Abstract
Background:
Treatment of patellofemoral pain syndrome (PFPS) has been extensively studied in physical therapy literature. Patients with PFPS demonstrate quadriceps and hip musculature weakness, altered lower extremity (LE) kinematics, and decreased LE flexibility. Psychosocial factors have also been identified as an important factor in patients with PFPS. The authors hypothesize that an ordered approach addressing each of these impairments sequentially will result in greater improvement in PFPS symptoms. The purpose of this pilot study was to assess the feasibility of performing a randomized trial and to determine the sample size necessary to examine the validity of this hypothesis.
Methods:
Patients received a sequential treatment approach using a PFPS treatment algorithm (PFPS Algorithm) designed by the authors. Patients were evaluated assessing psychosocial factors, flexibility, LE kinematics, and LE strength. Impairments that were found in the evaluation were addressed sequentially over the episode of care. Patients were prescribed therapy two times per week for six weeks. Pain, Anterior Knee Pain Scale (AKPS), and Global Rating of Change (GROC) were measured at evaluation and discharge.
Results:
Thirty consecutive patients with PFPS who were referred to physical therapy were enrolled in the pilot study. All phases of the feasibility study including recruitment, treatment protocols and data collection were effectively carried out. One hundred percent of patients treated with the PFPS algorithm who completed the prescribed treatment had a clinically significant improvement in the AKPS and GROC. A floor effect was noted with NPRS with 38% of patients unable to achieve clinically significant improvement.
Conclusions:
With minor changes to the protocol and outcome measures used, a full randomized trial is feasible and merited. Steps must be taken to reduce the high drop‐out rate among both groups.
Level of Evidence:
1b
Keywords: Patellofemoral pain, knee pain, physical therapy
INTRODUCTION
Patellofemoral pain syndrome (PFPS) accounts for 25 to 40% of knee pain in young and active individuals.1‐3 PFPS is described as anterior knee pain around the patella which is aggravated by activity, particularly activities that increase patellofemoral forces such as squatting, ascending or descending stairs, running, and jumping.4,5 It is common in adolescents and physically active adults.6 Females are more likely to experience PFPS than males.7 PFPS is a multifactorial condition with no clear etiology and is considered a syndrome and not a diagnosis. Dye8 has described PFPS as one of the most difficult orthopedic conditions to manage.
Multiple theories exist regarding a cause for PFPS pain. A primary theory for the cause of PFPS is abnormal patellar tracking which results in excessive patellofemoral joint compressive forces.9,10 Many factors contributing to abnormal patellar tracking have been suggested including; hip and quadriceps weakness, delayed or diminished activation of vastus medialis obliquus, increased Q‐angle, altered lower extremity mechanics and decreased lower extremity flexibility. Due to the number of suggested contributory factors to PFPS pain, a vast amount of interventions exist and are frequently used by clinicians. Although, physical therapy interventions have been shown to be effective over sham interventions, many individuals will have recurrent or chronic pain. Ninety‐six percent of patients report having problems four years following their diagnosis of PFPS.11 A possible reason for the continued pain is that PFPS is a multifactorial condition and the treatments may not address all of the contributing factors in each individual.
If all of the contributing factors for the patient’s PFPS are identified, addressing all of these factors at once may not be the best approach. Performing hip strengthening prior to quadriceps strengthening results in decreased levels of pain with exercise.12 Individuals with reduced flexibility are more likely to have impaired lower extremity mechanics.13 Performing traditional lower extremity strengthening exercises when there is impaired lower extremity mechanics results in increased patellofemoral joint contact forces.14
In an attempt to better treat individuals with PFPS, classification systems to subgroup patients with PFPS have been proposed, but their effectiveness has not been evaluated.15‐20 An important clinical question with classification systems is what to do when a patient does not nicely fit into one subgroup. If a patient does not meet or meets the criteria for multiple subgroups, how is the patient treated? No evidence exists on the relative frequency with which patients with PFPS fall into each of these proposed subgroups and whether these subgroups are mutually exclusive.
The clinical classification systems reported in literature only address physical impairments. Psychosocial factors have also been identified as important when treating patients with PFPS. In a study by Piva et al21 fear avoidance beliefs were the strongest predictor of outcomes for function and pain. Mental health status on the Medical Outcomes Short Form‐36 is correlated with severity of patellofemoral symptoms in athletes.22 The results of these studies highlight the necessity of addressing psychosocial factors when treating PFPS.
Therefore, the authors have designed a new classification system (PFPS algorithm) for subgrouping patients based on the patient’s clinical presentation. There are four subgroups in the new PFPS algorithm: Fear‐Avoidance, Flexibility, Functional Malalignment, and Strengthening with function progression. The criteria and intervention of each subgroup is addressed sequentially over the episode of care. This classification system aims to address problems encountered if individuals meet the criteria for multiple subgroups. There is also a psychosocial component to address the needs of individuals with activity avoidance. The PFPS algorithm is goal‐based, where meeting the criteria to pass through each subgroup is the focus of the treatment. Clinicians can provide whichever physical therapy intervention that allows an individual patient to meet the criteria of each subgroup. Interventions used in the PFPS algorithm are based on best available evidence, clinician’s experience, and the patient’s individual response to the intervention.
The authors hypothesize that an ordered approach addressing each of these impairments sequentially will result in greater improvements in function and pain than traditional treatment for PFPS symptoms. The clinical application of the new PFPS algorithm and the ability to recruit patients with PFPS has not been assessed. Therefore, it was decided to complete a pilot study to assess the feasibility of successfully implementing and completing a randomized controlled trial (RCT) to examine the validity of this hypothesis. The purpose of this pilot study was to determine the feasibility of (1) Appropriately delivering the PFPS algorithm in the clinic setting; (2) Recruiting enough patients to perform a full RCT; (3) Outcome measures appropriate to assess the outcomes of a full RCT.
METHODS
The design of this study is a randomized controlled pilot trial using consecutive patients referred to two physical therapy clinics. Patients with peripatellar pain who were referred to Nationwide Children’s Hospital’s physical therapy clinics for treatment were considered for participation. The institutional review board approved this study prior to recruitment and data collection. All patients and guardians provided written informed consent prior to participation. This study was registered at ClinicalTrials.gov (Identifier number NCT01767246).
ELIGIBILITY CRITERIA
Patients were eligible for this study if a clinical diagnosis of PFPS was made by the evaluating therapist. Patients were considered to have a clinical diagnosis of PFPS if the inclusion and exclusion criteria were met. (Table 1) Patients were also excluded from the study if they were unable to follow directions, had a history of activity limiting illness, were pregnant or nursing, or stated they were unable to attend follow‐up appointments.
Table 1.
Inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
INTERVENTIONS
Patients received physical therapy treatments two times per week for six weeks for a total of 12 treatments. Patients were also given a home exercise program that was to be performed daily.
PFPS Algorithm
Patients were evaluated using the PFPS algorithm assessing psychosocial factors, flexibility, LE kinematics and LE functional strength sequentially. (Figure 1) Patients were placed in treatment subgroups based upon the impairments that were found in the evaluation and impairments were addressed in this ordered approach over the episode of care.
Figure 1.
PFPS Algorithm
The first impairment assessed in the PFPS algorithm was fear avoidance. Patients were placed in this treatment subgroup if they scored ≥ 15 on the Fear Avoidance Belief questionnaire‐physical activity subscale (FABQ‐PA) modified for the knee.21 Patients who scored > 15 on the FABQ‐PA were treated using a combination of education and a graded exercise program that incorporated gradual exposure to stimuli. Fear avoidance education de‐emphasized anatomic findings and encouraged the patients to take an active role in their recovery. Therapeutic exercise focused on functional gains with exercise instead of pain.
Lower extremity flexibility was the second impairment assessed in the PFPS algorithm. Patients were considered to have flexibility deficits if they demonstrated tightness in one of the three primary muscles. Flexibility testing of the primary muscles was assessed for quadriceps (Figure 2) gastrocnemius (Figure 3) and soleus (Figure 4 and Figure 5). If a patient demonstrated tightness of three of the four secondary muscles the patient was also considered to have flexibility deficits. Secondary muscle flexibility tests were performed using the Thomas test for hip flexors, Ober’s test for the iliotibial band, straight leg raise test for hamstring flexibility, and adductor flexibility in supine. Treatment in this subgroup focused on stretching of the tight muscles. Low‐level quadriceps and hip strengthening was also incorporated during this treatment phase as stretching alone has been found to be non‐effective.
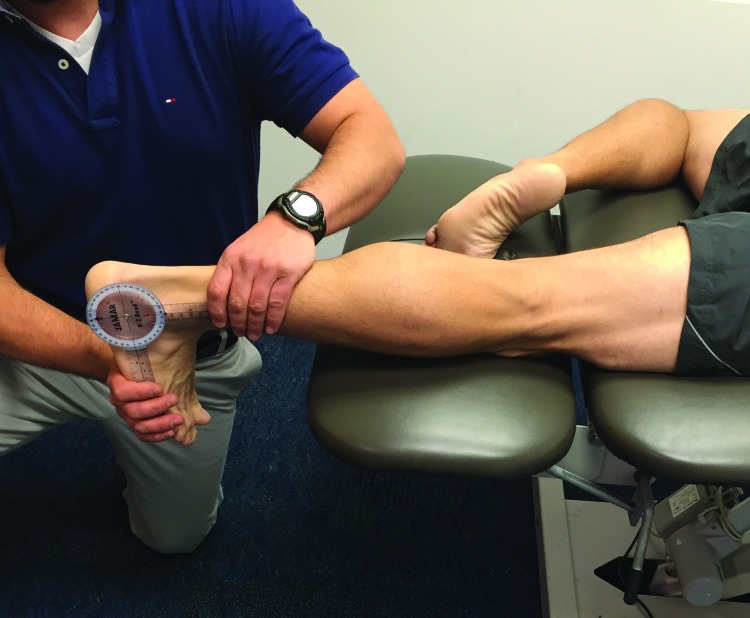
Figure 2.
Quadriceps flexibility testing
Quadriceps muscle length was assessed with the patient lying prone on the table while the therapist locked hip into place by pushing down on the PSIS region. The measurement was taken by passively flexing the patient’s knee into flexion end range. A measurement of less than 130 degrees was considered restricted.
Figure 3.
Gastrocnemius flexibility testing
The gastrocnemius was measured in the prone position with the tested knee extended and the opposite hip in the figure 4 position. The therapist manually verified sub‐talar neutral and passively flexed the foot into dorsiflexion. A gastrocnemius measurement of less than 12 degrees was considered restricted.
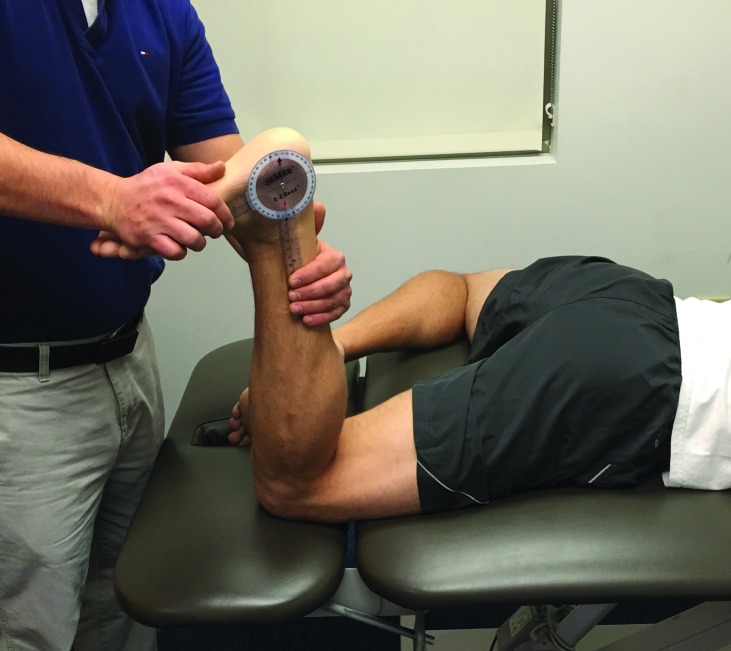
Figure 4.
Soleus flexibility testing
The soleus was measured in the prone position with the tested knee flexed to 90 degrees and the opposite hip in the figure 4 position. The therapist manually verified sub‐talar neutral and passively flexed the foot into dorsiflexion. A soleus measurement of less than 20 degrees was considered restricted.
Figure 5.
Weight bearing dorsiflexion flexibility testing
Weight bearing dorsiflexion was measured using the lung test. The patient lunged forward bringing the affected patella as close to a wall as possible without either heel coming up off the floor. Once maximum dorsiflexion was reached, measurement was taken using a digital inclinometer. The procedure was performed 3 times with the average being recorded. A measurement of less than 50 degrees was considered limited.
The functional malalignment subgroup addressed neuromuscular control of the core and lower extremities during dynamic movement. Functional malalignment was assessed using the lateral step down test (Figure 6) and the single leg squat test (Figure 7). Scoring for both tests were based on criteria previously reported in literature.13 A score of moderate or poor placed the participant in the functional malalignment subgroup. Treatment in this subgroup focused on LE mechanics during dynamic activity and strengthening of muscles necessary to maintain proper alignment. The focus of LE mechanics was individualized to match the functional activity in which each patient participated.
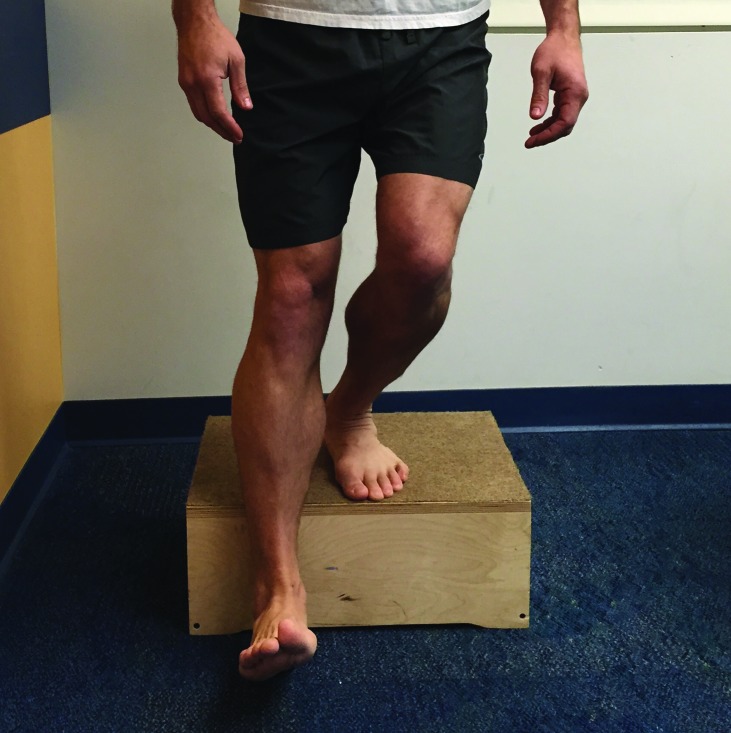
Figure 6.
Lateral Step Down Test
The lateral step down test was performed by having the patient stand on a 20 cm (8 inch) step and perform a squat to approximately 60 degrees. The patient was instructed to keep the trunk straight, hands on waist and bend the knee on the tested side until the heel on the opposite side touches the small step or floor. The patient was instructed to keep the knee of the tested leg over the second toe and lightly touch the heel to the floor and then return to the starting position. Patients repeated the step down 5 times.
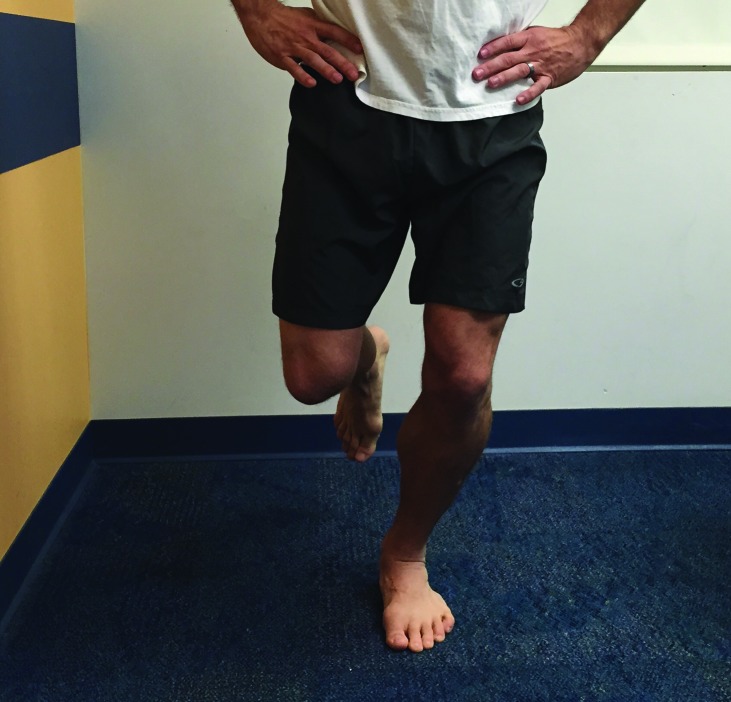
Figure 7.
Single Leg Squat Test
The single leg squat test is performed by having the patient squat while standing on one leg. The patient was instructed to keep the knee of the tested leg over the second toe and squat until he/she is no longer able to see their toes then return to the starting position. The patient repeated the squat 5 times.
The strengthening subgroup, the final classification in the PFPS algorithm, focused on high level strengthening of the lower quarter with emphasis on the quadriceps, hip abductors and external rotators and functional progression back to full activity. Strength was assessed by performing the single leg hop for distance, the triple leg hop for distance, the cross‐over hop for distance as described by Myer et al23 and the timed step down test (Figure 8).24 All tests were assessed using the Limb Symmetry Index. A score of less than 90% placed the participant in this group.
Figure 8.
Timed step down test
Unilateral test performed from a platform 20 cm (8 inch) high. The patient stepped forward and down toward the floor. The lowered limb only brushes the floor with the heel and then returns to starting position. This is counted as one repetition. The number of repetitions the patient performs in 30 seconds is recorded. Both limbs are tested.
OUTCOME MEASURES
The outcome measures utilized in this study included the Numeric Pain Rating Scale (NPRS), the Anterior Knee Pain Scale (AKPS) and the Global rating of Change Scale (GROC). Each were collected and measured at evaluation and discharge. Additionally, the Fear Avoidance Belief Questionnaire physical activity subscale (FABQ‐PA) was collected at initial evaluation.
Pain was measured using the NPRS. The use of the Numerical Pain Rating Scale for assessing pain has been validated for use in this patient population and has been found to have a minimal detectable change of two points.25 The NPRS is an 11‐point pain‐rating scale ranging from 0 (no pain) to 10 (worst imaginable pain) to assess current pain intensity and the best and worst level of pain during the last 24 hours.26 An average of the three ratings was used.
The AKPS and GROC have been utilized in clinical outcome studies and recommended for use with PFPS patients. The AKPS is a self‐reported 13‐item questionnaire with discrete categories related to various levels of current knee function. Categories within each item are weighted, and responses are summed to provide an overall score of 0‐100, with 100 representing no disability. The AKPS is found to be valid and reliable in patients from 12‐50 years of age presenting with anterior knee pain with a test‐retest reliability of .95.27 A change of 10 points represents the minimal clinical difference.28 The GROC is a 15‐point Likert type scale (−7 to +7). A score of 0 represents no change from initial injury, +7 represents a great deal better, and −7 represents a great deal worse. A score of +/− 3 represents a minimal clinical difference.29
Patient’s fear of pain and beliefs about avoiding activity was measured with the FABQ‐PA subscale.30 Higher FABQ physical activity subscale scores have been associated with greater activity limitation in the adolescent population.31 The FABQ also includes a work subscale component that is scored separately from the FABQ‐PA. The work subscale was not included because many patients with PFPS are younger and may not participate in regular work activity and the score would likely not be valid.
RELIABILITY TESTING
To assess the inter‐rater reliability of the subgrouping of the PFPS algorithm, two therapists evaluated each patient on the same day during their course of treatment. Each therapist separately tested and sub‐grouped the patient into the treatment group they deemed appropriate. Therapists were blinded to the evaluation and decision of the other therapist.
RECRUITMENT AND SAMPLE SIZE
No external strategies were used to recruit patients into this study. A sample of males and females 12 years and older were to be drawn from two pediatric outpatient orthopedic physical therapy clinics over the course of one year. There was no predetermined sample size; assessing the ability to recruit an appropriate number of eligible patients was an objective of this pilot study.
DATA ANALYSIS
Descriptive statistics and other exploratory analysis were calculated using SPSS version 21.0 (SPSS Inc. Chicago, IL). Inter‐rater reliability was calculated using Cohen’s Kappa statistic.
RESULTS
Patients were recruited from two outpatient pediatric physical therapy facilities. Eligible patients were recruited from February 2013 to February 2014. Thirty patients met the inclusion criteria and agreed to participate in the study, with 21 patients completing the study protocol (Figure 9).
Figure 9.
Flow diagram for patient recruitment and randomization
SAFETY
No serious adverse reactions were noted with treatment using the PFPS algorithm. There were a few reports of mild adverse reactions consistent with side effects of exercise and manual therapy, such as soreness and mild knee pain with exercise. No patient dropped out of the pilot study due to the side effects of the treatment.
ELIGIBLE PATIENTS
During the one‐year period of recruitment for the pilot study 157 patients were referred to participating physical therapists with knee pain. 19.1% of those patients met the inclusion criteria to participate in the pilot study. No eligible patient refused to participate in the pilot study. On average, 2.5 patients per month were recruited to participate in the pilot study. Thirty percent of the patients who consented to participate in the pilot study did not complete the prescribed treatment.
APPROPRIATE DELIVERY OF INTERVENTIONS
A subjective review of the treatment notes indicated that the treating therapist adhered to the PFPS algorithm in 100% of patients. Cohen’s κ was run to determine if there was agreement between therapists’ subgrouping of patients using the PFPS algorithm. Fifteen total patients were assessed by two therapists each. Therapists agreed on the treatment subgroup in 14 patients. There was very good inter‐rater reliability with a Kappa Score of 0.90 (95% CI, 0.72 to 1.00), p < .0005.
BASELINE DEMOGRAPHICS (TABLE 2)
Table 2.
Baseline Demographics
| Variable | All patients (n=21) |
|---|---|
| Age | 14.10(1.38) |
| Gender (% female) | 14(66.7%) |
| FABQ physical activity | 12.05(5.57) |
| Pain | 2.20 (0.99) |
| Anterior Knee Pain Scale | 77.90(8.99) |
*Data are means (SD) or numbers (%); FABQ= Fear Avoidance Behavior Questionnaire.
There were no outliers found in baseline variables, as assessed by inspection of a boxplot. The AKPS, NPRS and GROC scores were normally distributed at each time point, as assessed by Shapiro‐Wilk’s test (p > .05).
AKPS CHANGE
Clinically significant change with the PFPS algorithm treatment was noted at the six‐week follow‐up, with a mean change of 18.00 (SD 6.27) on the AKPS. One hundred percent of patients treated with the PFPS algorithm who completed the prescribed treatment had a clinically significant improvement in their AKPS score.
NPRS CHANGE
No clinically significant changes were noted at the six‐week follow up for pain. The mean change in NPRS for the PFPS algorithm group was −1.84 (SD 1.04). Only 33% of patients had a clinically significant improvement in NPRS. Thirty‐eight percent of patients had an initial NPRS of <2 making a clinically significant improvement impossible in these patients.
GLOBAL RATING OF CHANGE
Clinically significant change on the GROC was noted at the six‐week follow‐up with the PFPS algorithm, with a mean change of 5.30 (SD 1.42). One hundred percent of patients treated with the PFPS algorithm experienced a clinically significant improvement at the six‐week follow‐up on the GROC.
SAMPLE SIZE CALCULATION FOR FULL TRIAL
Calculations regarding sample size were conducted using the formula recommended by Noordjiz et al32 for randomized controlled trials. The AKPS was considered the primary outcome. The calculations were made using alpha = .05, beta = .20, a minimal clinically important difference between groups of 10, and a within‐group standard deviation (SD) of 12.4. These parameters were based on the findings of previous research.28,33 Twenty‐five subjects per group will be required to adequately achieve statistical power for the primary outcome of AKPS during a full study.
DISCUSSION
The cause of PFPS is not clearly understood. Current evidence suggests that it is multifactorial, with patients presenting with quadriceps and hip musculature weakness, altered lower extremity kinematics, decreased flexibility and psychosocial stressors. This pilot study suggests that a full RCT using an ordered treatment approach addressing soft tissue tightness, altered lower extremity kinematics, neuromuscular deficits and psychosocial stressors in a sequential manor may be feasible provided using the following modifications to the protocol.
The PFPS algorithm was properly implemented into physical therapy clinics. The pilot study used five sports and orthopedic physical therapists from 2 physical therapy clinics. Patients were treated, whenever possible, by the evaluating therapist for the duration of the treatment. After receiving training in the PFPS algorithm all therapists were able to evaluate and treat patients in a consistent manner. There was a high level of inter‐rater reliability, 90%, when placing patients into their subgrouping categories indicating a high level of agreement between therapists for progressing patients through the PFPS algorithm. Clinicians reported the PFPS algorithm was easily performed in a normal clinic setting and no deviations from treatment protocol were noted
Recruitment of patients for a full RCT was deemed feasible with patients referred to these physical therapy clinics if the number of physical therapists participating in the full RCT is increased. Currently, only 20% of the clinics’ physical therapist participated in recruitment. Recruitment rates can be increased even further by training additional physical therapists in the implementation of the research protocol. Training can be completed without disrupting patient care. The sample size necessary for a full RCT is 25 patients in each group with the comparator group being typical PFPS treatment. Accounting for dropout rates, a total number of 58 patients are necessary, based on a goal of limiting the dropout rate to 15%. By increasing the number of participating therapists, recruitment of the necessary patients could be completed in less than one year. No barriers were found to patients agreeing to participate in the study, with all eligible patients and parents consenting to participate.
The PFPS algorithm was deemed clinically effective for treating patients with PFPS. Clinically significant changes in function were noted in 100% of patients treated with the PFPS algorithm, with a mean change of 18.00 (SD 6.27) on the AKPS. Clinically significant change was also noted on the GROC in the PFPS algorithm group, with a mean change of 5.30 (SD 1.42). No clinically significant changes were noted at the six‐week follow up for pain. A floor effect was noted in a third of all patients, suggesting that an average of the three NPRS scores is not the best measure to assess clinically significant change in this population.
Two of the three outcome measures used in this pilot study were appropriate for assessing outcomes in a full RCT. The AKPS appears appropriate to measure function in patients with PFPS. The AKPS has been widely used and has been demonstrated to be valid and reliable for patients with PFPS. The AKPS scale is appropriate for patients with PFPS 12 years of age and older and is the gold standard for measuring function in those with PFPS. No patient was unable to demonstrate clinically significant improvement on the AKPS due to a ceiling effect. The GROC also appears to be an appropriate measure to assess the patient’s perceived level of overall improvement. This measure has been previously researched in patients with knee injuries and a cut score of +3 was found to indicate important change.29 The NPRS using an average of three scores (highest, lowest, and current pain) was deemed inappropriate for use in a full RCT. A floor effect was noted in a significant number of patients who had an initial NPRS of <2, making clinically significant improvement impossible. Using the highest level of pain or pain during activity on the NPRS might be more appropriate than an average measure of pain.
LIMITATIONS AND FUTURE TRIAL CONSIDERATIONS
The high dropout rate was a significant limitation of the current pilot study. This dropout rate would be unacceptable in a full RCT, introducing bias into the results. Two patients did not complete their assigned intervention for unavoidable reasons (concussion and unrelated surgery), but the dropout rate in the remaining patients should be decreased. To decrease the dropout rate changes have been proposed. First, the authors will attempt to secure a small source of funds to compensate research study patients for their time in order to help reduce the financial burden of participating and to motivate patients to participate for the entire study duration. Second, the expectations for participation in the study will be more clearly defined on the consent form, so all eligible patients have a clear understanding of what is needed to complete their prescribed course of treatment.
Another limitation of this pilot study was that the treating therapist and patient were not blinded. With the constant evaluation necessary to progress patients through the PFPS algorithm, blinding would be impractical, but there is the possibility of treatment bias with this approach. All outcomes were based on patient self‐report measures reducing this limitation. Treatment duration and contact with the physical therapist will be the same for both groups in the full RCT.
Minor changes to the PFPS algorithm have been proposed to improve the ability of patients to advance from one subgroup to the next. The need was most evident when patients were attempting to advance out of the flexibility subgroup to the functional malalignment subgroup. Due to the high cutoff score for standing DF motion required to advance, it was difficult for many patients to meet this requirement. This may be resolved by decreasing the cutoff criteria for standing dorsiflexion from 50 degrees to 48 degrees. Although this decrease is small, many patients peaked at 48 or 49 degrees of WB DF and had difficulty achieving 50 degrees. The change in cutoff score is based on using the upper limit of the 95% CI for patients that would fail the lateral step down criteria compared with using the lower limit of patients who would have passed.
Although clinically meaningful results were found with the PFPS algorithm, this pilot study was performed to guide future trial design. The results of this pilot study should not be generalized to patients until a fully powered RCT can be performed.
CONCLUSION
The primary aims of this pilot study were met. The therapists and clinic personnel successfully worked together to carry out all treatments required to conduct a future full scale RCT. The ordered treatment approach used in the PFPS algorithm, addressing soft tissue tightness, altered lower extremity kinematics, neuromuscular deficits and psychosocial factors in a sequential manor, resulted in clinically significant improvements in AKPS and GROC scores. With minor changes to the protocol and outcome measures used, a full RCT assessing the effectiveness of the PFPS algorithm is feasible.
REFERENCES
- 1.Chesworth BM Culham E Tata GE Peat M Validation of outcome measures in patients with patellofemoral syndrome. J Orthop Sports Phys Ther. 1989;10(8):302‐308. [DOI] [PubMed] [Google Scholar]
- 2.Insall J Current Concepts Review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147‐152. [PubMed] [Google Scholar]
- 3.Rubin B CR Runner’s knee. Phys Sportsmed. 1980;8:49‐58. [DOI] [PubMed] [Google Scholar]
- 4.Crossley K Bennell K Green S Cowan S McConnell J Physical therapy for patellofemoral pain: a randomized, double‐blinded, placebo‐controlled trial. Am J Sports Med. 2002;30(6):857‐865. [DOI] [PubMed] [Google Scholar]
- 5.Collins N Crossley K Beller E Darnell R McPoil T Vicenzino B Foot orthoses and physiotherapy in the treatment of patellofemoral pain syndrome: randomised clinical trial. Br J Sports Med. 2009;43(3):169‐171. [DOI] [PubMed] [Google Scholar]
- 6.Davis IS Powers CM Patellofemoral pain syndrome: proximal, distal, and local factors, an international retreat, April 30‐May 2, 2009, Fells Point, Baltimore, MD. J Orthop Sports Phys Ther. 2010;40(3):A1‐16. [DOI] [PubMed] [Google Scholar]
- 7.Witvrouw E Lysens R Bellemans J Cambier D Vanderstraeten G Intrinsic risk factors for the development of anterior knee pain in an athletic population. A two‐year prospective study. Am J Sports Med. 2000;28(4):480‐489. [DOI] [PubMed] [Google Scholar]
- 8.Dye S Patellofemoral pain current concepts: an overview. Sports Med Arthrosc Rev. 2001;9:264–272. [Google Scholar]
- 9.Doucette SA Goble EM The effect of exercise on patellar tracking in lateral patellar compression syndrome. Am J Sports Med. 1992;20(4):434‐440. [DOI] [PubMed] [Google Scholar]
- 10.Fulkerson JP Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447‐456. [DOI] [PubMed] [Google Scholar]
- 11.Price AJ Jones J Allum R Chronic traumatic anterior knee pain. Injury. 2000;31(5):373‐378. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda TY Rossetto FM Magalhaes E Bryk FF Lucareli PR de Almeida Aparecida Carvalho N Short‐term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. J Orthop Sports Phys Ther. 2010;40(11):736‐742. [DOI] [PubMed] [Google Scholar]
- 13.Rabin A Kozol Z Measures of range of motion and strength among healthy women with differing quality of lower extremity movement during the lateral step‐down test. J Orthop Sports Phys Ther. 2010;40(12):792‐800. [DOI] [PubMed] [Google Scholar]
- 14.Powers CM The influence of altered lower‐extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639‐646. [DOI] [PubMed] [Google Scholar]
- 15.Wilk KE Davies GJ Mangine RE Malone TR Patellofemoral disorders: a classification system and clinical guidelines for nonoperative rehabilitation. J Orthop Sports Phys Ther. 1998;28(5):307‐322. [DOI] [PubMed] [Google Scholar]
- 16.Witvrouw E Werner S Mikkelsen C Van Tiggelen D Vanden Berghe L Cerulli G Clinical classification of patellofemoral pain syndrome: guidelines for non‐operative treatment. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):122‐130. [DOI] [PubMed] [Google Scholar]
- 17.Selfe J Callaghan M Witvrouw E Richards J Dey MP Sutton C, et al. Targeted interventions for patellofemoral pain syndrome (TIPPS): classification of clinical subgroups. BMJ Open. 2013;3(9):e003795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes SW Jr. Clancy WG Jr Clinical classification of patellofemoral pain and dysfunction. J Orthop Sports Phys Ther. 1998;28(5):299‐306. [DOI] [PubMed] [Google Scholar]
- 19.Schutzer SF Ramsby GR Fulkerson JP Computed tomographic classification of patellofemoral pain patients. Orthop Clin North Am. 1986;17(2):235‐248. [PubMed] [Google Scholar]
- 20.Naslund J Naslund UB Odenbring S Lundeberg T Comparison of symptoms and clinical findings in subgroups of individuals with patellofemoral pain. Physiother Theory Pract. 2006;22(3):105‐118. [DOI] [PubMed] [Google Scholar]
- 21.Piva SR Fitzgerald GK Wisniewski S Delitto A Predictors of pain and function outcome after rehabilitation in patients with patellofemoral pain syndrome. J Rehabil Med. 2009;41(8):604‐612. [DOI] [PubMed] [Google Scholar]
- 22.Cheung RT Zhang Z Ngai SP Different relationships between the level of patellofemoral pain and quality of life in professional and amateur athletes. PM R. 2013;5(7):568‐572. [DOI] [PubMed] [Google Scholar]
- 23.Myer GD Schmitt LC Brent JL Ford KR Barber Foss KD Scherer BJ, et al. Utilization of modified NFL combine testing to identify functional deficits in athletes following ACL reconstruction. J Orthop Sports Phys Ther. 2011;41(6):377‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loudon JK Wiesner D Goist‐Foley HL Asjes C Loudon KL Intrarater Reliability of Functional Performance Tests for Subjects With Patellofemoral Pain Syndrome. J Athl Train. 2002;37(3):256‐261. [PMC free article] [PubMed] [Google Scholar]
- 25.Childs JD Piva SR Fritz JM Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976). 2005;30(11):1331‐1334. [DOI] [PubMed] [Google Scholar]
- 26.Jensen MP Turner JA Romano JM What is the maximum number of levels needed in pain intensity measurement? Pain. 1994;58(3):387‐392. [DOI] [PubMed] [Google Scholar]
- 27.Watson CJ Propps M Ratner J Zeigler DL Horton P Smith SS Reliability and responsiveness of the lower extremity functional scale and the anterior knee pain scale in patients with anterior knee pain. J Orthop Sports Phys Ther. 2005;35(3):136‐146. [DOI] [PubMed] [Google Scholar]
- 28.Crossley KM Bennell KL Cowan SM Green S Analysis of outcome measures for persons with patellofemoral pain: which are reliable and valid? Arch Phys Med Rehabil. 2004;85(5):815‐822. [DOI] [PubMed] [Google Scholar]
- 29.Wang YC Hart DL Stratford PW Mioduski JE Baseline dependency of minimal clinically important improvement. Phys Ther. 2011;91(5):675‐688. [DOI] [PubMed] [Google Scholar]
- 30.Waddell G Newton M Henderson I Somerville D Main CJ A Fear‐Avoidance Beliefs Questionnaire (FABQ) and the role of fear‐avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157‐168. [DOI] [PubMed] [Google Scholar]
- 31.Wilson AC Lewandowski AS Palermo TM Fear‐avoidance beliefs and parental responses to pain in adolescents with chronic pain. Pain Res Manag. 2011;16(3):178‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noordzij M Tripepi G Dekker FW Zoccali C Tanck MW Jager KJ Sample size calculations: basic principles and common pitfalls. Nephrol Dial Transplant. 2010;25(5):1388‐1393. [DOI] [PubMed] [Google Scholar]
- 33.Collins NJ Bierma‐Zeinstra SM Crossley KM van Linschoten RL Vicenzino B van Middelkoop M Prognostic factors for patellofemoral pain: a multicentre observational analysis. Br J Sports Med. 2013;47(4):227‐233. [DOI] [PubMed] [Google Scholar]