Abstract
Background:
One of the probable etiologic risk factors of oral squamous cell carcinoma (OSCC) is Candidal infection, especially by Candida albicans, whose role has not definitely been confirmed. Some have assigned a primary role to Candida, whereas others consider it as a transient inhabitant. The debate may be due to lack of an accurate and sensitive revealing technique. By identifying the presence of Candida, especially in deeper parts of OSCC, the etiologic role may be verified. The present study was conducted to detect the presence of Candida in OSCC by fluorescence staining technique.
Materials and Methods:
This study was descriptive experimental. Calcofluor-white, which is applied in fluorescence staining, is a specific staining substance for Candida and has a higher accuracy compared with other common methods. 100 specimens of well-differentiated OSCC with adequate amount of tissue were retrieved from the archive and two serial sections were obtained from each one. The first section was stained using the popular histochemical (periodic acid-Schiff [PAS]) method and then evaluated under a light microscope to detect the presence of Candida. The second section was stained using fluorescence staining technique. The sum of counted Candida in each technique was fed into SPSS software and analyzed by McNamara test. P < 0.001 was considered as significant.
Results:
The amount of Candida present in OSCCs was 74% measured by fluorescence technique. The sensitivity and specificity of the two staining techniques were significantly different. These parameters in the fluorescence technique were higher than those of the histochemical (PAS) method, confirmed by McNamara test showing significantly different results for them (P < 0.001). The results obtained from the fluorescence technique had higher accuracy compared with the histochemical (PAS) method.
Conclusion:
Some researchers couldn’t find a considerable number of fungi in OSCC, while our results revealed more presence of Candida, especially in deeper parts of tissue samples and probably a more important role for Candida as an etiologic risk factor for OSCC. However, since the fluorescence technique had a higher accuracy in the identification of Candida and it was nearly evident in two-third of the samples, the role of fungi as a primary cause is suggested to be studied in future investigations.
Keywords: Candida albicans, fluorescence, oral squamous cell carcinoma
INTRODUCTION
Squamous cell carcinoma of the oral cavity (oral squamous cell carcinoma [OSCC]) accounts for 5% of all cancers in men and 2% of all cancers in women.[1,2,3,4] Candida is among the etiologic risk factors of OSCC, which is part of normal oral flora.[5] The weakening of the immune system changes the normal flora and Candidiasis infection may occur.[6] Candida albicans is the most causal agent in oral fungal infections.[7] It can reproduce in two forms:
Hypha and
yeast.
Yeast is relatively harmless, but hypha usually attacks the host tissues.[5] Consequently, the contaminated epithelial cells begin to change morphologically[8] due to the:
These morphological changes may lead to carcinogenic evolvement and the subsequent OSCC.[14] Some have assigned a primary role to Candida, while others consider it as a transient inhabitant. The debate might be due to lack of an accurate and sensitive revealing technique.[5] By identifying the presence of Candida in deeper parts of OSCC, its etiologic role might be confirmed. The present study was conducted to evaluate the presence of Candida in OSCC using the fluorescence staining and to compare it with histochemical (periodic acid-Schiff [PAS]) staining.[5]
Evidently, the presence of C. albicans should be confirmed prior to evaluating any mechanism. A number of detecting techniques have been used to identify C. albicans in oral tissues:[5]
PAS.
Potassium hydroxide.
Immunohistochemistry (IHC).
Fluorescence.
Polymerase chain reaction (PCR).
Molecular and genetic techniques.[5]
Among these techniques, PAS has been used for many years as the most common technique to identify fungi. It stains the carbohydrates in the cell walls of fungi and produces a light red color.[5,15] However, in fluorescence technique (calcofluor-white), the fungi can be seen in apple green by fluorescent light. The dye contains β1-3 and β1-4 polysaccharides, which can bind to cellulose and chitin, present in the cell walls of the fungi.[16] It is a fast and accurate method to recognize the fungi and microorganisms with cellulose walls. It has a higher accuracy for C. albicans due to its specific cell wall characteristics.[17]
MATERIALS AND METHODS
In this descriptive in vitro study, we evaluated the presence of C. albicans in OSCC. A total of 100 samples were obtained from the patient specimens available in Department of Oral Pathology, Faculty of Dentistry and in Department of Pathology, Kashani Hospital, Isfahan University of Medical Sciences. The selection criteria included adequate tissue amount and well-differentiated OSCC. Any sample which did not fulfill these criteria was excluded. Retrieved paraffin blocks were sectioned (3 μm thick) by microtome (Slee-Germany). Two serial sections were obtained from each block, so that the tissue depth error, while comparing the two methods, would be minimized. Afterwards, the PAS method (Merck-Germany) was applied and staining of salivary acinus basement membrane and epithelial basement membrane was considered as an internal control. A urine sample containing adequate amounts of Candida, shining under a fluorescence microscope, was used as positive control for the fluorescence technique. First, the histochemical method (PAS) was performed and the samples were observed under a light microscope (Olympus-Japan BX 41, magnification ×400). The fluorescence technique (Calcofluor-white) (Sigma-USA) was also applied on each sample and evaluated using a fluorescence microscope with ultraviolet light (Olympus-Japan BX40, 290-340 nm). Each sample was coded (from 1 to 100) and the results of the presence of C. albicans, using both techniques, were recorded by a pathologist who was not aware of the results of the previous staining techniques. Data were analyzed by SPSS 10 software (SPSS Inc., Chicago, IL, USA) using McNamara test (P < 0.001).
RESULTS
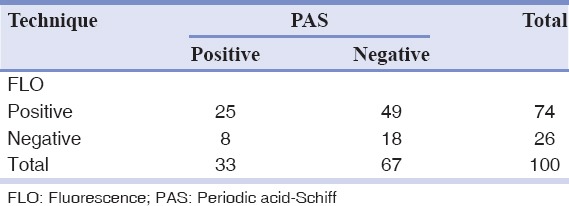
In this study, we applied fluorescence staining technique as a gold standard. The presence of C. albicans in OSCC was 74% according to this technique. One hundred samples were stained, of which 26 turned out as negative. In the PAS technique, however, of the 100 samples stained, 33 turned out as positive and 67 as negative. 25 out of the 74 positive samples, observed in the fluorescence method, were also positive using the PAS method, which means 33.8% sensitivity for the PAS method in terms of identification of C. albicans. From the 26 negative samples observed in the fluorescence method, 18 were also negative using the PAS method, which is indicative of 69.2% specificity for PAS in identification of C. albicans. The positive predictive value (PPV) for the PAS method was 75.8% and the negative predictive value (NPV) was 26.9%. On the whole, the PAS method showed low sensitivity and NPV in identifying C. albicans. Moreover, it did not have high specificity or a considerable PPV. The findings of McNamara analysis indicated a statistically significant difference between the two methods (P < 0.001). All of the data are presented in Table 1.
Table 1.
FLO-PAS cross tabulation
DISCUSSION
Candida can harm the epithelium in two ways:
By producing lytic enzymes (aspartic protease). These enzymes are produced by hypha, which digest the epithelial cells and interfere with the immune cells.[18]
By inducing endocytosis in epithelial cells and forming pseudo pods around the fungi.[18] Both the yeast and hypha can induce endocytosis and kill the epithelial cells, although hypha is more prone to induce the process.[18]
E-cadherin is one of the major adhesive proteins in epithelial cells. C. albicans has a similar protein in its cell wall named agglutinin-like sequence 3.[19] This protein can bind to oral epithelial cells and form a pseudopod adhesion complex leading to malfunction of E-cadherin and consequently decreasing cellular adhesion and facilitating cell mobility[20,21] and finally invading the connective tissue. In addition, the cell wall of this organism can block laminins, fibronectin and collagen types 4 and 1 and change the structure of extracellular matrix proteins, which, in turn, affects the epithelial cell mobility and provides the signals needed to induce cancer and metastasis.[16,17] In contrast, some researchers believe that C. albicans has a secondary role and in patients with OSCC, as a result of weakening the immune system, the fungi are able to rapidly multiply and grow more colonies, especially in superficial epithelium.[22] This is believed to occur at the time of diagnosis or secondary to treatment of patients.[22] However, as mentioned previously, there are scientists who claim that C. albicans has a primary role in the occurrence of OSCC.
Based on this assumption, the presence of C. albicans in the deeper epithelial layers is given more attention for investigation than its presence in the superficial layers.[23]
In the present study, because the time of biopsy, which was done as an incision before or after the surgery of the patients, was not clear, the primary or secondary role of C. albicans could not be determined at all times.
In this study, another considerable problem was uncertainty about positive or negative staining. Obviously, C. albicans has morphological properties that make it appear as rod-shaped bacteria in pink to reddish color.[24]
Considering the morphology of bacteria and the way they are stained (using the PAS method in this case), there are other types of species and even substances with similar characteristics that make the process more challenging. For example, when collagen fibers bind to form short subgroups, they form a netlike appearance which makes them similar to the mycelium of Candida and consequently very difficult to differentiate the two from each other. Moreover, in the epithelium itself, the existing keratinized fibers have high similarity to the mycelia of Candida. Therefore, the PAS technique is not a very reliable procedure to study and determine the presence or absence of fungi, because it involves many false positive and false negative results. This study investigated 100 cases of OSCC, of which 67 indicated negative results using the PAS technique [Figure 1]. This was because no fungi were observed from the top layer of the epithelium to the deeper layers and even in the connective stroma. There were 33 positive cases, although the positive results [Figure 2], determined through this technique, were questionable as mentioned above. Although we were confident about what we observed at the superficial epithelial layers in few cases, we excluded them from the study as our study was intended to investigate the deeper layers. On the other hand, we used the immediate section of the specimen for the fluorescence technique. However, we found 74 positive [Figure 3] and 26 negative cases [Figure 4] and detection of fungi was done very easily and quickly. In this study, some cases were positive in the PAS technique, but negative in the fluorescence technique.
Figure 1.
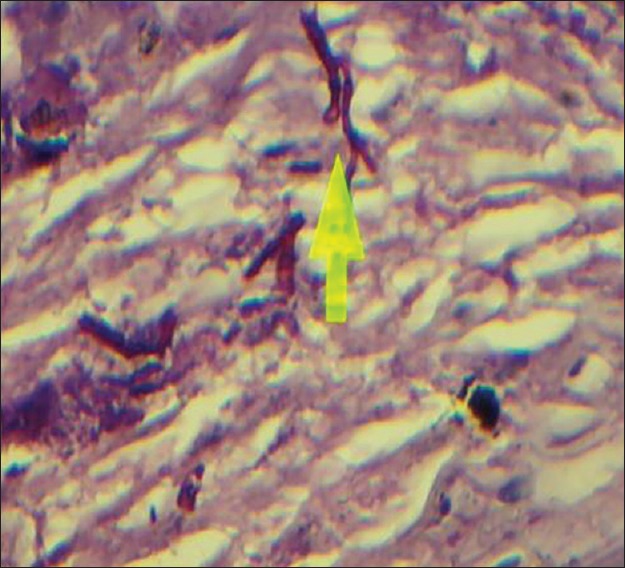
Positive (periodic acid-Schiff technique) magnification ×400
Figure 2.
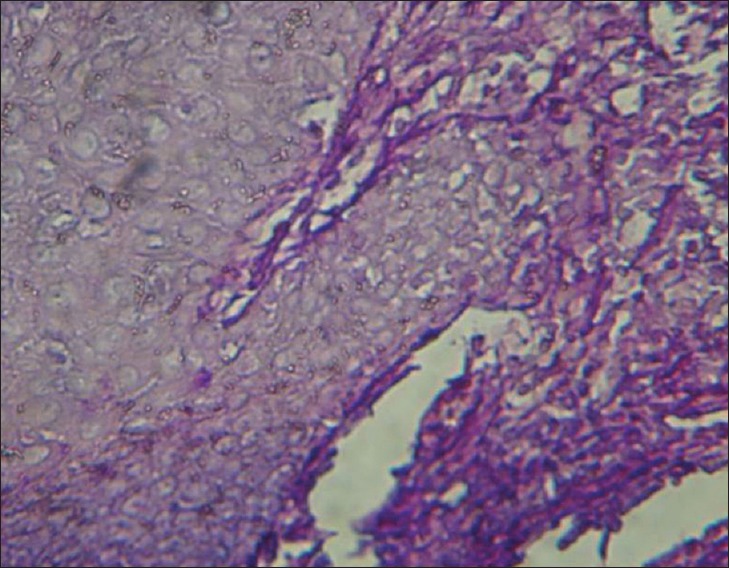
Negative (periodic acid-Schiff technique) magnification ×400
Figure 3.
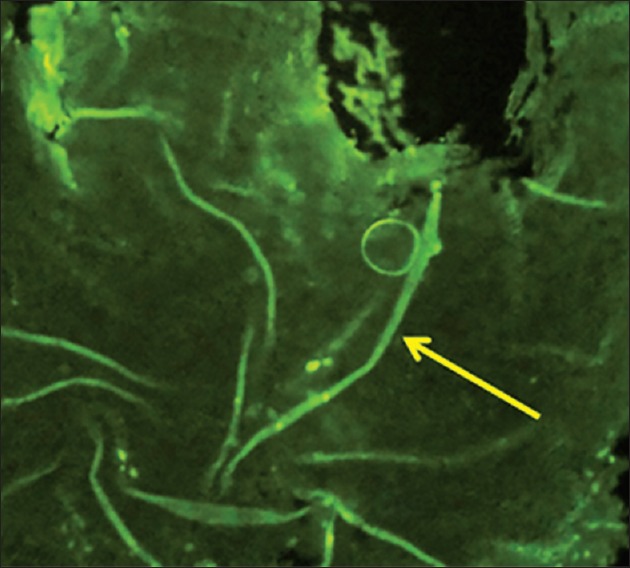
Positive (fluorescence technique) magnification ×400
Figure 4.
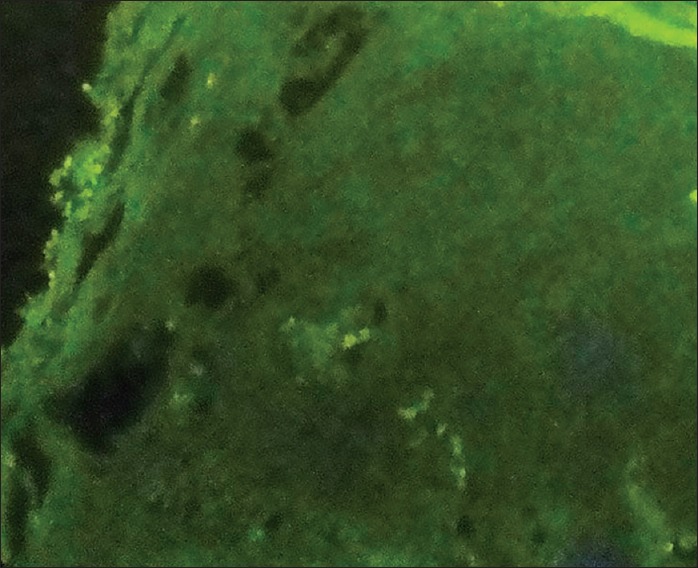
Negative (fluorescence technique) magnification ×400
In the fluorescence procedure, C. albicans appeared apple green in the black background of the microscopic field[25] and hence the florescence technique was very easy and more accurate to identify the C. albicans in OSCC. The PAS technique confirmed only 25 out of the 74 positive cases of the fluorescence technique as positive. Therefore, the sensitivity of this method in the identification of fungi was 33.8%. Furthermore, out of the 26 negative cases in the fluorescence technique, 18 turned out as negative in PAS method. Thus, the specificity of this technique to identify the Candida was 69.2%.
On the whole, the accuracy and negative results of the PAS technique were less than those of the fluorescence staining technique. These two methods had a major difference, so that one could confidently argue that the results of the PAS and fluorescence techniques were significantly different (P < 0.001).
Another limitation in this study was that the samples were not totally serially sectioned. This was mostly because it was not known which samples were obtained from the initial biopsy and which ones from the surgical removal of tumor.
O’Grady and Reade[23] have reported high amounts of Candida in OSCC, which is similar to the results obtained in the present study. However, they used a different method which was based on the evaluation of the patients’ response to anti-fungal treatment. Lipperheide et al.[26] reported that the amount of Candida presence was 82% in leukoplakia, 37% in lichen plan and 33.33% in all the samples, indicating they were prone to malignancy. Malic et al.,[27] using laser microscope, found that the amount of Candida presence in epithelium was 80%. Dwivedi et al.[28] used IHC and reported the amount of Candida presence in oral dysplastic lesion similar to what we found (75%). Silverman et al.,[29] using cell culture, found the C. albicans presence of 27% in OSCC. Nagy et al.[30] used the same method and reported 38% for the presence of Candida in OSCC. The reported findings for the presence of Candida in OSCC in both studies were lower than those of our study, which could be due to the lower accuracy of cell culture compared with the fluorescence technique. Mohd Bakri et al.,[31] using agar technique, reported 6.7% for the presence of Candida. Since agar technique did not provide a specific media for Candida growth and had less sensitivity compared with fluorescence method, it might not be a reliable technique.[31]
Regarding the results of this study and considerable amounts of Candida found in OSCC, future studies are suggested to focus on the primary or secondary role of Candida in OSCC. Furthermore, it should be noted that the high cost of fluorescence technique may be a limiting factor.
Finally, studies with a larger research population are recommended to be conducted to shed more light on this area of research, taking advantage of sensitive methods like PCR.
CONCLUSION
Since the fluorescence technique did not have the limitations of the histochemical method (PAS) and had a higher accuracy in identifying Candida, our results revealed more presence and probably a more important role for Candida as an etiologic risk factor for OSCC. Thus, future studies are recommended to analyze the role of fungi as a primary cause.
ACKNOWLEGMENTS
The authors would like to thank the Pathology Department of Alzahra Hospital, Isfahan University of Medical Sciences for their kind support. This paper was taken from a postdoctoral thesis submitted to the School of Dentistry, Isfahan University of Medical Sciences in partial fulfillment of the requirements for the M.S. degree (Project code 391155).
Footnotes
Source of Support: Isfahan University of Medical Sciences.
Conflict of Interest: None declared.
REFERENCES
- 1.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: Increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103:1843–9. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 2.Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya KA. Squamous cell carcinoma of the oral cavity in patients aged 45 years and under: A descriptive analysis of 116 cases diagnosed in the South East of England from 1990 to 1997. Oral Oncol. 2003;39:106–14. doi: 10.1016/s1368-8375(02)00026-x. [DOI] [PubMed] [Google Scholar]
- 3.Jahanshahi G, Sabaghian M. Comparative immunohistochemical analysis of angiogenesis and mast cell density in oral normal mucosa and squamous cell carcinoma. Dent Res J (Isfahan) 2012;9:8–12. doi: 10.4103/1735-3327.92920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razavi M, Sajadi S. Epidemiological study of oral and perioral cancers in Isfahan. Dent Res J (Isfahan) 2007;4:18–25. [Google Scholar]
- 5.Neville BW. 3rd ed. Philadelpha: Saunders/Elsevier; 2009. Oral and Maxillofacial Pathology; pp. 409–21. [Google Scholar]
- 6.Claveau I, Mostefaoui Y, Rouabhia M. Basement membrane protein and matrix metalloproteinase deregulation in engineered human oral mucosa following infection with Candida albicans. Matrix Biol. 2004;23:477–86. doi: 10.1016/j.matbio.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R. Oral candidiasis - A review. Sch J Med. 2012;2:26–30. [Google Scholar]
- 8.Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5:366–71. doi: 10.1016/s1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 9.Yang YL. Virulence factors of Candida species. J Microbiol Immunol Infect. 2003;36:223–8. [PubMed] [Google Scholar]
- 10.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–35. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 11.Pendrak ML, Yan SS, Roberts DD. Hemoglobin regulates expression of an activator of mating-type locus alpha genes in Candida albicans. Eukaryot Cell. 2004;3:764–75. doi: 10.1128/EC.3.3.764-775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korting HC, Schaller M, Eder G, Hamm G, Böhmer U, Hube B. Effects of the human immunodeficiency virus (HIV) proteinase inhibitors saquinavir and indinavir on in vitro activities of secreted aspartyl proteinases of Candida albicans isolates from HIV-infected patients. Antimicrob Agents Chemother. 1999;43:2038–42. doi: 10.1128/aac.43.8.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monod M, Capoccia S, Léchenne B, Zaugg C, Holdom M, Jousson O. Secreted proteases from pathogenic fungi. Int J Med Microbiol. 2002;292:405–19. doi: 10.1078/1438-4221-00223. [DOI] [PubMed] [Google Scholar]
- 14.Silva S, Hooper SJ, Henriques M, Oliveira R, Azeredo J, Williams DW. The role of secreted aspartyl proteinases in Candida tropicalis invasion and damage of oral mucosa. Int J Med Microbiol. 2002;292:405–19. [Google Scholar]
- 15.Bahadori M. 1st ed. Ch. 29, 17. Tehran University Medical Science publication; 1990. Histopathological Techniques; p. 387. 244. [Google Scholar]
- 16.Chaffin WL, López-Ribot JL, Casanova M, Gozalbo D, Martínez JP. Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiol Mol Biol Rev. 1998;62:130–80. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington BJ, Hageage JG. Calcoflur white: A review of its uses and applications in clinical mycology and parasitology. Lab Med J. 2003;34:361–7. [Google Scholar]
- 18.Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006;2:e129. doi: 10.1371/journal.ppat.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murciano C, Moyes DL, Runglall M, Tobouti P, Islam A, Hoyer LL, et al. Evaluation of the role of Candida albicans agglutinin-like sequence (ALS) proteins in human oral epithelial cell interactions. PLoS One. 2012;7:e33362. doi: 10.1371/journal.pone.0033362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown V, Sexton JA, Johnston M. A glucose sensor in Candida albicans. Eukaryot Cell. 2006;5:1726–37. doi: 10.1128/EC.00186-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Järvensivu A, Hietanen J, Rautemaa R, Sorsa T, Richardson M. Candida yeasts in chronic periodontitis tissues and subgingival microbial biofilms in vivo. Oral Dis. 2004;10:106–12. doi: 10.1046/j.1354-523x.2003.00978.x. [DOI] [PubMed] [Google Scholar]
- 22.Razavi M. 1st ed. Ch. 7. Iran: Isfahan University Medical Science publication; Isfahan; 2007. Essentials of Oral Pathology; p. 17. 58. [Google Scholar]
- 23.O’Grady JF, Reade PC. Candida albicans as a promoter of oral mucosal neoplasia. Carcinogenesis. 1992;13:783–6. doi: 10.1093/carcin/13.5.783. [DOI] [PubMed] [Google Scholar]
- 24.Shadzi SH. 1st ed. Ch. 1, 3. Tehran, Iran: Tehran University Medical Science publication; 2001. Medical Mycology; pp. 11–20. 44-55. [Google Scholar]
- 25.Harrington BJ, Hageage GJ. Calcofluor white: Tips for improving its use. Clin Microbiol Newsl J. 1991;13:3–5. [Google Scholar]
- 26.Lipperheide V, Quindós G, Jiménez Y, Pontón J, Bagán-Sebastián JV, Aguirre JM. Candida biotypes in patients with oral leukoplakia and lichen planus. Candida biotypes in leukoplakia and lichen planus. Mycopathologia. 1996;134:75–82. doi: 10.1007/BF00436868. [DOI] [PubMed] [Google Scholar]
- 27.Malic S, Hill KE, Ralphs JR, Hayes A, Thomas DW, Potts AJ, et al. Characterization of Candida albicans infection of an in vitro oral epithelial model using confocal laser scanning microscopy. Oral Microbiol Immunol. 2007;22:188–94. doi: 10.1111/j.1399-302X.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 28.Dwivedi PP, Mallya S, Dongari-Bagtzoglou A. A novel immunocompetent murine model for Candida albicans-promoted oral epithelial dysplasia. Med Mycol. 2009;47:157–67. doi: 10.1080/13693780802165797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman S, Jr, Luangjarmekorn L, Greenspan D. Occurrence of oral Candida in irradiated head and neck cancer patients. J Oral Med. 1984;39:194–6. [PubMed] [Google Scholar]
- 30.Nagy KN, Sonkodi I, Szöke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34:304–8. [PubMed] [Google Scholar]
- 31.Mohd Bakri M, Mohdw Bakri H, Rachelw Bakri A, Davidw Bakri R, Mary Rich A. Revisiting the association between candidal infection and carcinoma, particularly oral squamous cell carcinoma. J Oral Microbiol. 2010:2. doi: 10.3402/jom.v2i0.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]


