Abstract
Background:
Hypertension in pregnancy is one of the prevalent disorder resulting in maternal death. The aim of this study was to investigate the effect of stretching exercise and walking on changes of blood pressure in nulliparous women during pregnancy.
Materials and Methods:
This was a quasi-experimental trial that consisted three groups of women who took part in pre- and post-tests. We used a simple randomized sample, including 118 pregnant females (walking: 29 subjects, stretching exercise: 30 subjects control: 59 subjects). The data were collected using the demographic checklist and blood pressure was measured every week. SPSS 16 was used to analyze the data by one-way analysis of variance (ANOVA) and repeated measure ANOVA.
Results:
No significant difference was found in the demographic characteristics of the three groups of women. Mean systolic and diastolic blood pressure in the three groups (stretching exercises, walking, and routine care) at three intervals (pre-test, first post-test, and second post-test) were significantly different (P < 0.05). In this case, Tukey's test showed significant improvement of systolic and diastolic blood pressure in stretching exercise group. Walking and control groups showed no change or significant reduction (P < 0.05). No significant difference was found between the walking and control groups (P > 0.05).
Conclusions:
The results of the study showed that stretching exercise versus walking reduces systolic and diastolic blood pressure in the second trimester of pregnancy and controls it in the third trimester of pregnancy. In contrast, walking has no effect on blood pressure during pregnancy.
Keywords: Blood pressure, stretching exercise, walking
INTRODUCTION
Pregnancy is often a stressful period with great biochemical, physiological, and anatomical changes in the body. Sometimes, these physiological changes cause pathological conditions, and problems and diseases for the pregnant mother. Hypertensive disorder is one of the most common complications during pregnancy and it occurs in 5–10% of pregnancies.[1] Based on the latest classification system, these disorders are divided into five classes of gestational hypertension, preeclampsia, eclampsia, chronic hypertension, and superimposed preeclampsia.[2]
Gestational hypertension and preeclampsia can occur with pregnancy. Only definitive treatment is termination of the pregnancy.[3] Small numbers of risk factors for hypertensive disorders during pregnancy are known. Recent studies have suggested that height body mass index and weight gain during pregnancy are important risk factors for hypertensive disorders in pregnancy. Studies have shown that the risk of these disorders increases with obesity, being overweight before pregnancy, and excessive weight gain during pregnancy.[4,5] These conditions are associated with increased risk of preterm delivery, neonatal intensive care unit (NICU) admission, and fetal death. Moreover, these conditions form the second leading cause of death for pregnant women.[6] It was also shown that 19% of maternal deaths after childbirth and 20% of deaths after stillbirth are due to these disorders.[7]
High blood pressure during pregnancy can cause serious problems during childbirth, such as stroke, premature birth, or low birth weight.[8] In addition, gestational hypertension and preeclampsia are the risk factors for future metabolic syndrome, insulin resistance, and cardiovascular diseases.[9] Antihypertensive agents that seem to have no effect on pregnancy outcome are used for gestational hypertension treatment. However, it must be borne in mind that these studies were limited to the use of these drugs during pregnancy. On the other hand, these treatments are used after mid-pregnancy, when the potential risks of congenital anomalies have passed. However, complications such as fetal intrauterine growth restriction, fetal and neonatal bradycardia, and rare cases of thrombocytopenia have been reported due to the use of antihypertensive medications.[10] Today, due to the limitations of therapies, much attention has been paid to the prevention of hypertensive disorders in pregnancy.[11] Recently, a large number of women, according to the recommendations of the American Board of Obstetrics and Gynecologists and other organizations, engage in exercise during pregnancy.[12] Evidence shows that exercise during pregnancy, even for women who have been sedentary before pregnancy, has beneficial effects.[13] Exercise is one of the most effective ways to reduce the adverse effects of pregnancy, such as excessive weight gain[14,15] and hypertension.[16] Most pregnant women choose walking as their exercise because it is easy to implement,[17] but stretching exercise as physical activity could be done easier than walking; therefore, women adherence to this activity.[18] Stretching exercises increase muscle flexibility that was measured in a previous study and could reduce the most common complaints such as back pain after childbirth.[19]
Some studies on the relationship between physical activity and hypertensive disorders in pregnancy have shown that physical activity and exercise before or during pregnancy can reduce the risk of hypertensive disorders and preeclampsia.[20,21,22] However, Heggard et al.[23] and Vollebregt et al.[24] have not reported any relationship between these factors in their studies. In addition, the study results of Østerdal et al. showed that high-intensity physical activity in the first trimester of pregnancy may increase the risk of preeclampsia.[25] In total, there are discrepancies between the present studies, and most of the studies are cohort and case–control studies. To investigate the effect of physical activity on the prevention of high blood pressure during pregnancy, further studies are needed with better quality and semi-experimental trial designs. The purpose of the present study was to investigate the effects of walking and stretching exercises on blood pressure during pregnancy.
MATERIALS AND METHODS
The present study was a quasi-experimental study with pre- and post-test design. The participants were randomly selected from two health centers in Isfahan, Iran, from April 2011 until November 2012. 118 healthy pregnant women in their second trimester were selected. Furthermore, they were randomly placed in three groups of walking (n = 29), stretching exercises (n = 30), and routine care (n = 59). A written informed consent was obtained from every participant. This study was approved by the Ethics Committee of Isfahan University of Medical Sciences. The inclusion criteria of subjects in the present study included: First pregnancy, gestational age 18-22 weeks, willingness to participate in the study, a live fetus, women aged between 19 and 35 years, body mass index less than 30 kg/m2, absence of a family history of preeclampsia (mother and sister), no history of stretching exercises before pregnancy, and noprohibition to participating in stretching and walking exercises including movement disabilities, obstetric contraindications (history of threatened abortion, preterm birth risk in the current pregnancy, vaginal bleeding, history of preterm delivery, placenta previa, any problem in the waist or hips, hypertension, cardiovascular disease, incompetent cervix, leaking amniotic fluid, dizziness, headache,), and any kind of problem due to which inactivity is prescribed by the physician. Subjects from two public health centers in Isfahan were interviewed via phone calls, and were updated regarding the exercises and walking after the completion of the questionnaire and consent form. They were placed in three groups of stretching exercises, walking and routine care, based on their own preference.
For the group with stretching exercises, the program lasted 20 weeks; each week consisted of a maximum of five and a minimum of three sessions and each session lasted about 45 minutes. Each session consisted of three parts: Warm-up, stretching (1. back stretch to correct the curvature, 2. unilateral neck stretch, 3. neck rotating, 4. up and down movement of the head, 5. moving the chin upward with stretch, 6. stretching the chest with the hands crossing over the chest, 7. hand and arm stretch, 8. unilateral stretch of the body while seated, 9. child pose, 10. cat pose, 11. hamstring stretch, 12. pelvis tilt, 13. groin stretching, and 14. V stretching of the thighs), and cooling down (including relaxation and breathing exercises). Stretching exercises were copied on a compact disc and were handed to the group with stretching exercises. Pressure and the repetition of each exercise were determined based on individual abilities. Incremental exercise was performed in three stages. Due to lack of space, two training sessions per week were performed at home and one session per week at the health center by exercise rehabilitator. Emergency exercise stopping criteria were: Shortness of breath, palpitations, dizziness, headache, nausea, severe or sudden abdominal pain, chest pain, back pain, pain in pubic area, vaginal bleeding, and reduced fetal movements after training.[26] Due to lack of space in the health centers, the 3-days-a-week exercises were limited to one session training in the health center and a minimum of two and maximum of four sessions at home. The exercises along with the warnings and necessary awareness were provided for the subjects on a compact disc. The exercises were performed at home for 30 to 45 minutes. The participants visited the health centers 1 day a week and their blood pressure was measured before the exercise and 15 min after the exercise.
The group with walking exercise walked slowly for a maximum of 20 weeks, with a minimum of three sessions and a maximum of five sessions in each week, and each session lasted for 30–45 minutes. The pressure of walking was adjusted by a breathing test. The participants were taught to repeat a standard sentence while walking without shortness of breath. The sentence was “my Lord is the greatest, the most merciful, the most knowledgeable, and the most capable.” At the end of each week, the subjects referred to the related relevant health center for blood pressure measurement and monitoring for signs of preeclampsia and possible problems associated with it. The volunteers’ blood pressure was recorded after each weekly session. Moreover, the mean blood pressure was used for comparison of the second trimester and third trimester of pregnancy.
The routine care group was asked to refer to the health center as routine clinical visits for measurement of hypertension and severity of preeclampsia. Prenatal care performed by midwives or physician at health centers consisted of: Pressure control, body temperature, fetal heart rate, health and nutritional counseling and trainings, and routine tests during pregnancy according to the integrated national guidelines for maternal care. The frequency of meetings for low-risk mothers was eight times, during 6–10, 16–20, 26–30, 31–34, 35–37, 38, 39, and 40 weeks of gestation.[27] Since blood pressure had to be measured in the second half of the pregnancy to the end of pregnancy in this study, the researcher measured blood pressure of the control group seven times, and the subjects’ blood pressure was recorded and controlled based on their medical records in health centers. Data were analyzed using SPSS for Windows (version 16; SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) test was used to compare demographic characteristics and fertility. ANOVA with repeated data was used to compare blood pressure in the three groups.
RESULTS
In the present study, 118 patients were selected, of whom 2 in the stretching exercise group, 3 in the walking group, and 5 in the routine care group were excluded during the study. Thus, statistical analysis was performed on 108 subjects. Age, gestational age, body mass index, and systolic and diastolic blood pressure in all three groups showed no significant difference (P > 0.05) [Table 1]. Mean systolic and diastolic blood pressure in all three groups of stretching exercises, walking, and routine care during the three periods (pre-test, post-test I, and post-test II) are shown in Table 2. The results showed that systolic and diastolic blood pressure in the three study groups had a significant difference (P < 0.05). Tukey's test showed that the difference between systolic and diastolic blood pressure of stretching exercises group and both groups of walking (P < 0.05) and routine care (P < 0.05) was significant. Nevertheless, the difference between walking group and routine care (P > 0.05) was not significant. Figure 1 shows the comparison of changes in systolic and diastolic blood pressure of the three groups of stretching exercises, walking, and routine care in pre-test and post-test I and II.
Table 1.
Characteristics of the pregnant women included in the sample
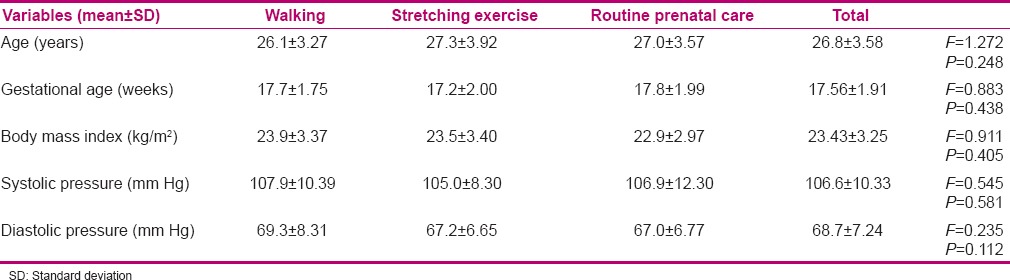
Table 2.
Repeated measure ANOVA results of systolic and diastolic blood pressure
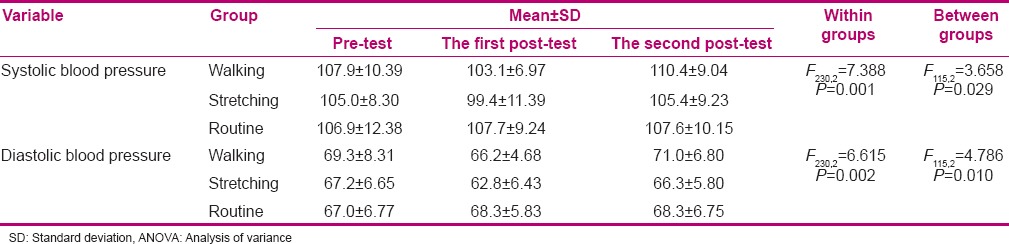
Figure 1.
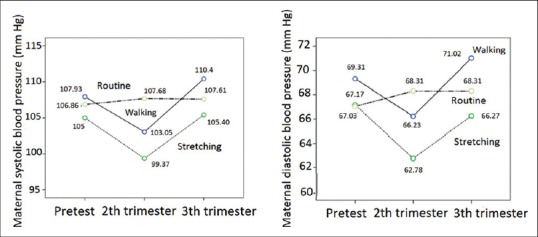
A comparison of the blood pressure changes during pregnancy among the research groups
DISCUSSION
Comparison of demographic characteristics revealed that the groups were similar in terms of age, gestational age, body mass index, and systolic and diastolic blood pressure. Therefore, the sampling method was good, and the results of this study seem reliable. To answer the aim of the present study, which was the comparative effectiveness of stretching exercises and walking on systolic and diastolic blood pressure of pregnant women during pregnancy, the results showed that stretching exercises compared to walking and routine care (no exercise) resulted in better control of systolic and diastolic blood pressure during pregnancy. In other words, it was shown that stretching exercises compared to walking could better decrease systolic and diastolic blood pressure in the second trimester and control their increase in the third trimester. In contrast, it was shown that the curve of systolic and diastolic blood pressure changes in the walking group was similar to the routine care group. Therefore, in this study it was concluded that walking does not affect systolic and diastolic blood pressure.
Pregnancy causes an increase in blood volume, an increase in cardiac output, and a slight increase in stroke volume; but with the increase in blood volume, blood pressure does not change significantly during pregnancy. However, studies have shown that a slight decrease in blood pressure occurs especially in the second trimester of pregnancy.[1] It was also observed in our study that the systolic and diastolic blood pressure had fallen slightly in the second trimester. This might be due to the fact that in addition to the physiological nature of the pregnancy in the second trimester, exercise decreases blood pressure. In contrast, during the third trimester, systolic and diastolic blood pressure in all three groups showed a slight increase. One possible reason for this was that the patients were approaching the time of delivery and, thus, their psychological stress and fear of childbirth increased. However, stretching exercises could control systolic and diastolic blood pressure better than walking in the third trimester of pregnancy. According to previous studies, oxidative stress will possibly increase in this period, which is expected to increase blood pressure. However, studies have shown that stretching reduces oxidative stress, or in other words, produces anti-oxidative activity.[28,29] Based on the present results, it seems that the proposed mechanism for the control of hypertension in the third trimester of pregnancy in the stretching exercises group is that regular stretching exercises is associated with increased endogenous antioxidants.[30]
Sorenson et al. concluded in their study that the increase in energy expenditure during mild to moderate exercise during pregnancy reduces the risk of preeclampsia.[20] The results of other studies show the fact that physical activity before and during pregnancy reduces the risk of preeclampsia and hypertensive disorders of pregnancy.[21,22,31,32,33] Yeo, in his clinical trials, experimented on the effects of walking and stretching exercises on the risk of preeclampsia. Systolic blood pressure had significantly increased in the walking group (P < 0.01), while for the stretching group, this change was not significant. Diastolic blood pressure decreased significantly in the stretching group (P < 0.05), but there was no significant change in the walking group.[30] In another report of Yeo et al., the incidence of preeclampsia in the walking group was 14.6% and in the stretching exercise group was 2.6%. In addition, the mean transferrin levels, as an indicator of antioxidants, were significantly higher in the stretching group compared to the walking group (P < 0.05).[33] This indicates that the stretching exercises had better effect on reducing blood pressure during pregnancy. This can be due to the endogenous antioxidant produced by regular stretching exercises, and was consistent with the current research.
Tyidum et al.,[34] Rudra et al.,[35] Heggard et al.,[23] and Vollebregt et al.[24] showed that physical activity before pregnancy does not lower the risk of preeclampsia or gestational hypertension. This was consistent with the results of the present study in the walking group. The reasons for the inconsistency of the results of these researches with that of the stretching exercises group could be due to not having a single definition for physical activity, different instruments used to measure physical activity, and the study method which was the prospective cohort method.
CONCLUSION
The results of this study have shown that stretching exercise intervention can prevent the risk of hypertensive disorders during pregnancy. Accordingly, it is recommended that pregnant women engage in stretching exercises during pregnancy, and use these exercises more often during this period. Since it is easier to perform stretching exercises than to walk, they can be more committed to these exercises. As mentioned, despite the ease of performing stretching exercises, they have benefits such as controlling hypertension during pregnancy. Therefore, health and fitness professionals should pay more attention to preparation and delivery of a variety of sports programs with different stretching techniques and encourage pregnant women to engage in this kind of exercise.
ACKNOWLEDGEMENT
The researcher greatly appreciates all mothers who warmly helped her with this research. This article was derived from a research project No. 390434, which was funded by Vice- Chancellor for Research in Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Source of Support: This study was funded and supported by Deputy of Research, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: Nil.
REFERENCES
- 1.Gary CF, Leveno KJ, Bloom SL, Hauth JC, Rose DJ, Spong CY. 23rd ed. New York: McGraw HIill Medical; 2010. Williams Obstetrics. [Google Scholar]
- 2.Fayyad AM, Harrington KF. Prediction and prevention of preeclampsia and IUGR. Early Human Development. 2005;81:865–76. doi: 10.1016/j.earlhumdev.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JM, Pearson G, Cutler J, Lindheimer M NHLBI Working Group on Research on Hypertension During Pregnancy. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–45. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 4.Taebi M, Sadat Z, Saberi F, Abedzadeh M. Early Pregnancy waist to hip ratio and risk of preeclampsia: A prospective cohort study. Hypertension Resaerch. 2015;38:80–3. doi: 10.1038/hr.2014.133. [DOI] [PubMed] [Google Scholar]
- 5.Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust. 2006;184:56–9. doi: 10.5694/j.1326-5377.2006.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 6.Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba’aqeel H, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194:921–31. doi: 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- 7.Chang J, Elam-Evans LD, Berg CJ, Herndon J, Flowers L, Seed KA, et al. Pregnancy-related mortality surveillance--united states, 1991--1999. MMWR Surveill Summ. 20031;52:1–8. [PubMed] [Google Scholar]
- 8.Ishikuro M, Obara T, Metoki H, Ohkubo T, Yamamoto M, Akutsu K, et al. Blood pressure measured in the clinic and at home during pregnancy among nulliparous and multiparous women: The BOSHI study. Am J Hypertens. 2013;26:141–8. doi: 10.1093/ajh/hps002. [DOI] [PubMed] [Google Scholar]
- 9.Gold RA, Gold KR, Schilling MF, Modilevsky T. Effect of age, parity, and race on the incidence of pregnancy associated hypertension and eclampsia in the United States. Pregnancy Hypertension: An International Journal of Women's Cardiovascular Health. 2014;4:46–53. doi: 10.1016/j.preghy.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Lindheimer MD, Taler SJ, Cunningham FG. American Society of Hypertension. ASH Position Paper: Hypertension in Pregnancy. J Clin Hypertens. 2009;11:214–25. doi: 10.1111/j.1751-7176.2009.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2013;209:327.e1–17. doi: 10.1016/j.ajog.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson D, Sikka RS, Hayman J, Novak M. Exercise in pregnancy. Curr Sports Med Rep. 2009;8:147–53. doi: 10.1249/JSR.0b013e3181a61d51. [DOI] [PubMed] [Google Scholar]
- 13.Kagan KO, Kuhn U. Sports and pregnancy. Herz. 2004;29:426–34. doi: 10.1007/s00059-004-2590-4. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen BK. Physical activity and pregnancy. In: Ovesen PG, Møller JD, editors. Maternal Obesity and Pregnancy. New York: Springer Berlin Heidelberg; 2012. pp. 63–74. [Google Scholar]
- 15.May L. Part 3: Special considerations for exercise during pregnancy. ACSM's Certified News. 2012;22:4–5. [Google Scholar]
- 16.Hassall J. Exercise in pregnancy: A review of current evidence and guidelines. Essentially MIDIRS. 2011;2:39–42. [Google Scholar]
- 17.Evenson KR, Savitz DA, Huston SL. Leisure-time physical activity among pregnant women in the US. Paediatr Perinat Epidemiol. 2004;18:400–7. doi: 10.1111/j.1365-3016.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- 18.Nelson AG, Kokkonen J. Champaign, IL: Human Kinetics; 2007. Stretching anatomy. [Google Scholar]
- 19.Yeo S. Adherence to walking or stretching, and risk of preeclampsia in sedentary pregnant women. Res Nurs Health. 2009;32:379–90. doi: 10.1002/nur.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorensen TK, Williams MA, Lee IM, Dashow EE, Thompson ML, Luthy DA. Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension. 2003;41:1273–80. doi: 10.1161/01.HYP.0000072270.82815.91. [DOI] [PubMed] [Google Scholar]
- 21.Fortner RT, Pekow PS, Whitcomb BW, Sievert LL, Markenson G, Chasan-Taber L. Physical activity and hypertensive disorders of pregnancy among Hispanic women. Med Sci Sports Exerc. 2011;43:639–46. doi: 10.1249/MSS.0b013e3181f58d3e. [DOI] [PubMed] [Google Scholar]
- 22.Rudra CB, Williams MA, Lee IM, Miller RS, Sorensen TK. Perceived exertion during pre-pregnancy physical activity and preeclampsia risk. Med Sci Sports Exerc. 2005;37:1836–41. doi: 10.1249/01.mss.0000175862.41620.41. [DOI] [PubMed] [Google Scholar]
- 23.Hegaard HK, Ottesen B, Hedegaard M, Petersson K, Henriksen TB, et al. The association between leisure time physical activity in the year before pregnancy and pre-eclampsia. J Obstet Gynaecol. 2010;30:21–4. doi: 10.3109/01443610903315686. [DOI] [PubMed] [Google Scholar]
- 24.Vollebregt KC, Wolf H, Boer K, van der Wal MF, Vrijkotte TG, Bonsel GJ. Does physical activity in leisure time early in pregnancy reduce the incidence of preeclampsia or gestational hypertension? Acta Obstet Gynecol Scand. 2010;89:261–7. doi: 10.3109/00016340903433982. [DOI] [PubMed] [Google Scholar]
- 25.Østerdal ML, Strøm M, Klemmensen AK, Knudsen VK, Juhl M, Halldorsson TI, et al. Does leisure time physical activity in early pregnancy protect against pre-eclampsia? prospective cohort in Danish women. BJOG. 2009;116:98–107. doi: 10.1111/j.1471-0528.2008.02001.x. [DOI] [PubMed] [Google Scholar]
- 26.Artal R, O’Toole M. Guidelines of the american college of obstetricians and gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37:6–12. doi: 10.1136/bjsm.37.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valafar SH. 6th ed. Iran: Ministry of Health and Medical Education of Iran. Office of Family Health and Population, Maternal Health Administration; 2009. National program of safe motherhood: Integrated care of maternal health for midwife- General Physician. [Google Scholar]
- 28.Moretti M, Phillips M, Abouzeid A, Cataneo RN, Greenberg J. Increased breath markers of oxidative stress in normal pregnancy and in preeclampsia. Am J Obstet Gynecol. 2004;190:1184–90. doi: 10.1016/j.ajog.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Yeo SA, Davidge ST. Possible beneficial effect of exercise, by reducing oxidative stress, on the incidence of preeclampsia. J Womens Health Gend Based Med. 2001;10:983–9. doi: 10.1089/152460901317193558. [DOI] [PubMed] [Google Scholar]
- 30.Yeo SA, Davidge S, Ronis DL, Antonakos CL, Hayashi R, O’Leary S. A comparison of walking versus stretching exercises to reduce the incidence of preeclampsia: A randomized clinical trial. Hypertens Pregnancy. 2008;27:113–30. doi: 10.1080/10641950701826778. [DOI] [PubMed] [Google Scholar]
- 31.Yeo SA. Adherence to walking or stretching, and risk of preeclampsia in sedentary pregnant women. Res Nurs Health. 2009;32:379–90. doi: 10.1002/nur.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazemi A, Ahmadi P. Relationship between physical activity during the first 20 weeks of gestation and hypertension in pregnancy. J Shahrekord Univ Med Sci. 2007;9:20–7. [Google Scholar]
- 33.Magnus P, Trogstad L, Owe KM, Olsen SF, Nystad W. Recreational physical activity and the risk of preeclampsia: A prospective cohort of norwegian women. Am J Epidemiol. 2008;168:952–7. doi: 10.1093/aje/kwn189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyldum EV, Romundstad PR, Slørdahl S A. Pre-pregnancy physical activity and preeclampsia risk: A prospective population-based cohort study. Acta Obstet Gynecol Scand. 2010;89:315–20. doi: 10.3109/00016340903370106. [DOI] [PubMed] [Google Scholar]
- 35.Rudra CB, Sorensen TK, Luthy DA, Williams MA. A prospective analysis of recreational physical activity and preeclampsia risk. Med Sci Sports Exerc. 2008;40:1581–8. doi: 10.1249/MSS.0b013e31817cab1. [DOI] [PubMed] [Google Scholar]


