Abstract
Background:
The objective of this study was to evaluate the antiinflammatory, antiinfective and clinical properties of amniotic membrane (AM) when used for guided tissue regeneration (GTR) in contained interdental defects.
Materials and Methods:
A total of 30 subjects participated in this study. Two sites in each subject were randomly assigned into each of the following experimental groups; test group: AM with bone graft and control group: Bone graft only. Clinical parameters included recording site-specific measures of plaque, gingivitis, probing pocket depth (PPD), and clinical attachment loss (CAL). The levels of interleukin-1β (IL-1β) and human beta-defensin-2 (hBD-2) levels in gingival crevicular fluid (GCF) from the test and control sites were measured by using commercially available enzyme linked immunosorbent assay kits. The evaluation of bone fill was performed by using digital subtraction technique and morphometric area analysis. One-way analysis of variance followed by the post-hoc test was used for intragroup and intergroup comparison. A P < 0.05 was considered as statistically significant.
Results:
Combination therapy using an AM increased bone fill and reduced PPD and CAL when compared to controls. AM also resulted in a significant reduction of GCF IL-1β levels and insignificant increase in the hBD-2 levels.
Conclusion:
From this trial conducted over a period of 24 weeks, AM demonstrated a marked antiinflammatory effect and its use resulted in an improvement in periodontal parameters. AM has the potential to function as a barrier for GTR and the unique properties associated with this material can augment its potential as a matrix for periodontal regeneration.
Keywords: Amnion, guided periodontal tissue regeneration, periodontal bone loss, periodontitis
INTRODUCTION
Wound healing studies seem to indicate that bone defects resulting from periodontitis heal by the formation of collagenous scar tissue and is accompanied by the apical migration of gingival epithelium on the root surface.[1,2] This healing process does not fully restore either the form or function of the lost structures and hence does not constitute regeneration.[2] The necessity of exclusion of epithelial and connective tissue cells of the gingiva from the wound led to development and application of guided tissue regeneration (GTR) membranes.[3,4] The clinical application of amniotic membrane (AM) for GTR, while fulfilling the current goals of GTR (cell exclusion, space maintenance, tissue integration, and ease of use) is also in line with the modern concept of biomechanical GTR.[5] AM strongly resembles the oral mucosal basement membrane and possesses several types of laminins, which can promote regeneration, accelerate tissue adhesion, and preserve tissues, all of which play a key role in improved healing of periodontal lesions and might result in reduction in probing pocket depth (PPD) and decrease in clinical attachment loss (CAL).[6] It also contains growth factors that may aid in neovascularization.[7] While working on the principles of GTR, AM is a rich source of stem cells and exhibits antiinflammatory and antimicrobial effects thus enhancing healing through reduction of postoperative scarring.[6,7]
AM demonstrates a potent antimicrobial effect due to the production of human beta-defensins (hBD)[8] and by forming an early physiologic “seal” with the host tissue thus acting as a physical barrier against the external environment.[8] Defensins play an important role in antibacterial activity and tissue proliferation, and the production of these antimicrobial peptides by the AM may promote periodontal regeneration.[9] AM exerts potent antiinflammatory actions as well; using AM is known to inhibit pro-inflammatory cytokine production including interleukin-1β (IL-1β).[10] It is conceivable that this effect, at least in part, may contribute toward periodontal regeneration as IL-1β and other pro-inflammatory cytokines may counteract various mechanisms contributing towards periodontal regeneration.[11] Good biocompatibility,[12,13] ideal mechanical properties[14] such as permeability, stability, elasticity, flexibility, plasticity, and resorbability,[15,16] makes it a promising barrier material in GTR.
The objective of this study was to evaluate the antiinflammatory, antiinfective and clinical properties of AM when used for GTR in contained interdental defects. Primary outcome measures were the antiinflammatory and antiinfective properties of AM based on the changes in inflammatory and antimicrobial peptide biomarkers. Improvements in the measures of periodontal disease as measured clinically and radiographically were the secondary outcomes.
MATERIALS AND METHODS
Sample size calculation
As the trial's primary outcome measure was a difference in levels of enzyme linked immunosorbent assay (ELISA)-dependent biomarkers, sample size was calculated and validated as follows: Proportional power calculation was used to determine the sample size and according to the analysis, a minimum of 26 subjects were needed to detect a sensitivity of 0.90 using the gingival crevicular fluid (GCF) IL-1β/hBD-2 kits when the power of the test is 0.80 at a significance level of 0.05. Once the data were obtained from both the tests from 26 subjects, the exact binomial test estimated the sensitivity of the test to be 0.892 and 0.919 at 95% confidence interval for the IL-1β and hBD-2 kits, respectively.
Source of data
A total of 30 subjects (16 males; mean age: 40.27 ± 9.66) were selected from the outpatient section of the Department of Periodontology, SVS Institute of Dental Sciences from March 2012 to February 2013. Systemically healthy chronic periodontitis patients within the age group of 30-55 years having at least two periodontal pockets ≥5 mm with at least one pocket in each quadrant showing radiographic evidence of vertical bone loss were included in the study. Assessment of suitability for GTR membrane placement was confirmed by transgingival probing to verify the presence of two well-contained interdental bone defects in as many quadrants. Approval from the Institutional Review Board (no: SVS031101) was obtained and the study is listed on http://www.clinicaltrials.gov (NCT02033226). Smokers, medically compromised patients, pregnant women and lactating mothers and subjects receiving periodontal therapy in the 24 weeks period leading to the study were not included. All the included subjects received an initial treatment of SRP and occlusal therapy if required. Sites selected for surgery in these subjects had to demonstrate an absence of bleeding on probing on the day of surgery.
Study design including randomization and blinding
The study was designed as a split-mouth, double-blind, and randomized controlled clinical trial. Randomization and blinding included computerized generation of the allocation sequence in random permuted blocks (Saghei's block randomization) and blinded disbursement of medication. Allocation was performed by assigning the block of sites to study groups according to the specified sequence. Based on the sequence, the first operator (AN) selected two sites for each of the following experimental sites; the test site, in which AM with the graft was placed and the control site, which was treated by graft placement only. All the surgeries were performed by a designated operator (AK) for the sake of uniformity, whereas the relevant readings were recorded by a set of calibrated operators (AAR, BHR and CR) who were blinded to the nature of the site. The blind was not broken until this clinical trial was completely finished. A total number of 30 subjects were recruited, in which, three subjects were lost during follow-up, limiting the statistical analysis to 27 subjects and 54 teeth (27 sites each in the test and control group).
Study protocol
After the prospective interdental areas were probed buccally and lingually/palatally, the site was considered for the study if the average PPD was ≥5 mm. All baseline values were recorded before the surgical procedure. GCF samples for IL-1β were taken at baseline and 1 week and for hBD-2 were taken at baseline, 1 week and 4 weeks. Noninvasive site-specific measures of plaque index (PI) and modified gingival index (MGI) at baseline, 1, 12, and 24 weeks were recorded. PPD and CAL were recorded at baseline and at the end of 12 and 24 weeks using a UNC-15 color-coded periodontal probe. A custom acrylic stent limited to the occlusal 2/3rd of the clinical crown was prepared from a cast obtained from an alginate impression. A groove was prepared in the stent to standardize the probing angulation throughout the procedure and also during the follow-up visits.
Radiograph standardization
Standard digital radiographs were taken at baseline, 12 and 24 weeks by the paralleling/long-cone technique at preset parameters using a commercially available RVG system (Kodak RVG 5100® Digital Radiography System, Carestream Health, Rochester, USA). After the imaging plate was placed in the film holder for paralleling technique (XCP Kits for Digital Sensors®, BlueDent, Chennai, India), addition silicon impression material (Elite HD + Regular Body Normal Set®, Zhermack, Badia Polesine, Italy) was added around the biting surface and allowed to set. This arrangement ensured standardized alignment of the aiming device and the holder ensuring correct positioning of the collimator in subsequent radiographs.
Collection of gingival crevicular fluid samples
After isolation, 6 mm diameter filter paper circles (Medium flow filter paper, Whatman®, Mumbai, India) were used for collecting samples by the intra-crevicular method. The fluid seeping out of the sulcus was collected and any paper contaminated with blood or saliva was discarded, and collection was repeated after 30 min. GCF samples were eluted from the strips by placing them in Eppendorf tubes that contained 500 μL of buffer (Trizma®, Sigma-Aldrich, Hyderabad, India) and stored at −80°C.
Enzyme linked immunosorbent assay for interleukin-1β and human beta-defensin-2
Commercially available ELISA kits were used to quantify GCF levels of IL-1β (Bender MedSystems GmbH, Vienna, Austria) and hBD-2 (BD-2 ELISA Kit®, Life Technologies, New Delhi, India). All assay procedures were conducted according to the manufacturer's instructions and in both the tests, 100 μl of the samples or standards were added into the respective wells in duplicate. Color development was stopped using 1% H2SO4 and absorbance of the entire plate was measured within 30 min by an ELISA reader (Multiskan®, Vapi, Gujarat, India) at 450 nm. The amounts of IL-1β and hBD-2 were calculated based on the dilutions, and the results were expressed as pg/30 s and pg/site in the 30 s GCF sample.
Amniotic membrane
Lyophilized and irradiated AM (3 × 3 cm) was obtained from a commercial tissue bank (Tata Memorial Hospital-Tissue Bank, Mumbai, India). The tissue was dispatched for clinical use from the tissue bank. The tissue conforms to the International Atomic Energy Agency recommendations and the Asia Pacific Association of Surgical Tissue Banks standards. AM is stable and can be stored at room temperature.
Surgical phase
All surgical procedures were performed by one clinician. After recording relevant parameters, and following routine local anesthesia, sulcular incisions were given, and a full thickness Kirkland flap was elevated for defect access. Thorough degranulation of the bone defect was done and root planning was done by using appropriate site-specific curettes.
The test site was treated as follows; after placement and proper condensation of natural Hydroxyapatite (HA) graft (G-graft®, Surgiwear, Shahjahanpur, India) in the defect, hydrated AM was cut based on defect anatomy of surgical site and then adapted over the bone graft and alveolar bone extending from the base of the flap reflection to the tooth surface. Flap was approximated and stabilized by placing direct loop sutures. Periodontal dressing was placed subsequently. For the control site, the procedure was identical except that the membrane was not placed. Patients received postoperative instructions and were prescribed medication. The silk sutures were removed at 1 week postsurgery and subsequent measures were recorded. All the patients received a uniform course of antibiotics and analgesics during the interim 1 week period leading to suture removal.
Radiographic assessment
A single operator (RVC) evaluated the bone fill by using digital subtraction technique and morphometric area analysis by using specific tools in two image processing software.
Digital subtraction technique
The radiographs obtained at 12 and 24 weeks were subtracted from the radiograph taken at the baseline by using commercially available image processing software (Adobe Photoshop® 6.0, Adobe Systems, San Jose, USA). To reduce brightness and contrast variations, both images were adjusted based on the levels and curves in the software. Before digital subtraction, both radiographs were moved in appropriate directions as needed, to reduce geometric distortion. These images were then superimposed and subtracted by selecting the image > calculation > exclusion > new channel tools. The excluded interdental layer was outlined by using the polygonal lasso tool and the layer was copied and saved as a separate joint photographic expert group document at low compression [Figure 1].
Figure 1.

Pre (a) and post treatment (b) radiographs were superimposed (c) and subtracted (d) in Adobe Photoshop®
Morphometric area analysis
After digital subtraction, the digitized and excluded interdental layer was transferred to open source software for area calculation (ImageJ®, Research Services Branch, NIH, Bethesda, Maryland, USA) for area calculation. The layer was converted into a grayscale image, and the measurement scale was set to account for any magnification/reduction of the radiograph because of RVG. The area of the layer was calculated (in mm2) by initially enclosing the entire area with the rectangular selection tool and then by using Analyze > Analyze Particles tool [Figure 2].
Figure 2.

Morphometric area analysis was performed after digital subtraction by selecting (a), isolating (b) and transferring the excluded interdental layer to ImageJ. The area of the layer (c and d) was calculated (in mm2) after converting the layer into a grayscale image (e)
Statistical analysis
An analysis based upon knowledge of the expected outcomes in periodontitis patients was used to assess the treatment effect. A site-specific intragroup comparison of PPD, CAL, bone fill, PI, MGI, and levels of IL-1β and hBD-2 between various groups was performed using repeated measures analysis of variance (ANOVA) followed by multiple comparisons using Bonferroni correction. One-way ANOVA followed by the post-hoc test was used for intragroup and intergroup comparison. A P < 0.05 was considered statistically significant. Data were analyzed using a commercially available software program (SPSS Statistics 17.0, SPSS Inc., Chicago, IL).
RESULTS
A total of 30 subjects (mean age: 40.27 ± 9.66) were included in the initial phase of the study. Of the 30, three subjects were excluded due to sampling errors, thus limiting the final sample size to 27 subjects. No untoward side-effects such as membrane exposure and flap dehiscence were observed in any of the cases.
Intragroup comparisons
Primary outcomes
The mean levels of IL-1β (pg/30 s) at baseline and at the end of 1 week were 136.88 ± 40.7 and 102.19 ± 48.9 in the control group and 136.84 ± 42.27 and 89.34 ± 38.86 in the test group, respectively. This reduction in IL-1β levels at 1 week, when compared with baseline was statistically significant (P < 0.05) in the control group, whereas it was highly significant (P < 0.001) in the test group [Figure 3]. hBD-2 levels (pg/site) at baseline and at the end of 1 week and 4 weeks in the control group were 69.76 ± 10.2, 64.5 ± 18.93 and 66.5 ± 13.09 and were 70.03 ± 12.6, 67.15 ± 12.9 and 69.76 ± 15.89 in the test group, respectively. These minimal changes, when compared with baseline were not significant in both the treatment groups [Figure 4].
Figure 3.
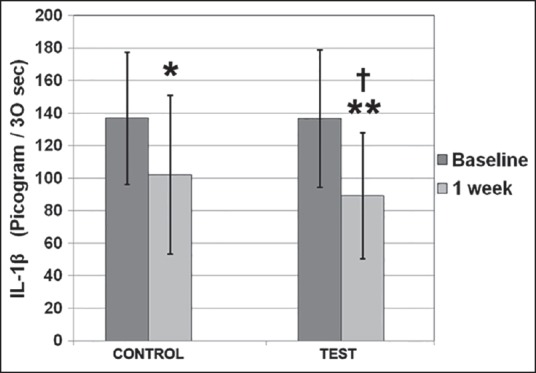
This reduction in interleukin-1β (IL-1β) levels at 1 week, when compared with baseline was statistically significant* in the control group, where as it was highly significant** in the test group. Intergroup comparison at different time based intervals showed highly significant changes in levels of IL-1β at 1 week when test site was compared to control site†
Figure 4.
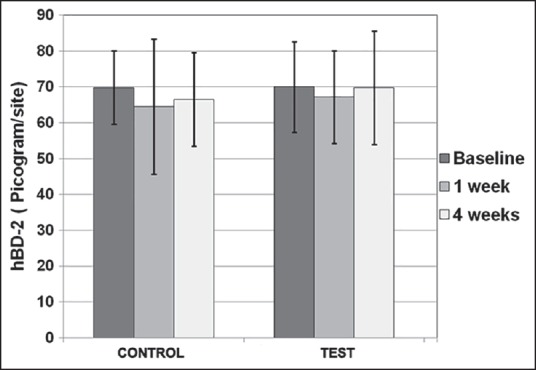
No significant intra-or inter-group differences were observed for human beta-defensin-2 at different time intervals
Secondary outcomes
The mean PPDs (in mm) in the control group were 7.26 ± 1.18, 5.19 ± 1.09, 5.11 ± 0.90 and in the test group were 7.57 ± 1.33, 4.34 ± 0.74, 3.73 ± 0.91 at baseline, at the end of 12 and 24 weeks, respectively. The intragroup reduction in pocket depth, when compared from baseline to 12 and 24 weeks was statistically highly significant in both the treatment groups (P < 0.001). The mean CAL (in mm) in the control group was 6.80 ± 1.05, 5.00 ± 1.16, 5.07 ± 1.05 and in the test group was 6.88 ± 1.2, 3.69 ± 0.88, 3.19 ± 1.13 at baseline, at the end of 12 and 24 weeks, respectively. This intragroup reduction in CAL when compared from baseline to end of 12 and 24 weeks was statistically highly significant in both the treatment groups (P < 0.001).
The change in mean bone fill (in mm2) when compared from baseline to 12 weeks and 24 weeks in the control group was 12.61 ± 4.2 and 13.9 ± 3.8 and in the test group was 11.92 ± 4.6 and 15.2 ± 4.7, respectively. This intragroup gain in bone fill when compared from baseline to end of 12 weeks and from baseline to 24 weeks was statistically highly significant in both the treatment groups (P < 0.001).
The mean site-specific PI scores in the control group was 0.49 ± 0.25, 1.02 ± 0.60, 0.79 ± 0.30, 1.08 ± 0.38 and in the test group was 0.45 ± 0.28, 0.94 ± 0.56, 1.00 ± 0.46, 1.11 ± 0.45 at baseline and at the end of 1, 12 and 24 weeks, respectively. This increase in plaque scores when compared from baseline to end of 1, 12, and 24 weeks was statistically highly significant (P < 0.001) in both the treatment groups. The mean site-specific MGI scores in the control group was 0.29 ± 0.19, 0.68 ± 0.48, 0.79 ± 0.30, 1.08 ± 0.38 and in the test group was 0.24 ± 0.20, 0.53 ± 0.29, 1.00 ± 0.47, 1.11 ± 0.46 at the baseline, 1, 12, and 24 weeks. The increase in the gingival index (GI) scores when compared from baseline to the end of 1, 12, and 24 weeks was statistically highly significant (P < 0.001) in both the study groups.
Pair-wise comparisons
Primary outcomes
The mean difference in levels of hBD-2 at the control site were 5.192, −1.923, 3.269 and at the test site were 2.885, −2615, 0.269 between baseline to 1 week, 1-4 weeks and from baseline to 4 weeks, respectively. These mean differences between any of the two groups, at different time intervals were statistically not significant.
Secondary outcomes
The mean difference in PPD at the control site was 2.07, 0.07, and 2.15 and at the test site was 3.2, 0.615, and 3.8 between baseline to 12 weeks, 12-24 weeks, and from baseline to 24 weeks, respectively. These mean differences between baseline to 12 weeks, baseline to 24 weeks were statistically highly significant (P ≤ 0.001) in both study groups, whereas it was not significant between 12 and 24 weeks. The mean difference in CAL at the control site was 1.80, −0.077, and 1.73 and at the test site was 3.11, 0.5, 3.61 between baseline to 12 weeks, 12-24 weeks and from baseline to 24 weeks, respectively. These mean differences between baseline to 12 weeks, baseline to 24 weeks were statistically highly significant (P ≤ 0.001) in both study groups, whereas it was not significant between 12 and 24 weeks.
The mean difference in PI at the control site was –0.53, 0.23, –0.29, –0.59 and at the test site was –0.49, –0.05, –0.11, –0.66 between baseline to 1 week, 1-12 weeks, 12-24 weeks and from baseline to 24 weeks, respectively. The mean difference between baseline to 1 week for the both the study groups was statistically significant (P ≤ 0.05), whereas, this difference from baseline to 24 weeks is statistically highly significant (P ≤ 0.001) for both groups. This mean difference was not significant between other time intervals. The mean difference in MGI scores at the control site was –0.39, –0.11, –0.29, –0.79 and at the test site was –0.29, –0.47, –0.11, –0.87 between baseline to 1 week, 1-12 weeks, 12-24 weeks, and from baseline to 24 weeks, respectively. The mean difference between baseline to 1 week and from 1 week to 12 weeks was statistically significant (P ≤ 0.05) in both the treatment groups. Between 12 weeks and 24 weeks, only the control group showed statistically significant (P ≤ 0.05) difference. The mean difference between the baseline and 24 weeks was statistically highly significant (P ≤ 0.001) in both treatment groups.
Intergroup comparisons
Intergroup comparison at different time based intervals using ANOVA showed highly significant (P ≤ 0.001) changes in levels of IL-1β at 1 week when test site was compared to control site [Figure 3]. This difference was nonsignificant at the baseline. No significant differences were observed among the two groups for PI, MGI, and hBD-2 at different time intervals [Figure 4 and Table 1].
Table 1.
Intergroup comparison of various parameters at different time based intervals using ANOVA

Significant reduction in PPD at the test site was observed at 12 weeks, whereas this reduction was highly significant at 24 weeks (P ≤ 0.001) when compared to the control site. The test group showed a highly significant reduction in the CAL both at the 12 and 24 weeks (P ≤ 0.001). The significant gain in bone fill was observed at test group from baseline to 24 weeks (P ≤ 0.001) [Table 1].
DISCUSSION
Indications of AM usage continue to expand and encompass varying range of procedures even within periodontics itself. There are three representative forms of AM available; fresh, cryopreserved and dried AM. Studies seem to indicate that freeze dried-air preserved AMs show no qualitative changes in growth factor and cell contents even after 5 years of storage as compared to the other two types.[17,18,19] In any form, the membrane is easy to handle as an oral-dressing material and adheres well to the bare connective and osseous tissue.[20,21,22] Mechanical properties such as permeability, stability, elasticity, flexibility, plasticity, and resorbability,[14,15] makes it a promising barrier material in GTR.
AM demonstrates a direct suppressive effect on IL-1β[23] and has an upregulatory effect on the expression of hBD-2.[14,24] The significant reduction of GCF IL-1β levels in sites treated with AM seems to indicate that AM has a significant anti-inflammatory effect on periodontal tissues. However, this reduction in IL-1β did not show a considerable impact on gingival inflammation as there was no difference in the GI scores between the AM and the control group. This can have important clinical implications as results of flap surgery are largely dependent on the reduction of inflammation caused by periodontal disease.[25,26,27] During infections, AMs represent a mechanical and immunological barrier against infection by secreting defensins.[14,24] Increased hBD-2 may play an important role in defense from periodontopathogens in human gingival tissues.[28] In this study, there was a minimal insignificant increase in the hBD-2 levels in sites treated with AM. IL-1β results in an up regulation of hBD-2[29] expression and the significant reduction in the IL-1β levels must have resulted in the relatively modest rise in the hBD-2 levels. The PI scores between the two groups was not significant which is in agreement with the observation of Tehrani et al.[30] and others[31,32] who stated that antibacterial property is a sum function of the amniotic cells and the other components of extracellular matrix such as defensins, elafin, and secretory leukocyte proteinase inhibitor. To what extent the reduction of hBD-2 expression affects the overall antimicrobial properties of AM properties must be further investigated.
AM showed a marked impact on periodontal clinical parameters including PPD, CAL and bone fill as compared to the control site which is an agreement with the previous studies supporting the use of chorioamniotic membranes in periodontal pocket therapy.[33,34,35] This can be attributed to various factors. It's thin, self-adherent nature does not compromise blood flow and AM does not displace from underneath the flap thus oblivating the use of sutures or tacks.[36] Collectively, AM's unique biological and physical attributes reduce the complexity in trimming, suturing, and placement of barriers effectively minimizing the chances of postoperative complications.[36] AM also contributes to periodontal regeneration through the presence of intense concentrations of growth factors[15,16,37] and laminins,[6,7] a strong anti-adhesive property that induces an arrest in tissue proliferation,[17,38] its lack of immunogenicity[39] and through its ability to decrease the host immunologic response via mechanisms such as localized suppression of polymorphonuclear cell migration.[10] In this study, bone fill was greatest for the AM group; this correlates to previous studies demonstrating new bone formation.[16,37] The bone inductive potential of AM is primarily due to the existence of mesenchymal progenitor cells which demonstrate osteogenic and adipogenic differentiation.[40] At the same time, AM shows excellent acceptability with bone grafts by demonstrating excellent containment of the material and resorbs without the formation of voids and detritus.[41] The role of some unknown mechanism in inducing bone formation cannot be discounted as well.[40,41]
However, this study has some limitations. Limited literature exists on the resorption dynamics of AM, although general literature seems to suggest that AM completely resorbs into the wound in about 2-4 weeks.[17] The resorption rate is important as GTR membranes must function for a period of 4-6 weeks to achieve ideal periodontal regeneration.[42] Thus, we had to limit the observation period for IL-1β and hBD-2 to a maximum period of 4 weeks to account for changes before the anticipated resorption of AM. However, one study has stated that AM may induce rapid epithelialization and acceptable collagen formation in as early as 10 days, suggesting that AM transplantation may promote rapid gingival wound healing.[43] While radiographic findings at 12 and 24 weeks demonstrated significant bone gain with AM, the mechanism can be further explored and studied at a histologic level to better explain the findings. In addition, image subtraction was done in commercially available image analysis software by using a modification of a method described by Carvalho et al.[44] The tool, while exhibiting good sensitivity to bone fill,[44] is not specific for digital subtraction and can be affected by the competency of the evaluator especially during the demarcation of the excluded bone fill slice. This study compared a GTR versus non-GTR intervention and no attempt was made to compare AM with commonly used GTR materials such as collagen membranes.[45] As an example, the data for collagen is very contradictory with some studies indicating that collagen placement either elicits a pro-inflammatory reaction[46] or exhibits a strong anti-inflammatory activity.[47] As comparable GTR material could not be utilized, HA bone graft was placed as it may result in improved clinical outcomes compared with those achieved with flap-debridement alone.[48] At the same time, AM is very thin and may collapse into the defect when placed in a site with nonsupportive anatomy; this necessitated using a dense graft such as HA.
CONCLUSION
To conclude, AM demonstrated a marked anti-inflammatory effect and its use resulted in an improvement in periodontal parameters. Newer GTR materials are expected to display biological and biomechanical properties vastly superior and complementary to “conventional” GTR materials. AM has the potential to function as a barrier for GTR and the unique properties associated with this material can augment its potential as a matrix for periodontal regeneration.
ACKNOWLEDGMENTS
The authors would like to thank Dr. R. V. Venkat Satish for the ELISA analysis of IL-1β and hBD-2 and J Rahul for his help on the use of image processing software. This study received no external support and was funded by the authors’ institutions. The authors declare that there are no conflicts of interest in this study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Novak MJ. Classification of diseases and conditions affecting the periodontium. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Clinical Periodontology. 10th ed. Philadelphia: Saunders; 2007. pp. 100–9. [Google Scholar]
- 2.Caton J, Nyman S, Zander H. Histometric evaluation of periodontal surgery. II. Connective tissue attachment levels after four regenerative procedures. J Clin Periodontol. 1980;7:224–31. doi: 10.1111/j.1600-051x.1980.tb01965.x. [DOI] [PubMed] [Google Scholar]
- 3.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–60. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 4.Aurer A, Jorgie-Srdjak K. Membranes for periodontal regeneration. Acta Stomatol Croatol. 2005;39:107–12. [Google Scholar]
- 5.Lafzi A, Farahani RM, Shoja MM, Tubbs RS. Amniotic membrane: A potential candidate for periodontal guided tissue regeneration? Med Hypotheses. 2007;69:454. doi: 10.1016/j.mehy.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Ghahroudi AA, Khorsand A, Rokn AR, Sabounchi SS, Shayesteh YS, Soolari A. Comparison of amnion allograft with connective tissue graft for root coverage procedures: A double-blind, randomized, controlled clinical trial. J Int Acad Periodontol. 2013;15:101–12. [PubMed] [Google Scholar]
- 7.Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–7. [PubMed] [Google Scholar]
- 8.King AE, Paltoo A, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta. 2007;28:161–9. doi: 10.1016/j.placenta.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Watanabe H, Ogita M, Ichinose S, Izumi Y. Effect of human beta-defensin-3 on the proliferation of fibroblasts on periodontally involved root surfaces. Peptides. 2011;32:888–94. doi: 10.1016/j.peptides.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19:348–52. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Nokhbehsaim M, Deschner B, Winter J, Bourauel C, Rath B, Jäger A, et al. Interactions of regenerative, inflammatory and biomechanical signals on bone morphogenetic protein-2 in periodontal ligament cells. J Periodontal Res. 2011;46:374–81. doi: 10.1111/j.1600-0765.2011.01357.x. [DOI] [PubMed] [Google Scholar]
- 12.Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42:1539–46. [PubMed] [Google Scholar]
- 13.Hammer A, Hutter H, Blaschitz A, Mahnert W, Hartmann M, Uchanska-Ziegler B, et al. Amnion epithelial cells, in contrast to trophoblast cells, express all classical HLA class I molecules together with HLA-G. Am J Reprod Immunol. 1997;37:161–71. doi: 10.1111/j.1600-0897.1997.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 14.Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 15.Samandari MH, Yaghmaei M, Ejlali M, Moshref M, Saffar AS. Use of amnion as a graft material in vestibuloplasty: A preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:574–8. doi: 10.1016/S107921040400006X. [DOI] [PubMed] [Google Scholar]
- 16.Samandari MH. Human amniotic membrane, best healing accelerator, and the choice of bone induction for vestibuloplasty technique (an animal study) Transplant Res Risk Manage. 2011;3:1–8. [Google Scholar]
- 17.Kesting MR, Loeffelbein DJ, Classen M, Slotta-Huspenina J, Hasler RJ, Jacobsen F, et al. Repair of oronasal fistulas with human amniotic membrane in minipigs. Br J Oral Maxillofac Surg. 2010;48:131–5. doi: 10.1016/j.bjoms.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Chacharkar MP. Dried gamma-irradiated amniotic membrane as dressing in burn wound care. J Tissue Viability. 2011;20:49–54. doi: 10.1016/j.jtv.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Chopra A, Thomas BS. Amniotic membrane: A novel material for regeneration and repair. J Biomim Biomater Tissue Eng. 2013;18:1–8. [Google Scholar]
- 20.Kim SS, Song CK, Shon SK, Lee KY, Kim CH, Lee MJ, et al. Effects of human amniotic membrane grafts combined with marrow mesenchymal stem cells on healing of full-thickness skin defects in rabbits. Cell Tissue Res. 2009;336:59–66. doi: 10.1007/s00441-009-0766-1. [DOI] [PubMed] [Google Scholar]
- 21.Sharma Y, Maria A, Kaur P. Effectiveness of human amnion as a graft material in lower anterior ridge vestibuloplasty: A clinical study. J Maxillofac Oral Surg. 2011;10:283–7. doi: 10.1007/s12663-011-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai N, Tsuno H, Okabe M, Yoshida T, Koike C, Noguchi M, et al. Clinical application of a hyperdry amniotic membrane on surgical defects of the oral mucosa. J Oral Maxillofac Surg. 2012;70:2221–8. doi: 10.1016/j.joms.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 23.Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol. 2001;85:444–9. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King AE, Critchley HO, Sallenave JM, Kelly RW. Elafin in human endometrium: An antiprotease and antimicrobial molecule expressed during menstruation. J Clin Endocrinol Metab. 2003;88:4426–31. doi: 10.1210/jc.2003-030239. [DOI] [PubMed] [Google Scholar]
- 25.Wang HL, Greenwell H, Fiorellini J, Giannobile W, Offenbacher S, Salkin L, et al. Periodontal regeneration. J Periodontol. 2005;76:1601–22. doi: 10.1902/jop.2005.76.9.1601. [DOI] [PubMed] [Google Scholar]
- 26.Villar CC, Cochran DL. Regeneration of periodontal tissues: Guided tissue regeneration. Dent Clin North Am. 2010;54:73–92. doi: 10.1016/j.cden.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Tonetti MS, Prato GP, Cortellini P. Factors affecting the healing response of intrabony defects following guided tissue regeneration and access flap surgery. J Clin Periodontol. 1996;23:548–56. doi: 10.1111/j.1600-051x.1996.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 28.To M, Kamata Y, Saruta J, Shimizu T, Sato T, Kondo Y, et al. Induction of β-defensin expression by porphyromonas gingivalis-infected human gingival graft transplanted in nu/nu mouse subdermis. Acta Histochem Cytochem. 2013;46:25–34. doi: 10.1267/ahc.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Du X, Chen J, Hu L, Chen L. The induction expression of human β-defensins in gingival epithelial cells and fibroblasts. Arch Oral Biol. 2013;58:1415–21. doi: 10.1016/j.archoralbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Tehrani FA, Ahmadiani A, Niknejad H. The effects of preservation procedures on antibacterial property of amniotic membrane. Cryobiology. 2013;67:293–8. doi: 10.1016/j.cryobiol.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Kanyshkova TG, Buneva VN, Nevinsky GA. Lactoferrin and its biological functions. Biochemistry (Mosc) 2001;66:1–7. doi: 10.1023/a:1002817226110. [DOI] [PubMed] [Google Scholar]
- 32.Gomes MF, dos Anjos MJ, Nogueira TO, Guimarães SA. Histologic evaluation of the osteoinductive property of autogenous demineralized dentin matrix on surgical bone defects in rabbit skulls using human amniotic membrane for guided bone regeneration. Int J Oral Maxillofac Implants. 2001;16:563–71. [PubMed] [Google Scholar]
- 33.Kothiwale SV, Anuroopa P, Gajiwala AL. A clinical and radiological evaluation of DFDBA with amniotic membrane versus bovine derived xenograft with amniotic membrane in human periodontal grade II furcation defects. Cell Tissue Bank. 2009;10:317–26. doi: 10.1007/s10561-009-9126-3. [DOI] [PubMed] [Google Scholar]
- 34.Velez I, Parker WB, Siegel MA, Hernandez M. Cryopreserved amniotic membrane for modulation of periodontal soft tissue healing: A pilot study. J Periodontol. 2010;81:1797–804. doi: 10.1902/jop.2010.100060. [DOI] [PubMed] [Google Scholar]
- 35.Kothiwale SV. The evaluation of chorionic membrane in guided tissue regeneration for periodontal pocket therapy: A clinical and radiographic study. Cell Tissue Bank. 2014;15:145–52. doi: 10.1007/s10561-013-9386-9. [DOI] [PubMed] [Google Scholar]
- 36.Holtzclaw DJ, Toscano NJ. Amnion-Chorion allograft barrier used for guided tissue regeneration treatment of periodontal intrabony defects: A retrospective observational report. Clin Adv Periodontics. 2013;3:131–7. [Google Scholar]
- 37.Najafabadi MH, Farahani SH, Kheirollahi H. Histological evaluation of human amniotic membrane graft on oral keratinizing mucosa in the rabbit model (a pilot study) J Dent Med. 2007;20:144–49. [Google Scholar]
- 38.Bauer F, Hingsammer LM, Wolff KD, Kesting MR. Case report temporomandibular joint arthroplasty with human amniotic membrane: A case report. Eplasty. 2013;13:e17. [PMC free article] [PubMed] [Google Scholar]
- 39.Colocho G, Graham WP, 3rd, Greene AE, Matheson DW, Lynch D. Human amniotic membrane as a physiologic wound dressing. Arch Surg. 1974;109:370–3. doi: 10.1001/archsurg.1974.01360030022006. [DOI] [PubMed] [Google Scholar]
- 40.In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–45. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 41.Gomes JA, Romano A, Santos MS, Dua HS. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol. 2005;16:233–40. doi: 10.1097/01.icu.0000172827.31985.3a. [DOI] [PubMed] [Google Scholar]
- 42.Tonetti MS, Cortellini P, Suvan JE, Adriaens P, Baldi C, Dubravec D, et al. Generalizability of the added benefits of guided tissue regeneration in the treatment of deep intrabony defects. Evaluation in a multi-center randomized controlled clinical trial. J Periodontol. 1998;69:1183–92. doi: 10.1902/jop.1998.69.11.1183. [DOI] [PubMed] [Google Scholar]
- 43.Rinastiti M, Harijadi, Santoso AL, Sosroseno W. Histological evaluation of rabbit gingival wound healing transplanted with human amniotic membrane. Int J Oral Maxillofac Surg. 2006;35:247–51. doi: 10.1016/j.ijom.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho FB, Gonçalves M, Tanomaru-Filho M. Evaluation of chronic periapical lesions by digital subtraction radiography by using Adobe Photoshop CS: A technical report. J Endod. 2007;33:493–7. doi: 10.1016/j.joen.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Crigger M, Bogle GC, Garrett S, Gantes BG. Repair following treatment of circumferential periodontal defects in dogs with collagen and expanded polytetrafluoroethylene barrier membranes. J Periodontol. 1996;67:403–13. doi: 10.1902/jop.1996.67.4.403. [DOI] [PubMed] [Google Scholar]
- 46.Stone PJ, Korn JH, North H, Lally EV, Miller LC, Tucker LB, et al. Cross-linked elastin and collagen degradation products in the urine of patients with scleroderma. Arthritis Rheum. 1995;38:517–24. doi: 10.1002/art.1780380409. [DOI] [PubMed] [Google Scholar]
- 47.Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727–30. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 48.Rosen PS, Reynolds MA, Bowers GM. The treatment of intrabony defects with bone grafts. Periodontol 2000. 2000;22:88–103. doi: 10.1034/j.1600-0757.2000.2220107.x. [DOI] [PubMed] [Google Scholar]


