Abstract
Background:
Polycystic ovary syndrome (PCOS) is a polygenic endocrine disorder in women of reproductive age that lead to infertility. The aim of this study was to investigate the effects of probiotic on pancreatic β-cell function and C-reactive protein (CRP) in PCOS patients.
Methods:
This randomized double-blind placebo-controlled clinical trial was conducted among 72 women aged 15–40 years old diagnosed with PCOS. Participants were randomly assigned to two groups receiving: (1) Probiotic supplements (n = 36), (2) placebo (n = 36) for 8-week. Fasting blood samples were taken at baseline and after 8-week of intervention.
Results:
Probiotic supplementation, compare with placebo, reduced fasting blood sugar (−4.15 ± 2.87 vs. 2.57 ± 5.66 mg/dL, respectively P = 0.7), serum insulin levels in crude model (−0.49 ± 0.67 vs. 0.34 ± 0.82 μIU/mL, respectively, P = 0.09), homeostasis model of assessment-insulin resistance score (−0.25 ± 0.18 vs. −0.05 ± 0.18, respectively, P = 0.14) nonsignificantly. Serum insulin levels after adjustment with covariates reduced significantly in probiotic group (P = 0.02). We did not found any significant differences in mean changes of CRP between groups (−0.25 ± 0.18 vs. −0.05 ± 0.18, respectively, P = 0.14).
Conclusions:
A 8-week multispecies probiotics supplementation had nonsignificantly beneficial effect on pancreatic β-cell function and CRP in PCOS patients. After adjustment for some covariates, serum insulin changes were significantly different between groups.
Keywords: C-reactive protein, pancreatic β-cell function, polycystic ovary syndrome, probiotic supplementation
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a polygenic endocrine disorder in women of reproductive age that lead to infertility.[1,2] Complications of PCOS are ovarian enlargement, hyperandrogenism and hirsutism, acne, alopecia, menstrual irregularity,[3] endometrial cancer,[4] and some metabolic complications including glucose metabolism disturbance,[5] β-cell dysfunction[6] and elevated C-reactive protein (CRP) as an inflammation marker.[7] The prevalence of PCOS was estimated 12–21% in Western countries[8,9] and 15.2% in Iranian women.[10] Although the pathogenesis of PCOS is not completely known, the potential factors involve in gonadotropin releasing hormone secretion abnormalities and insulin resistance.[11]
Lifestyle modification, weight loss, and exercise would be beneficial in the management of PCOS.[12] According to previous findings, it seems that lifestyle change and diet manipulation improve most chronic diseases.[13,14] Probiotics as living microorganisms[15] synergism with gut microbiota and probably influence on metabolic and inflammatory conditions.[16] Dairy products including yoghurts, fermented foods and some cheeses are known as a valuable resources of probiotic cultures in diet. However, the gut microbiome alteration and biological effects of each source are ambiguous. Available probiotic supplements are influenced by initial dose strain, quality, temperature and anaerobic storage conditions.[17] Previous investigations have indicated the beneficial role of probiotics in various diseases as gastrointestinal diseases, infections, diabetes and atopic diseases.[14] Several animal studies reported that probiotics improved blood sugar and insulin resistance in diabetic rats.[18,19] A meta-analysis study showed that probiotic ameliorated insulin resistance in nonalcoholic fatty liver disease patients.[20] Despite many evidences about the association between insulin resistance and probiotics in various diseases, a little information is available about probiotic and CRP.
Considering the effects of probiotic supplementation on some metabolic disorders including insulin resistance and inflammation and these disorders are associated with PCOS, it seems that probiotic supplementation has favorable effects on PCOS. To the best of our knowledge and belief, this study is the first that investigated the effect of probiotic supplementation on pancreatic β-cell function and CRP in women with PCOS.
METHODS
Study design
Seventy-two PCOS patients aged 15–40 years old were assigned to this randomized double-blind placebo-controlled clinical trial that was performed in Isfahan, Iran, during May 2013 to December 2013.
Exclusion criteria were age below 15 and more than 40 years, those with history of chronic heart, kidney, liver, lung or pancreatic disease specially cardiovascular diseases, thyroid disorder, small bowel syndrome, autoimmune disease, allergy to probiotic capsules or placebo, current or previous (within the last 6 months) use of chemotherapy, corticosteroid (insulin injection, statins, diuretics), antibiotic, multivitamin mineral supplements and omega-3 medications and women with specific diet or physical activity programs.
Participants were stratified according to body mass index (BMI) (<18.5, 18.5–24.9, 25–29.9, >30 kg/m2) and age (15–20, 21–25, 26–30, 31–35, 36–40 years) in order to matching participants and after getting informed consent were randomly allocated to one of the two groups: (1) Probiotic supplement (n = 32), (2) placebo (n = 33) for 8-week. Diagnosis of PCOS was done according to the 2003 Rotterdam criteria:[10] those with two of the following features were considered as PCOS: Oligo-ovulation and/or anovulation, clinical and biochemical hyper- and rogenism, and polycystic ovaries in ultrasonography. Subjects that admitted to the infertility centers of two hospitals affiliated to Isfahan University of Medical Sciences, Isfahan, Iran, as Beheshti and the Alzahra Hospital were screened for PCOS. Those who have inclusion criteria were entered to the study. Their blood samples were transferred on dry ice to laboratory of Sedigheh Tahereh Metabolism and Endocrinology Research Center, Isfahan, Iran for analysis.
Patients in probiotic group received one Familact probiotic capsule (500 mg) daily that each capsule contained the following bacterial strains: Lactobacillus casei 7 × 109 CFU/g, Lactobacillus acidophilus 2 × 109 CFU/g, Lactobacillus rhamnosus 1.5 × 109 CFU/g, Lactobacillus bulgaricus 2 × 108 CFU/g, Bifidobacterium breve 2 × 1010 CFU/g, Bifidobacterium longum 7 × 109 CFU/g, Streptococcus thermophiles 1.5 × 109 CFU/g. Participants in the placebo group received the placebo that contained starch and maltodextrins but no bacteria. The placebo was indistinguishable in color, shape, size, and packaging, smell and taste from the probiotic supplement. All capsules were provided by fermented biological company of Tehran University of Medical Sciences that was recorded and approved by Food and Drug Administration. The study was approved by the Ethical Committee of Isfahan University of Medical Sciences, Isfahan, Iran. Patients take capsules with water after lunch.
Demographic characteristics of participants were collected by questionnaire. Three days food records were taken (2-week days and 1-week end) and Nutritionist IV software (First Databank, San Bruno, CA, USA) modified for Iranian foods was used to obtain nutrient intake. Three physical activity records were taken at 2, 4, and 6 weeks. Compliance to supplements was monitored by: (1) Participant interview (2) follow-up by frequent short message service and phone call (3) bring the medication containers. The registration ID in the Iranian website (www.irct.ir) was: IRCT2013081111763N11.
Anthropometric assessment
Body weight was assessed with minimal clothing and without shoes by standard scale (Seca, Germany) to the nearest 0.1 kg. Height was measured by a wall mounted stadiometer to the nearest 0.5 cm. BMI was computed as the weight in kilogram divided by the height in meters squared. Waist circumference (WC) was measured in the middle of the lowest gear and the top of the iliac crest with a nonstretched tape.
Biochemical assessment
The blood samples (10 cc) were taken at the baseline and after 8-week intervention after 12 h of fasting. Blood samples were analyzed at Laboratory of Metabolism and Endocrinology Research Center, Isfahan, Iran. Blood was immediately centrifuged (Hettich D-78532, Tuttlingen, Germany) at 3500 rpm for 10 min to separate serum and stored at −70°C before analysis. Commercial kits were used to determine fasting plasma glucose (FPG) and CRP, (Pars Azmun, Tehran, Iran). The inter-assay coefficients of variations (CVs) for serum CRP were 4.00%. The intra- and inter-assay CVs for FPG were 1.74 and 1.19%, respectively. Serum insulin was assayed by immunoassay system (Advia Centaur Up, USA). The homeostatic model of assessment for insulin resistance (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI) was calculated with formulas: HOMA-IR = fasting insulin (μU/mL) × fasting glucose (mg/dL)/405, QUICKI = 1/(log (fasting insulin μU/mL) + log (fasting glucose mg/dL).[21] Systematic error and inter-assay variability decreased by blinded fashion, duplicate, pairs (during intervention) at the same time, the same analytical run, and random order for glucose, insulin and CRP measurements.
Statistical analysis
T-test was used to detect differences in general characteristics and dietary intakes between the two groups. The effects of probiotic supplementation on fetal bovine serum (FBS), Insulin, HOMA, QUICKI, CRP, and variations were compared by paired t-test. The percentage of changes in variables after intervention determined by of the following formula: ([after values – before values]/before values) ×100. MANCOVA was applied to identify any differences between the two groups at the end of the study that analysis was adjusted with baseline values and some covariates including age, BMI, waist, marriage status, education and physical activity to omit the probably potential bias. All statistical analyses were done by the Statistical Package for Social Science software (SPSS Inc., Chicago, IL, USA version 16.0). All results were expressed as means ± standard error. P < 0.05 was considered as statistically significant. Smirnov–Kolmogorov test was used to check the normality of our data.
RESULTS
A total of 85 women were screened for PCOS in the infertility center of Beheshti and Alzahra Hospital Affiliated to Isfahan University of Medical Sciences, Isfahan, Iran. Totally, 72 patients had all the inclusion criteria and were ultimately included in the trial. Finally, 65 subjects (probiotic [n = 32], placebo [n = 33]) completed our study. Among individuals in the placebo group, three women (unwilling to continue [n = 2], become pregnant [n = 1]) and in the probiotic group, four women (unwilling to continue [n = 2], become pregnant [n = 1], health problems [n = 1]) did not complete the study [Figure 1]. There were no significant differences in terms of mean age, weight, BMI, WC, marriage status and physical activity between probiotic and placebo groups at the baseline of the study [Table 1].
Figure 1.
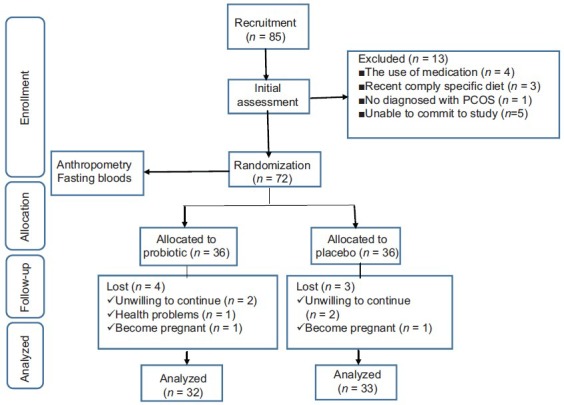
Summary of patient flow diagram, individuals received 500 mg probiotic supplements daily, polycystic ovary syndrome
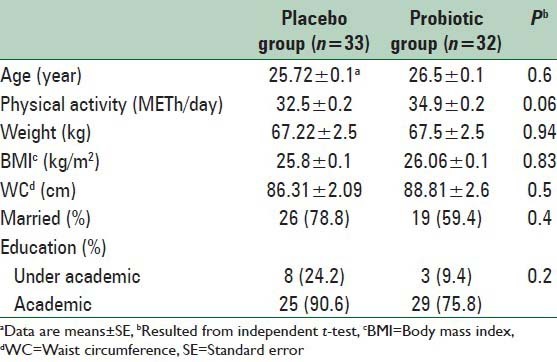
Table 1.
General characteristics of study participants in two groups at baseline
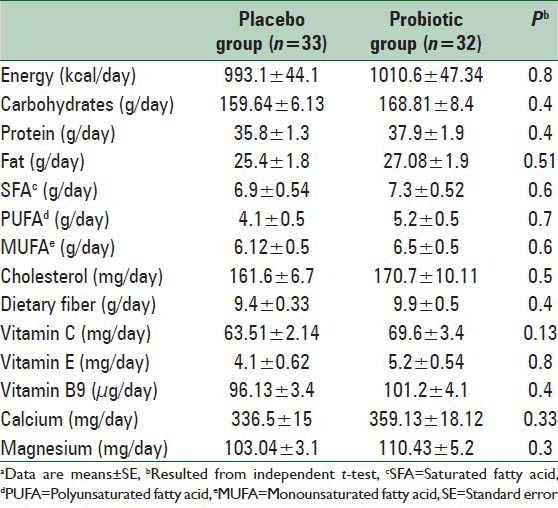
No statistically significant differences were seen between the two groups in terms of dietary intakes of energy, carbohydrates, proteins, fats, saturated fatty acids, polyunsaturated fatty acid, monounsaturated fatty acid, cholesterol, dietary fiber, Vitamin E, Vitamin C, calcium and magnesium based on dietary records that obtained throughout the intervention [Table 2].
Table 2.
Dietary intakes of study participants throughout the studya
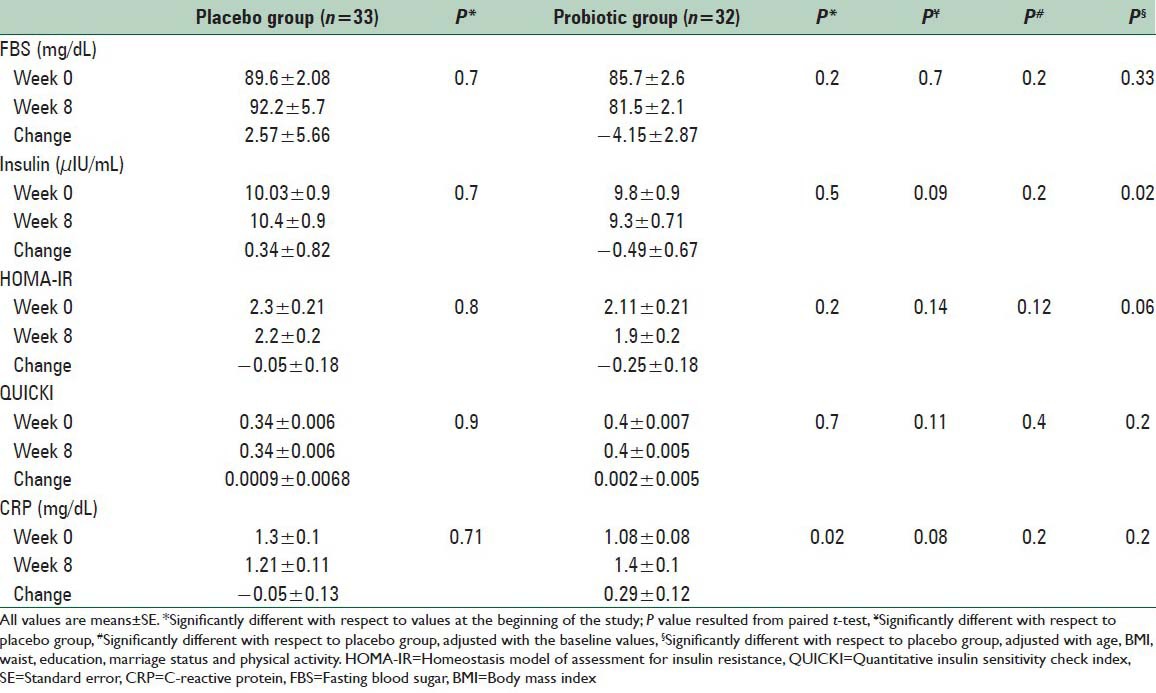
We found within probiotic group a nonsignificant reduction in FBS (85.7 ± 2.6 vs. 81.5 ± 2.1 mg/dL, respectively, P = 0.2), serum insulin levels (9.8 ± 0.9 vs. 9.3 ± 0.71 μIU/mL, P = 0.5) and HOMA-IR score (2.11 ± 0.21 vs. 1.9 ± 0.2, respectively, P = 0.2) in before intervention compared with after intervention. Fasting blood sugar (−4.15 ± 2.87 vs. 2.57 ± 5.66 mg/dL, respectively P = 0.7), serum insulin levels (−0.49 ± 0.67 vs. 0.34 ± 0.82 μIU/mL, respectively, P = 0.09) and HOMA-IR score (−0.25 ± 0.18 vs. −0.05 ± 0.18, respectively, P = 0.14) reduced nonsignificantly in probiotic group compared with placebo. Supplementation with probiotic or placebo had no significant effects on pancreatic β-cell function and CRP (0.29 ± 0.12 vs. −0.05 ± 0.13 mg/dL, respectively P = 0.08). We found a significant difference in mean change of serum insulin (P = 0.02) after adjustment with some covariate including age, BMI, waist, education, marriage status, physical activity. Adjustment with the above-mentioned covariates did not affect our findings about other variables. When we adjusted the analysis for baseline values findings remained nonsignificant [Table 3].
Table 3.
The effect of probiotic supplementations on glucose metabolism and CRP
DISCUSSION
The current study demonstrated that taking probiotic supplements for 8-week had no significant effect on CRP and pancreatic β-cell function among PCOS women. However, after adjustment with some covariates, only serum insulin level decreased significantly in the probiotic group compare with placebo group. Previous studies have assessed the effect of probiotic supplementation on insulin resistance and inflammation marker. To the best of our knowledge, the present study is the first study that investigated the effect of probiotic supplementation on pancreatic β-cell function and CRP in PCOS women.
Patients with PCOS are predisposed to insulin resistance and metabolic dysfunctions.[22] In agreement with our findings, previous studies showed supplementation with probiotic reduced insulin resistant in type II diabetes patients.[23,24,25,26,27] Several animal studies showed probiotics improved gut permeability, plasma endotoxin levels, inflammation, and insulin resistance.[28] According to Alokail et al., study, supplementation with probiotics verified circulating endotoxin and inflammation markers during 26 weeks in type II diabetes patients.[15] Nitert et al., showed probiotic supplementation with >1 × 109 CFU each of L. rhamnosus GG and Bifidobacterium lactis BB-12 per capsule from 16 weeks of gestation until delivery prevented gestational diabetes in high-risk group of pregnant women.[29] Laitinen et al., reported a risk reduction of elevated glucose concentration, (odds ratio: 0.31, 95% confidence interval 0.12–0.78, P = 0.013), Insulin concentration (P = 0.032), HOMA-IR and (P = 0.028) and QUICKI (P = 0.028) during probiotics supplementation compared with the placebo group in normo-glycemic pregnant women.[30] Study on 54 diabetic patients aged 35–70 years showed, multispecies probiotic administration for 8-week increased fasting blood sugar decreased serum high-sensitivity CRP (hs-CRP) and increased plasma total GSH, compared with placebo on.[31] Consumption of probiotic yoghurt containing L. acidophilus and Bifidobacterium animalis among pregnant women after 9 weeks led to decrease serum hs-CRP[32] as the same probiotic supplementation in colorectal cancer,[33] autoimmune[34,35] and chronic kidney disease.[36] However, different findings can be explained by the probiotics dosage and strains used in different studies. Combination of gut microbiota and probiotics can influence glucose metabolism via immune system modulation and prevent pancreatic β-cell dysfunction via bacteria endotoxins inhibition resulted from lipopolysaccharide and inflammation reduction[37,38] and produced short chain fatty acids explained enzymatic synthesis of hepatic CRP.[38]
This study was the first study that assessed the effect of probiotic supplementation on CRP and pancreatic β-cell function among PCOS women. Some limitations of our study included: We were not able to assay the effect of probiotic supplementation on oral glucose tolerance tests and hormonal tests in subjects. In addition, long-term intervention might verify the results in terms of probiotic beneficial effects on inflammatory or insulin resistance markers. Probiotic dosage that was used in the present study was not assured by any guidelines.[39] Treatment noncompliance is the risk of all randomized controlled trials.
CONCLUSIONS
Probiotic supplementation for 8-week had no beneficial effects on FBS, HOMA-IR, QUICKI and CRP significantly. After adjustment with some covariates, serum insulin changes were significantly different between groups.
ACKNOWLEDGEMENTS
This study was supported by Isfahan University of Medical Sciences, Isfahan, Iran. The financial support of this study was given by Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Source of Support: Isfahan University of Medical Sciences, Isfahan, Iran. The financial support of this study was given by Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Pasquali R, Gambineri A. Polycystic ovary syndrome: A multifaceted disease from adolescence to adult age. Ann N Y Acad Sci. 2006;1092:158–74. doi: 10.1196/annals.1365.014. [DOI] [PubMed] [Google Scholar]
- 2.Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23:462–77. doi: 10.1093/humrep/dem426. [DOI] [PubMed] [Google Scholar]
- 3.Wehr E, Möller R, Horejsi R, Giuliani A, Kopera D, Schweighofer N, et al. Subcutaneous adipose tissue topography and metabolic disturbances in polycystic ovary syndrome. Wien Klin Wochenschr. 2009;121:262–9. doi: 10.1007/s00508-009-1162-2. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 5.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–31. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 6.Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;96:520–7. doi: 10.1172/JCI118064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cussons AJ, Stuckey BG, Watts GF. Cardiovascular disease in the polycystic ovary syndrome: New insights and perspectives. Atherosclerosis. 2006;185:227–39. doi: 10.1016/j.atherosclerosis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Boyle JA, Cunningham J, O’Dea K, Dunbar T, Norman RJ. Prevalence of polycystic ovary syndrome in a sample of Indigenous women in Darwin, Australia. Med J Aust. 2012;196:62–6. doi: 10.5694/mja11.10553. [DOI] [PubMed] [Google Scholar]
- 9.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–11. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 11.Setji TL, Brown AJ. Polycystic ovary syndrome: Update on diagnosis and treatment. Am J Med. 2014;127:912–9. doi: 10.1016/j.amjmed.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Costello MF, Misso ML, Wong J, Hart R, Rombauts L, Melder A, et al. The treatment of infertility in polycystic ovary syndrome: A brief update. Aust N Z J Obstet Gynaecol. 2012;52:400–3. doi: 10.1111/j.1479-828X.2012.01448.x. [DOI] [PubMed] [Google Scholar]
- 13.Lindström J, Neumann A, Sheppard KE, Gilis-Januszewska A, Greaves CJ, Handke U, et al. Take action to prevent diabetes – The IMAGE toolkit for the prevention of type 2 diabetes in Europe. Horm Metab Res. 2010;42(Suppl 1):S37–55. doi: 10.1055/s-0029-1240975. [DOI] [PubMed] [Google Scholar]
- 14.Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23:62–8. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Alokail MS, Sabico S, Al-Saleh Y, Al-Daghri NM, Alkharfy KM, Vanhoutte PM, et al. Effects of probiotics in patients with diabetes mellitus type 2: Study protocol for a randomized, double-blind, placebo-controlled trial. Trials. 2013;14:195. doi: 10.1186/1745-6215-14-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen SJ, Jordan S, Storey M, Thornton CA, Gravenor M, Garaiova I, et al. Dietary supplementation with lactobacilli and bifidobacteria is well tolerated and not associated with adverse events during late pregnancy and early infancy. J Nutr. 2010;140:483–8. doi: 10.3945/jn.109.117093. [DOI] [PubMed] [Google Scholar]
- 17.Tanriover MD, Aksoy DY, Unal S. Use of probiotics in various diseases: Evidence and promises. Pol Arch Med Wewn. 2012;122(Suppl 1):72–7. [PubMed] [Google Scholar]
- 18.Harisa GI, Taha EI, Khalil AF, Salem MM. Oral Administration of Lactobacillus acidophilus restores nitric oxide level in diabetic rats. Aust J Basic Appl Sci. 2009;3:2963–9. [Google Scholar]
- 19.Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: A meta-analysis. World J Gastroenterol. 2013;19:6911–8. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musmar S, Afaneh A, Mo’alla H. Epidemiology of polycystic ovary syndrome: A cross sectional study of university students at An-Najah national university-Palestine. Reprod Biol Endocrinol. 2013;11:47. doi: 10.1186/1477-7827-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisprasert V, Ingram KH, Lopez-Davila MF, Munoz AJ, Garvey WT. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: Impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36:845–53. doi: 10.2337/dc12-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bargiota A, Diamanti-Kandarakis E. The effects of old, new and emerging medicines on metabolic aberrations in PCOS. Ther Adv Endocrinol Metab. 2012;3:27–47. doi: 10.1177/2042018812437355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamant M, Blaak EE, de Vos WM. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev. 2011;12:272–81. doi: 10.1111/j.1467-789X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 24.Caesar R, Fåk F, Bäckhed F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J Intern Med. 2010;268:320–8. doi: 10.1111/j.1365-2796.2010.02270.x. [DOI] [PubMed] [Google Scholar]
- 25.Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther. 2011;130:202–12. doi: 10.1016/j.pharmthera.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Delzenne NM, Cani PD. Nutritional modulation of gut microbiota in the context of obesity and insulin resistance: Potential interest of prebiotics. Int Dairy J. 2010;20:277–80. [Google Scholar]
- 27.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26:5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 28.Tuohy KM, Gougoulias C, Shen Q, Walton G, Fava F, Ramnani P. Studying the human gut microbiota in the trans-omics era – Focus on metagenomics and metabonomics. Curr Pharm Des. 2009;15:1415–27. doi: 10.2174/138161209788168182. [DOI] [PubMed] [Google Scholar]
- 29.Nitert MD, Barrett HL, Foxcroft K, Tremellen A, Wilkinson S, Lingwood B, et al. SPRING: An RCT study of probiotics in the prevention of gestational diabetes mellitus in overweight and obese women. BMC Pregnancy Childbirth. 2013;13:50. doi: 10.1186/1471-2393-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laitinen K, Poussa T, Isolauri E Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: A randomised controlled trial. Br J Nutr. 2009;101:1679–87. doi: 10.1017/S0007114508111461. [DOI] [PubMed] [Google Scholar]
- 31.Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63:1–9. doi: 10.1159/000349922. [DOI] [PubMed] [Google Scholar]
- 32.Asemi Z, Jazayeri S, Najafi M, Samimi M, Mofid V, Shidfar F, et al. Effects of daily consumption of probiotic yoghurt on inflammatory factors in pregnant women: A randomized controlled trial. Pak J Biol Sci. 2011;14:476–82. doi: 10.3923/pjbs.2011.476.482. [DOI] [PubMed] [Google Scholar]
- 33.Zhang JW, Du P, Chen DW, Cui L, Ying CM. Effect of viable Bifidobacterium supplement on the immune status and inflammatory response in patients undergoing resection for colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13:40–3. [PubMed] [Google Scholar]
- 34.Anderson AD, McNaught CE, Jain PK, MacFie J. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut. 2004;53:241–5. doi: 10.1136/gut.2003.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatakka K, Martio J, Korpela M, Herranen M, Poussa T, Laasanen T, et al. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis – A pilot study. Scand J Rheumatol. 2003;32:211–5. doi: 10.1080/03009740310003695. [DOI] [PubMed] [Google Scholar]
- 36.Ranganathan N, Friedman EA, Tam P, Rao V, Ranganathan P, Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: A 6-month pilot scale trial in Canada. Curr Med Res Opin. 2009;25:1919–30. doi: 10.1185/03007990903069249. [DOI] [PubMed] [Google Scholar]
- 37.Lye HS, Kuan CY, Ewe JA, Fung WY, Liong MT. The improvement of hypertension by probiotics: Effects on cholesterol, diabetes, renin, and phytoestrogens. Int J Mol Sci. 2009;10:3755–75. doi: 10.3390/ijms10093755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadrzadeh-Yeganeh H, Elmadfa I, Djazayery A, Jalali M, Heshmat R, Chamary M. The effects of probiotic and conventional yoghurt on lipid profile in women. Br J Nutr. 2010;103:1778–83. doi: 10.1017/S0007114509993801. [DOI] [PubMed] [Google Scholar]
- 39.Introduction of a Qualified Presumption of Safety [QPS] approach for assessment of selected microorganisms referred to EFSA. Opinion of the Scientific Committee. The EFSA J. 2007;587:1–16. [Google Scholar]


