaluation of apical microleakage and presence of voids using methylene blue dye penetration. Ann Stomatol (Roma) 2013;4:184–90. [PMC free article] [PubMed] [Google Scholar]
- 23.Camps J, Pashley D. Reliability of the dye penetration studies. J Endod. 2003;29:592–4. doi: 10.1097/00004770-200309000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Hirschberg CS, Patel NS, Patel LM, Kadouri DE, Hartwell GR. Comparison of sealing ability of MTA and EndoSequence bioceramic root repair material: A bacterial leakage study. Quintessence Int. 2013;44:e157–62. [PubMed] [Google Scholar]
- 25.Tapsir Z, Aly Ahmed HM, Luddin N, Husein A. Sealing ability of various restorative materials as coronal barriers between endodontic appointments. J Contemp Dent Pract. 2013;14:47–50. doi: 10.5005/jp-journals-10024-1268. [DOI] [PubMed] [Google Scholar]
- 26.Machado R, Silva Neto UX, Ignácio SA, Cunha RS. Lack of correlation between obturation limits and apical leakage. Braz Oral Res. 2013;27:331–5. doi: 10.1590/s1806-83242013000400007. [DOI] [PubMed] [Google Scholar]
- 27.Pommel L, Camps J. Effects of pressure and measurement time on the fluid filtration method in endodontics. J Endod. 2001;27:256–8. doi: 10.1097/00004770-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Nair U, Ghattas S, Saber M, Natera M, Walker C, Pileggi R. A comparative evaluation of the sealing ability of 2 root-end filling materials: An in vitro leakage study using Enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:e74–7. doi: 10.1016/j.tripleo.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 29.Mortensen DW, Boucher NE, Jr, Ryge G. A method of testing for marginal leakage of dental restorations with bacteria. J Dent Res. 1965;44:58–63. doi: 10.1177/00220345650440013101. [DOI] [PubMed] [Google Scholar]
- 30.Krakow AA, de Stoppelaar JD, Gron P. In vivo study of temporary filling materials used in endodontics in anterior teeth. Oral Surg Oral Med Oral Pathol. 1977;43:615–20. doi: 10.1016/0030-4220(77)90117-7. [DOI] [PubMed] [Google Scholar]
- 31.Karagenç B, Gençoglu N, Ersoy M, Cansever G, Külekçi G. A comparison of four different microleakage tests for assessment of leakage of root canal fillings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:110–3. doi: 10.1016/j.tripleo.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 32.da Silva-Neto JP, Nóbilo MA, Penatti MP, Simamoto PC, Jr, das Neves FD. Influence of methodologic aspects on the results of implant-abutment interface microleakage tests: A critical review of in vitro studies. Int J Oral Maxillofac Implants. 2012;27:793–800. [PubMed] [Google Scholar]


