Abstract
Background:
Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) is applied for remineralization of early caries lesions or tooth sensitivity conditions and may affect subsequent resin bonding. This in vitro study investigated the effect of CPP-ACP on the shear bond strength of dental adhesives to enamel.
Materials and Methods:
Sixty extracted human molar teeth were selected and randomly divided into three groups and six subgroups. Buccal or lingual surfaces of teeth were prepared to create a flat enamel surface. Adhesives used were Tetric N-Bond, AdheSE and AdheSE One F. In three subgroups, before applying adhesives, enamel surfaces were treated with Tooth Mousse CPP-ACP for one hour, rinsed and stored in 37°C temperature with 100% humidity. This procedure was repeated for 5 days and then adhesives were applied and Tetric N-Ceram composite was adhered to the enamel. This procedure was also fulfilled for the other three subgroups without CPP-ACP treatment. After 24 hour water storage, samples were tested for shear bond strength test in a universal testing machine. Failure modes were determined by stereomicroscope. Data were analyzed by t-test and one-way analysis of variance with P < 0.05 as the level of significance.
Results:
In comparison between applied and non-applied CPP-ACP subgroups, there was no significant decrease in the shear bond strength to enamel only in Tetric N-Bond (P > 0.05). In non-applied CPP-ACP subgroups, there were statistically significant differences among all subgroups. Tetric N-Bond had the highest and AdheSE One F had the lowest shear bond strength.
Conclusion:
CPP-ACP application reduces the shear bond strength of AdheSE and AdheSE One F to enamel but not Tetric N-Bond.
Keywords: Casein phosphopeptide-amorphous calcium phosphate, enamel, etch-and-rinse, self-etch, shear bond strength
INTRODUCTION
Prevention of dental caries formation or arresting white spot lesions is one of the important goals in operative dentistry. This elaborate prevention procedure depends on remineralization of enamel and dentin. Remineralization can occur in patients with nonacidic salivary pH, enough amounts of minerals especially calcium and fluoride in saliva content and a very good oral hygiene.[1] Recently, casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) has been introduced.[2] CPP-ACP has a therapeutic effect on dental caries, white spot lesions, hypomineralized or hypocalcified enamel, mild fluorosis, root hypersensitivity, erosion, hypersensitivity after vital bleaching[3] and also preventing demineralization around brackets and other orthodontic appliances due to its remineralization effects.[4] CPP-ACP is a bioactive substance based on milk products, composed of two parts: CPP and ACP. CPP is obtained from milk's Casein protein and can stabilize calcium and phosphate in “nanocluster” forms of ions in solution, resulting in increased level of calcium and phosphate in dental plaque.[5] Furthermore, calcium and phosphate enter enamel prisms and restore apatite crystals. Also, buffering characteristic of CPP can prevent demineralization of tooth structures. ACP, a biological active substance, releases calcium and phosphate and prepares a supersaturated level of them, decreases the demineralization and increases remineralization. Calcium and phosphate ions disperse through porous lesion easily and precipitate on partially demineralized enamel prisms and reform apatite crystals. Higher concentration of CPP-ACP may lead to a better remineralization.[6]
CPP-ACP properties such as inhibition of demineralization, increasing remineralization and its bacteriostatic/bactericidal effect are similar to fluoride. It has also a fluoride-free type which is safe to use in children younger than 2-year-old whom may swallow it during application. CPP-ACP is more efficient than fluoride-containing toothpastes in neutralizing bacterial acid attack and also it has a stronger remineralization effect comparing to fluoride alone. Problems such as fluorosis and poisoning which occur in over dosage of fluoride will not happen in CPP-ACP usage.[7,8] CPP-ACP can protect enamel surface from erosion and increases its wear resistance and helps better clinical management in tooth wear situations.[9] It can bind to pellicle, dental plaque and soft-tissues as well.[10] After CPP-ACP treatment, placement of composite on the enamel surfaces may become necessary and therefore evaluation of bonding effectiveness is important to gain a successful bonding phenomenon.
The aim of this study was to evaluate the influence of CPP-ACP on the shear bond strength of etch-and-rinse and self-etch adhesives to enamel. The null hypothesis was that there was no significant difference in shear bond strength of composite to enamel with CPP-ACP application.
MATERIALS AND METHODS
This was an in vitro experimental study including sixty freshly extracted human third molars without any cracks, decay or any other defects. After removing of remnant connective tissue and storing in a 0.5% chloramine-T solution (Fisher Chemical, Fair lawn, NJ, USA) for 24 h, the teeth were washed with saline solution and stored in distilled water at the room temperature during the study time.
A diamond fissure bur (SS White, Great White Series, Lakewood, NJ, USA) was used to freshen the buccal or lingual enamel surfaces of teeth (under air-water coolant spray) in order to make a flat surface. This flatness was checked by a surveyor's perpendicular rod. The prepared surfaces had to have full contact with the rod. The teeth were mounted up to the level of cement-enamel junction in chemically-cured acryl (Acropars, Marlic Co., Tehran, Iran) and the vertical position of flattened surfaces (perpendicular to horizon) was confirmed by using surveyor's rod.
In the next step, the specimens were randomly divided into three main groups based on bonding agents. Then, each of these three main groups were divided into two subgroups: Case and control. To mark the field of bonding area on each specimen, paper sticks with a punched hole, 3 mm-in-diameter, were attached to the prepared surfaces. In three case subgroups, small amounts of Tooth Mousse CPP-ACP (CP) (GC Corp., Tokyo, Japan) paste were placed using an oral spatula on the marked areas for one hour. Then specimens were rinsed with distilled water for 20 s and placed in an incubator (Incubator 6530, Behdad, Tehran, Iran) at the temperature of 37°C and humidity of 100%. This procedure was repeated for 5 consecutive days exactly in the same manner. The next step was applying bonding agents [Tetric N-Bond (TB), AdheSE (Ad) and AdheSE One F (Ad One F)] and placing composite materials in all six subgroups. The materials were used according to manufacturers’ instructions [Table 1].
Table 1.
Materials used in this study
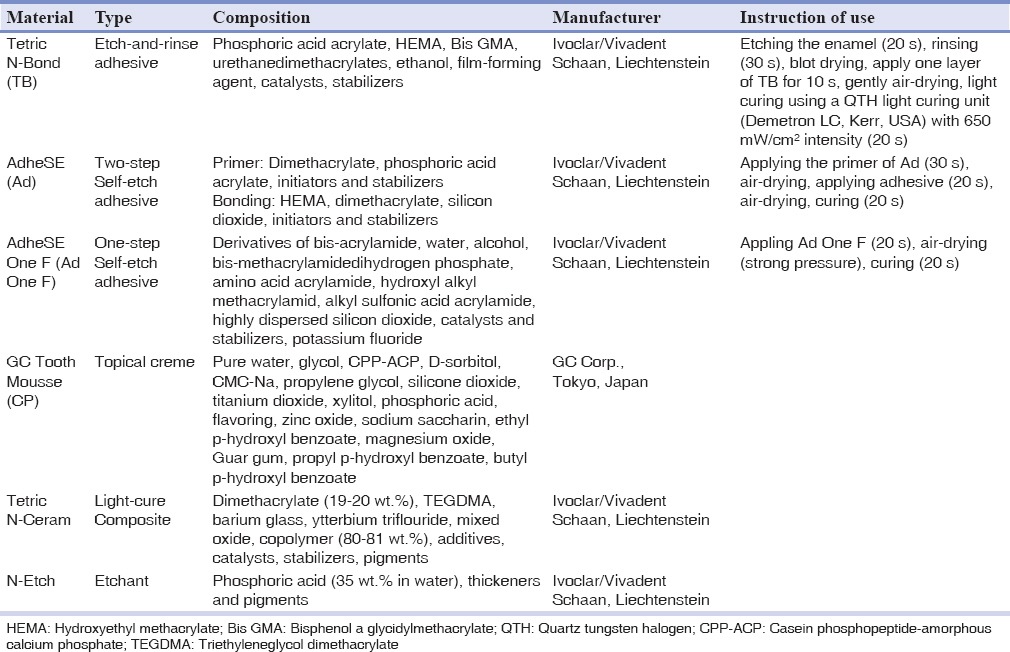
The light curing unit was being monitored periodically by a radiometer (Optilux, SdS, Kerr, USA). After bonding process, a clear plastic cylindrical mold (internal diameter of 2 mm and height of 3 mm) was used to attach Tetric-N Ceram composite (Ivoclar/Vivadent, Schaan, Liechtenstein) on the enamel surface in two increments and each layer was cured for 20 s from top of the mold. After removing the mold, the composite was cured for additional 40 s.
After storing all specimens in 37°C distilled water for 24 h, shear bond strength was measured by universal testing machine (Testometric M350-10 CT, England) with a crosshead speed of 0.5 mm/min and load cell of 50 kgF, using a steel parallel blade (1 mm in diameter) on the composite-enamel interface. The computer measured shear loading for each specimen and showed the results in a force deflection diagram.
The force required to dislodge the composite cylinder was recorded in Newton and converted to mega Pascal (MPa) with this equation:
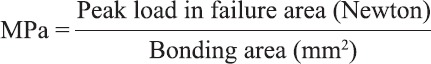
Mode of failure in each specimen was observed by two examiners under a stereomicroscope (Olympus, DP 12, Germany) at ×40 magnification and classified to four classes: Adhesive failure (90-100% of the bonded interface failed), cohesive failure in enamel (90-100% of the failure was in enamel), cohesive failure in composite (90-100% of the failure was in composite) and mixed failure (partially adhesive and partially cohesive).[11]
Data analysis was performed by one-way analysis of variance (t-test) at the P < 0.05 level of significance using SPSS version 13.0 (SPSS, Chicago, IL, USA).
RESULTS
The mean and standard deviation values of the shear bond strength measurements for all subgroups are summarized in Table 2.
Table 2.
Shear bond strength in MPa (mean ± SD) for each tested subgroup
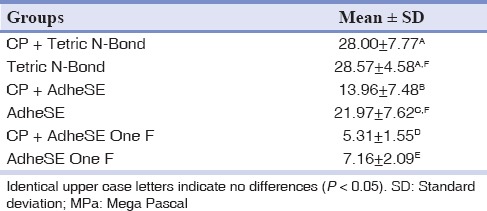
In Ad and Ad One F groups, there were statistically significant decreasing in the shear bond strength to enamel of case subgroups in comparison with their control subgroups (Ad: P = 0.029, Ad One F: P = 0.037) but not in TB group (P = 0.845).
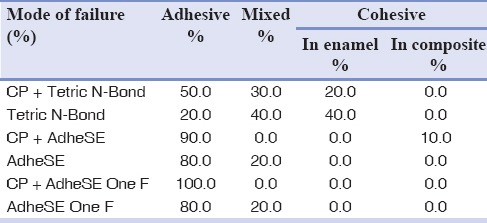
Among case subgroups, there were statistically significant differences among all. CP + TB had the highest and CP + Ad One F had the lowest shear bond strength. Modes of failures are presented in Table 3.
Table 3.
Mean percentage of failure mode after shear bond strength test
DISCUSSION
In local application of fluoride ions, deficiency of calcium and phosphate ions can be a limiting factor for remineralization, which is worse in xerostomic conditions.[12] Calcium and phosphate ions have low stability, especially in the presence of fluoride, so their clinical usage is not successful.[3] CPP-ACP is a safe material to use in the oral care products, professional dental products and also in nutrition.[13] CPP-ACP causes an increase in the level of calcium and phosphate ions in supra gingival plaque by bonding its CPP part to saliva pellicle and Streptococcus mutans bacteria surface. Its existence in plaque biofilm can lead to subsurface enamel lesions’ remineralization, and thus they would be more resistant to acid attacks in the future.[12,14]
The results of this study showed that only the shear bond strength of CP + TB subgroup has no significant difference compared with TB subgroup (P = 0.845). Phosphoric acid has a strong etching effect and has the ability to remove calcium and phosphate from the enamel surface and hence it will increase available bonding area which leads to increased adhesion. In addition, phosphoric acid can improve adhesive's wet ability by increasing the enamel surface energy and will create a stronger adhesion. Rinsing the etching agent makes the enamel surface free from any contaminant, thus improves the bonding quality. Etching with phosphoric acid can remove a thin layer of surface enamel and provides small porosities.[15] Several studies have showed that CPP-ACP has no effect on the shear bond strength of etch-and-rinse adhesives,[16,17] which are in agreement with this study.
In Adebayo et al. study, it was concluded that CPP-ACP application could not reduce the microshear bond strength of one etch-and-rinse adhesive, Single Bond, to enamel.[15] According to Tabrizi and Cakirer study, CPP-ACP could not affect shear bond strength of orthodontic brackets to enamel with application of etch-and-rise adhesives.[18] CPP-ACP can compensate the reduction of shear bond strength of demineralized enamel to orthodontic brackets with etch-and-rinse adhesives and increase it to the level of non-demineralized enamel.[19] Khoroushi and Keshani study demonstrated that CPP-ACP application reduces the shear bond strength of etch-and-rinse adhesive to bleached enamel.[20]
CPP-ACP can produce a hypermineralized enamel surface by depositioning calcium and phosphate, thus the weak acidity of these self-etch adhesives (Ad pH = 1.6,[21] Ad One F pH = 1.4[22]) cannot remove enriched surface layer and adhesive penetration is adversely affected. Furthermore, even after complete rinsing, some amount of CPP-ACP paste might be trapped in enamel surface porosities (residual layer), which primer and adhesive cannot penetrate through it, so it will decrease the impregnation of primer and consequently resin and resin tag formation.[15] This phenomenon can explain the more adhesive failures in case subgroups than their control subgroups [Table 3].
Moule et al. observed that CPP-ACP reduces the bond strength of self-etch adhesive to enamel and the treated teeth with CPP-ACP are more resistant to acid because of the enamel resistance to the etching effect of that self-etch adhesive and some CPP-ACP paste remain on the enamel surface acting as a contaminant, which can interfere with bonding to enamel.[16] Based on Cehreli et al. study, CPP-ACP application did not disturb the shear bond strength of the self-etch adhesive, Transbond Plus, to orthodontic bracket.[23] Furthermore, one study showed that CPP-ACP application does not significantly affect the shear bond strength of etch-and-rinse and self-etch adhesives. SEM analysis showed that etching patterns of groups treated with CPP-ACP is similar to non-treated groups.[24]
The acidity of self-etch adhesives does not necessarily cause higher bond strength to enamel.[25] The results of the present study showed that Ad with a “mild” acidity caused a higher bond strength compared to Ad One F with a “moderate” pH.
All-in-one adhesive systems possess highly hydrophilic acidic polymers and therefore, even after being light cured, they act as a permeable membrane during water storage.[25] Water presence in Ad composition beside hydroxyethyl methacrylate facilitates penetration to fresh enamel; however, it can induce earlier degradation after thermocycling.[26] On the other hand, TB possesses ethanol and separate etching and bonding steps increases its technique sensitivity.[27] These reasons can explain the results in control subgroups.
If an adhesive system has higher bond strength, usually the mode of failure will be cohesive rather than adhesive.[21] This matter is consistent with the results of this study, i.e., the most cohesive failures were observed in etch-and-rinse groups with higher bond strength and they had the lowest rate of adhesive failure. The most adhesive failures occurred in CP + Ad One F subgroup which had the lowest bond strength.
CONCLUSION
CPP-ACP treatment did not show any significant decrease of the shear bond strength in Tetric N-Bond to the enamel, but significantly reduced the shear bond strength of AdheSE and AdheSE One F to the enamel. Finally, by applying CPP-ACP, shear bond strength was significantly decreased in the following order: Tetric N-Bond, AdheSE and AdheSE One F. SEM studies are recommended to obtain more information about the presented results.
ACKNOWLEDGMENTS
This study was resulted from a student thesis which was supported by Kerman University of Medical Sciences. The authors thank Kerman University of Medical Sciences for financial support and Dr. Jahani for statistical analysis.
Footnotes
Source of Support: The authors thank Kerman University of Medical Sciences for financial support and Dr. Jahani for statistical analysis.
Conflict of Interest: None declared.
REFERENCES
- 1.Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: Role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1) J Clin Pediatr Dent. 2003;28:47–52. doi: 10.17796/jcpd.28.1.yg6m443046k50u20. [DOI] [PubMed] [Google Scholar]
- 2.Jayarajan J, Janardhanam P, Jayakumar P, Deepika Efficacy of CPP-ACP and CPP-ACPF on enamel remineralization —Chemistry and clinical applications. An in vitro study using scanning electron microscope and DIAGNOdent. Indian J Dent Res. 2011;22:77–82. doi: 10.4103/0970-9290.80001. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds EC. Casein phosphopeptide-amorphous calcium phosphate: The scientific evidence. Adv Dent Res. 2009;21:25–9. doi: 10.1177/0895937409335619. [DOI] [PubMed] [Google Scholar]
- 4.Xiaojun D, Jing L, Xuehua G, Hong R, Youcheng Y, Zhangyu G, et al. Effects of CPP-ACP paste on the shear bond strength of orthodontic brackets. Angle Orthod. 2009;79:945–50. doi: 10.2319/101108-573.1. [DOI] [PubMed] [Google Scholar]
- 5.Cochrane NJ, Saranathan S, Cai F, Cross KJ, Reynolds EC. Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res. 2008;42:88–97. doi: 10.1159/000113161. [DOI] [PubMed] [Google Scholar]
- 6.Rahiotis C, Vougiouklakis G. Effect of a CPP-ACP agent on the demineralization and remineralization of dentine in vitro. J Dent. 2007;35:695–8. doi: 10.1016/j.jdent.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Azarpazhooh A, Limeback H. Clinical efficacy of casein derivatives: A systematic review of the literature. J Am Dent Assoc. 2008;139:915–24. doi: 10.14219/jada.archive.2008.0278. [DOI] [PubMed] [Google Scholar]
- 8.Rose RK. Effects of an anticariogenic casein phosphopeptide on calcium diffusion in streptococcal model dental plaques. Arch Oral Biol. 2000;45:569–75. doi: 10.1016/s0003-9969(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 9.Tehrani MH, Ghafournia M, Samimi P, Savabi O, Parisay I, Askari N, et al. Effect of casein phosphopeptide-amorphous calcium phosphate and acidulated phosphate fluoride gel on erosive enamel wear. Dent Res J (Isfahan) 2011;8:S64–70. [PMC free article] [PubMed] [Google Scholar]
- 10.Somasundaram P, Vimala N, Mandke LG. Protective potential of casein phosphopeptide amorphous calcium phosphate containing paste on enamel surfaces. J Conserv Dent. 2013;16:152–6. doi: 10.4103/0972-0707.108199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unlu N, Gunal S, Ulker M, Ozer F, Blatz MB. Influence of operator experience on in vitro bond strength of dentin adhesives. J Adhes Dent. 2012;14:223–7. doi: 10.3290/j.jad.a22191. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV, et al. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2008;87:344–8. doi: 10.1177/154405910808700420. [DOI] [PubMed] [Google Scholar]
- 13.Cross KJ, Huq NL, Reynolds EC. Casein phosphopeptides in oral health – Chemistry and clinical applications. Curr Pharm Des. 2007;13:793–800. doi: 10.2174/138161207780363086. [DOI] [PubMed] [Google Scholar]
- 14.Poggio C, Lombardini M, Dagna A, Chiesa M, Bianchi S. Protective effect on enamel demineralization of a CPP-ACP paste: An AFM in vitro study. J Dent. 2009;37:949–54. doi: 10.1016/j.jdent.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Adebayo OA, Burrow MF, Tyas MJ. Effects of conditioners on microshear bond strength to enamel after carbamide peroxide bleaching and/or casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) treatment. J Dent. 2007;35:862–70. doi: 10.1016/j.jdent.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Moule CA, Angelis F, Kim GH, Le S, Malipatil S, Foo MS, et al. Resin bonding using an all-etch or self-etch adhesive to enamel after carbamide peroxide and/or CPP-ACP treatment. Aust Dent J. 2007;52:133–7. doi: 10.1111/j.1834-7819.2007.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 17.Keçik D, Cehreli SB, Sar C, Unver B. Effect of acidulated phosphate fluoride and casein phosphopeptide-amorphous calcium phosphate application on shear bond strength of orthodontic brackets. Angle Orthod. 2008;78:129–33. doi: 10.2319/122506-529.1. [DOI] [PubMed] [Google Scholar]
- 18.Tabrizi A, Cakirer B. A comparative evaluation of casein phosphopeptide-amorphous calcium phosphate and fluoride on the shear bond strength of orthodontic brackets. Eur J Orthod. 2011;33:282–7. doi: 10.1093/ejo/cjq062. [DOI] [PubMed] [Google Scholar]
- 19.Uysal T, Baysal A, Uysal B, Aydınbelge M, Al-Qunaian T. Do fluoride and casein phosphopeptide-amorphous calcium phosphate affect shear bond strength of orthodontic brackets bonded to a demineralized enamel surface? Angle Orthod. 2011;81:490–5. doi: 10.2319/090510-520.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoroushi M, Keshani F. Effect of CPP-ACP on shear bond strength of composite-resin to bleached enamel. J Islam Dent Assoc Iran (JIDA) 2010;22:42–8. [Google Scholar]
- 21.Britta LC, Martins M, França FM. Influence of different primer application times on bond strength of self-etching adhesive systems to unground enamel. Oper Dent. 2009;34:43–50. doi: 10.2341/08-35. [DOI] [PubMed] [Google Scholar]
- 22.Margvelashvili M, Goracci C, Beloica M, Papacchini F, Ferrari M. In vitro evaluation of bonding effectiveness to dentin of all-in-one adhesives. J Dent. 2010;38:106–12. doi: 10.1016/j.jdent.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Cehreli SB, Sar C, Polat-Özsoy O, Unver B, Ozsoy S. Effects of a fluoride-containing casein phosphopeptide-amorphous calcium phosphate complex on the shear bond strength of orthodontic brackets. Eur J Orthod. 2012;34:193–7. doi: 10.1093/ejo/cjq183. [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Cha JY, Kim KN, Hwang CJ. The effect of casein phosphopeptide amorphous calcium phosphate on the in vitro shear bond strength of orthodontic brackets. Korean J Orthod. 2013;43:23–8. doi: 10.4041/kjod.2013.43.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahveci O, Belli S. Composite bond strength to intact enamel with current simplified adhesives. J Adhes Dent. 2011;13:31–7. doi: 10.3290/j.jad.a18514. [DOI] [PubMed] [Google Scholar]
- 26.Tay FR, Gwinnett AJ, Wei SH. The overwet phenomenon: A transmission electron microscopic study of surface moisture in the acid-conditioned, resin-dentin interface. Am J Dent. 1996;9:161–6. [PubMed] [Google Scholar]
- 27.Osorio E, Toledano M, Aguilera FS, Tay FR, Osorio R. Ethanol wet-bonding technique sensitivity assessed by AFM. J Dent Res. 2010;89:1264–9. doi: 10.1177/0022034510376403. [DOI] [PMC free article] [PubMed] [Google Scholar]


