Abstract
Background and Purpose:
Lateral thigh pain, commonly referred to as greater trochanteric pain syndrome (GTPS) and/ or iliotibial band syndrome (ITBS) is commonly treated by the physical therapist. Lateral thigh pain is commonly treated by the physical therapist. The sources of lateral thigh pain are commonly attributed to GTPS and/ or ITBS though various pathologies may contribute to this pain, of which trigger points (TrPs) may be an etiology. Dry needling (DN) is an intervention utilized by physical therapists where a monofilament needle is inserted into soft tissue in order to reduce pain to improve range of motion/ motor control dysfunction. This can assist with facilitation of return to prior level of function. The purpose of this case report is to report the outcomes of a patient with lateral hip and thigh pain treated with DN as a primary intervention strategy.
Case Description:
The subject was an active 78‐year‐old female recreational walker who was referred to physical therapy for chronic left lateral hip and thigh pain of greater than one‐year duration without a clear mechanism of injury. She had a history of previous physical therapy treatment for the same condition, and previous therapeutic intervention strategies were effective for approximately two to three months duration prior to return of pain symptoms. Physical examination supported a diagnosis of GTPS/ ITBS. Subjective reports denoted sleep deficit due to pain lying on the left side at night and difficulty walking more than five minutes. Objective findings included decreased strength of the hip musculature and reproduction of pain symptoms upon flat palpation in specific locations throughout the lateral hip and thigh regions. She was treated for eight weeks using only DN to determine the effectiveness of DN as a primary intervention strategy, as previous physical therapy interventions were inconsistent and were only beneficial in the short‐term.
Outcomes:
Clinically meaningful improvements were noted in disability and pain, as measured by the Lower Extremity Functional Scale and Quadruple Visual Analog Scale. Improvement in strength was not an objective measure being assessed, however, lower extremity strength improvement was noted upon final physical examination. This case report focused on pain reduction for improved function rather than strength improvement. Improvements in pain and disability were subjectively reported. The subject was able to lie on her left side at night, which improved her ability to sleep. She was also able to tolerate walking approximately twenty to thirty minutes for improved community ambulation needs.
Discussion:
This case report presents promising outcomes for the use of DN in the treatment of chronic lateral hip and thigh pain. Further research is recommended to determine if DN is clinically beneficial independent of other therapeutic interventions such as exercise, myofascial release/ massage, non‐thrust mobilization, or manipulation.
Level of evidence:
Level 4
Keywords: Dry needling, hip pain, iliotibial band, trochanteric bursitis
INTRODUCTION
Lateral hip and thigh pain may be the result of a host of pathological etiologies including, but not limited to osteoarthritis of the hip joint, greater trochanteric bursitis, iliotibial band syndrome (ITBS)/ snapping hip syndrome, muscle weakness/ strength imbalances, flexibility deficits, spinal pathology, and leg length discrepancies.1‐15 “Trochanteric bursitis” (TB) is still common terminology used to identify lateral hip pain by medical providers. TB tends to occur between the fourth and sixth decades of life, though cases have been reported in all age‐groups.11 Trochanteric pain syndrome was originally thought to be caused by inflammation of the sub‐gluteus maximus bursa (i.e. bursitis), but recent MRI and ultrasound studies question the idea that bursitis is the primary source of trochanteric pain.13 A contemporary term, greater trochanteric pain syndrome (GTPS) encompasses a number of disorders of the lateral, peri‐trochanteric region of the hip, including trochanteric bursitis, tears of the gluteus medius and minimus and external coxa saltans (snapping hip).14 The incidence of GTPS is reported to be approximately 1.8 subjects per 1000 per year, with the prevalence being higher in women, and subjects with concomitant low back pain, osteoarthritis, ITB tenderness, and obesity.15 Symptoms consist of persistent pain in the lateral hip radiating distally down the lateral thigh to the knee, and occasionally below the knee and/or buttock. Physical examination typically indicates point tenderness in the posterolateral area of the greater trochanter.15
Iliotibial band (ITB) involvement, which is typically associated with lateral knee pain, is regularly observed concurrently with GTPS from a clinical perspective. From a diagnostic standpoint, the lateral knee is the most extensively researched region of ITB pain pathology, but clinically it is common to have palpable tenderness along the entire length of the ITB. There is a paucity of evidence supporting the effectiveness of treatment strategies for ITBS, which include non‐steroidal anti‐inflammatory drug (NSAID) administration, phonophoresis, corticosteroid injections, deep friction massage, and correction of hip strength abnormalities.4,5 The inconsistency with accurate diagnosis of chronic lateral hip and thigh pain sources leads to the possibility of TrPs in the affected hip and thigh musculature as being sources of pain.
Dry needling (DN) research continues to be in spotlight in the therapy community regarding validity/ effectiveness as a treatment strategy for a host of pathological conditions. Currently, no randomized control trial (RCT) studies have looked at the effectiveness of DN to the lateral hip and thigh for pain reduction. Various continuous education programs teach DN techniques, and some of the programs focus on trigger points (TrPs) as the primary justification for using DN intervention. TrPs have been studied extensively over the years as sources of pain,16‐28 and the literature suggests a TrP is identified clinically by palpation of a tender nodule in a taught band of muscle and subject pain recognition of tender spot palpation.28 However, accurate diagnosis of TrP location is difficult due to the lack of a clinician’s ability to reliably and repeatably identify a specific TrP.18,20,21,28 Two studies, one by Sciotti et al23 and one by Myburgh et al22 have shown positive inter‐rater reliability for identification TrPs in the upper trapezius muscle if the examiners are experienced, however, pairing experienced and inexperienced examiners caused a reduction in the ability to reliable identify TrPs.22
In regards to DN for intervention related to TrPs, some authors such as Hong et al29 suggest that the local twitch response (LTR) is necessary for maximum effectiveness of trigger point dry needling (TrP‐DN), however, Tough et al28 indicate that of the original four criteria most commonly used to diagnose TrPs (LTR, predicted pain referral pattern, palpable tender nodule in a taught band of tissue, and reproduction of pain symptoms), LTR and predicted pain referral pattern are no longer considered essential for diagnosis. It should be noted that DN is not limited to myofascial intervention, although this case report’s DN intervention was focused on treating myofascial TrPs in the local tissue.
Physical Therapists regularly attempt to determine the “why” of the root cause of pathology and how to “fix” the issue. Due to the already noted lack of research supporting diagnostic criterion and treatment strategies for lateral hip and thigh pain, the need for clinically effective intervention tools that can quickly improve pain, thereby improving general function that has become deficient due to chronic pain are necessary. The purpose of this case report is to determine the effectiveness of DN as a primary treatment strategy in a subject with chronic lateral hip and thigh pain. Informed consent was obtained from the patient prospectively prior to the start of intervention.
CASE DESCRIPTION
The subject in this study was an active 78‐year‐old female recreational walker, who was referred to physical therapy for evaluation of chronic non‐specific left lateral hip and thigh pain. The reports of pain affected her ability to negotiate stairs, walk for exercise and shopping needs, and also affected her sleeping patterns. She was treated a few years previously using “traditional” physical therapy interventions including exercise, neuromuscular re‐education techniques, deep friction tissue mobilization, and ultrasound. This provided temporary relief, but it was not immediate and her pain persisted (intermittently) over the years. Intermittent symptoms consistent with radiculopathy were reported. The reported radicular symptoms had been occurring for years and positional changes such as sitting down/ laying down always eliminated her pain immediately. She did not report regular bouts of radicular symptoms affecting her daily function. Her overall general health was good, though she reported having a pacemaker. The patient was assessed and cleared of contraindications to the use of DN. Given the fact she had a pacemaker; the use of electro‐stimulation was not utilized as an addition to DN in this case report. She was already taking anti‐inflammatory medication on an as needed basis for hip pain and she had received a cortisone injection six weeks prior to presentation to the clinic, which was reported to only reduce pain for a very short period. Her goal was to reduce pain to improve her ability to walk, sit, sleep, and travel.
The outcome measures employed in this case report were the Lower Extremity Functional Scale (LEFS) and the Quadruple Visual Analog Scale (QVAS) and are reported in Table 1. Upon initial evaluation per the QVAS, the subject reported her current (28 mm), average (35 mm), best (12 mm), and worst (90 mm) pain levels during the last 24‐hour period. The visual analog scale (VAS) has moderate to good reliability (correlation coefficient 0.60‐0.77)30 to detect disability and high reliability for pain (correlation coefficient 0.76‐0.84).31 The minimum clinically significant change has been estimated to be 11 points (mm) on a 100 point (mm) scale.32
Table 1:
Outcome measures
| Outcome Measures | Initial Exam | Upon Completion at 8 Weeks |
|---|---|---|
| LEFS | 24/ 80 | 59/80 |
| QVAS Current | 28 cm | 13 cm |
| QVAS Average | 35 cm | 20 cm |
| QVAS Best | 12 cm | 6 cm |
| QVAS Worst | 90 cm | 82 cm |
LEFS: Lower Extremity Functional Scale
QVAS: Quadruple Visual Analog Scale for pain.
The LEFS was used to assess functional disability. The LEFS is a patient reported functional tool that can be easily and quickly completed and has been found to be a reliable and sensitive to change when compared to the SF‐36 with a minimal detectible change being 9 scale points and the minimal clinically important difference being 9 scale points.33 Test‐ retest reliability per Watson et al34 was found to be high for subjects with anterior knee pain, and Yeung et al35 reported a large responsiveness to change as well as good reliability and validity in outpatient and inpatient orthopedic settings among subjects with revision joint replacements. The results of the LEFS are also shown in Table 1, and the subject had a baseline score of 24/ 80.
EXAMINATION
The subject in this case report was treated several years previously by the author. At that point, she was treated with exercise, myofascial release and deep tissue mobilization techniques, and ultrasound for the same issue. She improved gradually during that intervention period, but her pain returned relatively quickly (approximately 2‐3 months). She presented for this episode of care with reports of burning pain in the left lateral hip and thigh from the superior iliac crest region to the proximal lateral knee, and from the tensor fascia latae (TFL) region to the posterior superior iliac spine (PSIS) and lateral piriformis area of the hip and thigh. Pain increased with lying, sitting, standing, and walking.
She had a history of low back pain, and a previous radiographic study showed lumbar arthritic changes at the L3 through S1 levels. Given her history of back pain, it was necessary to rule out lumbar radiculopathy, pain of spinal origin, and sacroiliac joint (SIJ) involvement (given the SIJ is innervated from branches of L3‐S4). She reported intermittent left lower extremity radicular‐like symptoms, but this was a minor secondary issue that had no current impact on her daily laying, sitting, standing, and walking tolerance. She had an observable minimally shorter left lower extremity in standing, which, in the opinion of the author, could have been an issue affecting mechanical changes to gait patterns leading to the reported pain over the years. Based the subject’s subjective reports including her previous history, differential diagnoses included pain of discogenic origin, osteoarthritis of the hip, sacroiliac joint dysfunction, and GTPS/ ITBS.
Assessment of posture and gait mechanics was performed. This included assessment of lumbar, innominate, and global spinal positioning, and observation of gait mechanics. Physical examination revealed observable mild loss of lumbar lordosis, but given the layers of tissue covering the lumbar region including increased adiposity, accurate palpation and observation of lumbar spinal curvature was difficult and unreliable. There was observed rounded bilateral shoulder positioning. The left innominate was slightly inferior and asymmetric compared to the right upon observation. It is noted that the ability to properly assess pelvic symmetry with static or movement‐based positioning testing, including leg length discrepancy, is not valid or reliable,36‐38 therefore palpation assessment for positional faults of the SIJ were not performed. She limped on the left lower extremity and demonstrated a very mild Trendelenberg walking pattern indicating left hip abductor muscle weakness. No other postural abnormalities were noted.
Bilateral lower extremity (BLE) strength was assessed via manual muscle testing (MMT) in a short sitting position with her hips and knees flexed and the legs hanging off the table. The results are shown in Table 1. Note that hip flexion (4‐/ 5 bilateral), abduction (4/ 5 bilateral), and knee flexion (4/ 5 bilateral) weakness bilaterally was noted upon initial presentation. All other BLE MMT scores were 5/ 5 bilaterally.
A lower quarter neurological examination was performed to screen for symptoms of spinal origin. Dermatomal testing was normal for light touch sensory assessment of the T10‐S2 dermatomal regions of the trunk and lower extremities. Myotomal testing was assessed via MMT of the same nerve root levels, see results above. DTRs were assessed via testing of the L4 and S1 nerve roots in short sitting with the legs off the table and using a reflex hammer at the patellar tendon and Achilles tendon bilaterally. Patellar tendon reflex was 2+ and Achilles tendon reflex was 0 bilaterally. Lack of Achilles DTR could be attributed to chronic and intermittent radicular symptoms stemming from the L5‐S1 nerve root level, though no diagnostic images looking in detail at the nerve roots had been performed at the time of the intervention. Seated slump testing (sensitivity= 0.84; specificity= 0.83)39 was performed to assess for lumbar disc herniation at the L4‐S1 levels, and this did not show pathological involvement. There were no neurovascular abnormalities noted.
Symptom centralization testing for discogenic origin has been found to be valid and reliable.40 The subject was tested via repeated flexion and extension movements in standing for perihperalization/ centralization phenomenon, which was negative for discogenic pain. Sacroiliac joint involvement was ruled out using a multi‐test regimen as indicated by Van der Wurff et al41 and the Active Straight Leg Raise (ASLR) as described by Mens et al.42 Van der Wurff et al included five special tests in the multi‐test regimen: Distraction test, Compression test, Thigh Trust test, Patrick sign, and Gaenslen’s test. All testing of the SIJ was negative.
Palpation assessment revealed tender nodules in taut tissue bands in the gluteus medius, gluteus maximus, lateral piriformis, greater trochanteric region, and ITB regions of the left LE, indicative of the likelihood of TrPs in the affected musculature. Pain from this region likely caused her functional mobility deficit, as pain was the limiting factor in her intolerance to walking activities. There were no autonomic responses noted (e.g. temperature change, diaphoresis, etc.) and sensation was intact to light touch and deep pressure. Trophic changes of the skin were also absent.
EVALUATION/ DIAGNOSIS
Upon completion of subjective history and physical examination, TrPs in the aforementioned musculature were suspected as the underlying pathology causing pain. Strength deficit in the hip musculature and observable asymmetric innominate positioning were present and could possibly have contributed to the long term cause of the subject’s lateral hip and thigh pain. As mentioned prior, the ability to definitively ascertain reliable innominate positioning by palpation is poor; therefore the therapist could not reliably say that pain was due to improper pelvic symmetry. This leads to the likelihood of the possibility of TrPs as a source of pain. This decision was based upon the author’s three years of clinical experience utilizing DN for muscular pathology.
Clinical reasoning determined DN should be the intervention employed, due to the palpable taut bands and reported pain reproduction. Due to the subject’s reports of severe pain upon presentation, it was not believed that stretching and exercise interventions would provide the pain relief she was seeking.
INTERVENTION
Risks and potential complications were advised and written consent was obtained outlining common and serious adverse events associated with DN interventions. Common complications include muscle soreness, bruising, and vasovagal reaction. More serious (but rare) complications include infection, broken needle, and pneumothorax.43 There were no reported contraindications to the use of DN. Contraindications include, but are not limited to: local infection, recent cancer/ history of immune suppression, bleeding disorders, current/ chronic use of anti‐coagulant medications, pregnancy, compromised sterility of equipment, and lack of practitioner practical knowledge.43
The subject was treated for sixteen total sessions, two‐ times per week for eight weeks. She was positioned in right side lying with a pillow between her knees on a hi‐low table for subject and therapist comfort and to reduce the effects of vasovagal response. The following soft tissues were treated: gluteus maximus and medius, lateral piriformis, greater trochanteric bursa area, and four points on the lateral thigh (ITB/ vastus lateralis). These points are outlined in the following paragraphs.
The needles used for treatment of this subject were solid monofilament Seirin J‐type sterile needles, No. 5 (0.25 diameter) x 30 mm. in length; No. 8 (0.30 diameter) x 60 mm.; and No. 8 (0.30 diameter) x 50 mm. Needles were used one time and discarded, as the risk of needle injury to the therapist is increased with techniques that teach “re‐sheathing” of the needles to use in other locations on the same subject.43 Each needle was held in the therapist’s dominant hand for application of and manipulation of the needle within the tissue. Prior to insertion of the needles, an application of 70% isopropyl alcohol was performed to cleanse the treatment areas.
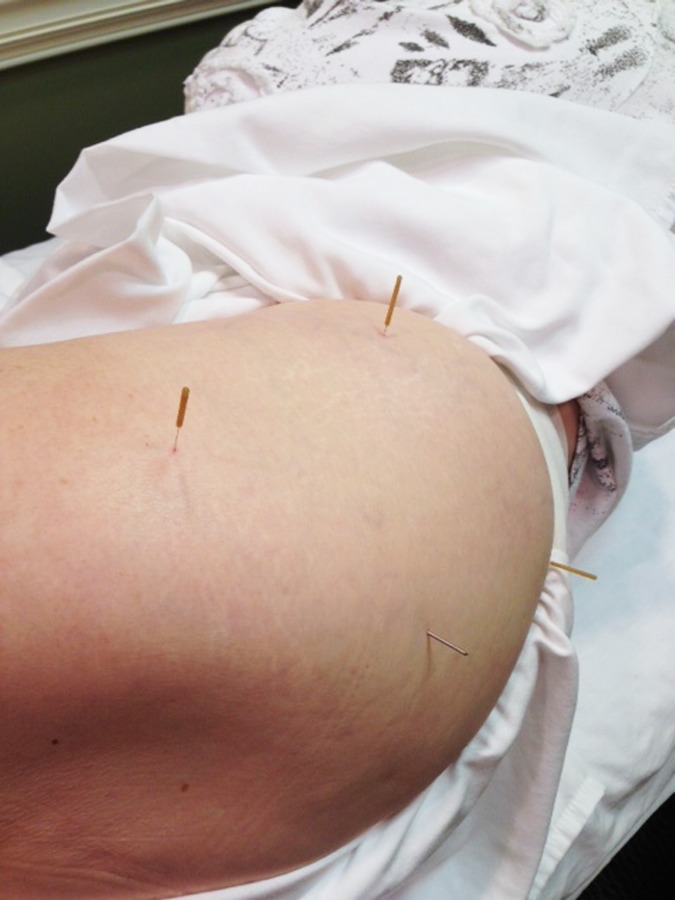
DN to the gluteus maximus and medius points were performed with 50 mm length needles. The needles were inserted into tender nodules in the tissue identified upon flat palpation, which were located three‐ fingerbreadths distal to the mid iliac crest (Figure 1) and three fingerbreadths lateral to the PSIS (Figure 2). The needles were inserted perpendicularly through the muscle bellies utilizing a fast‐in/ out movement technique in a cone pattern to attempt to target as many sensitive loci as possible within the tender nodule(s) in the taut band of the target musculature. The needles were then wound clockwise to attain needle grasp, and then were in turn left in‐situ for 15 minutes. DN techniques may have a local and/ or remote therapeutic effect based on mechanical coupling of connective tissue and the needle thereby causing a “downstream” effect on the generation of a mechanical signal caused by needle grasp pulling. These downstream effects may include cell secretion, modification of extracellular matrix, enlargement and propagation of the signal along connective tissue planes, and afferent input modulation by changes in the connective milieu.44‐47
Figure 1.
Needle placement for gluteus maximus
Figure 2.
Needle placements for gluteus maximus and gluteus medius points
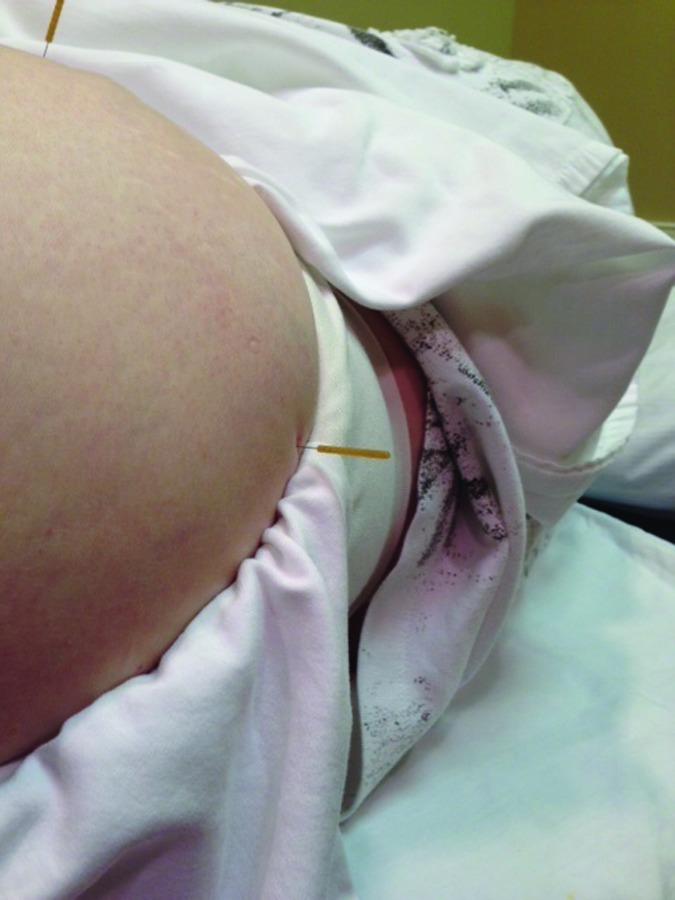
DN of the lateral piriformis musculo‐tendinous junction (Figure 3) was performed using a 60 mm needle inserted into the lateral piriformis region of the posterolateral hip region. The needle was inserted perpendicularly through the muscle belly angled slightly cephalad and towards the symphysis pubis. The needle was wound clockwise needle grasp caused a slight discomfort reported by the subject. This needle was then left in‐situ for 15 minutes.
Figure 3.
Needle placement for lateral piroformis
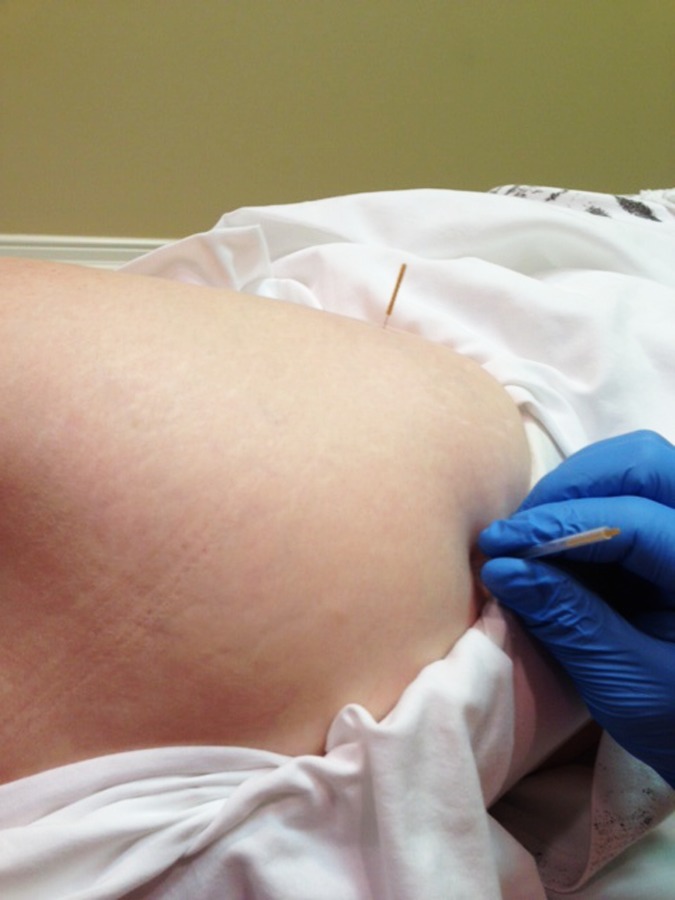
DN of the greater trochanteric region (Figure 4) was performed using a 50 mm needle that was inserted lateral to medial in the center of the greater trochanteric region to a depth of 50 mm. The needle was wound clockwise to attain needle grasp and left in‐situ for 15 minutes.
Figure 4.
Needling of the greater trochanteric region
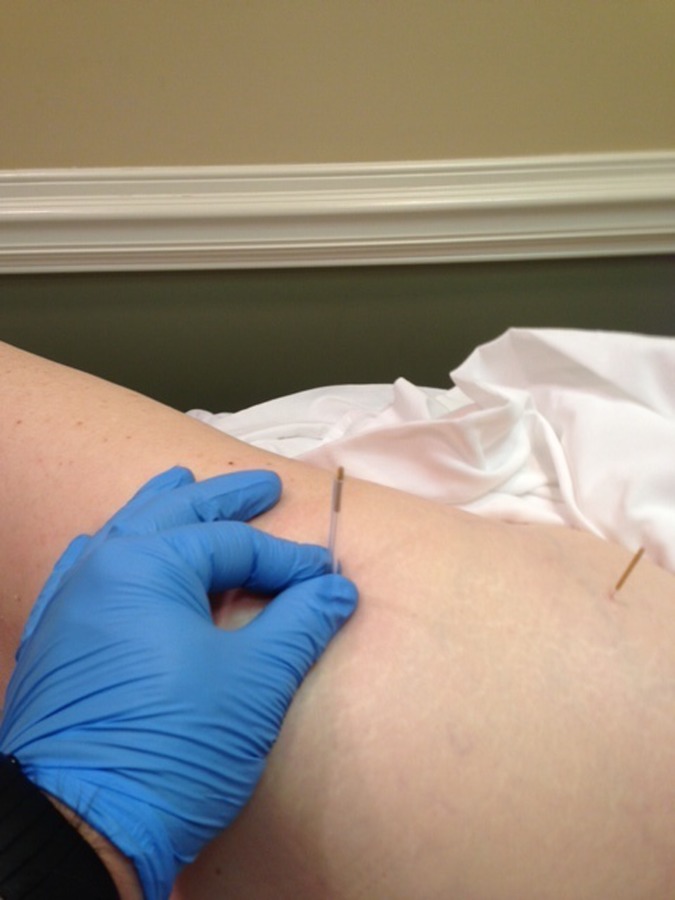
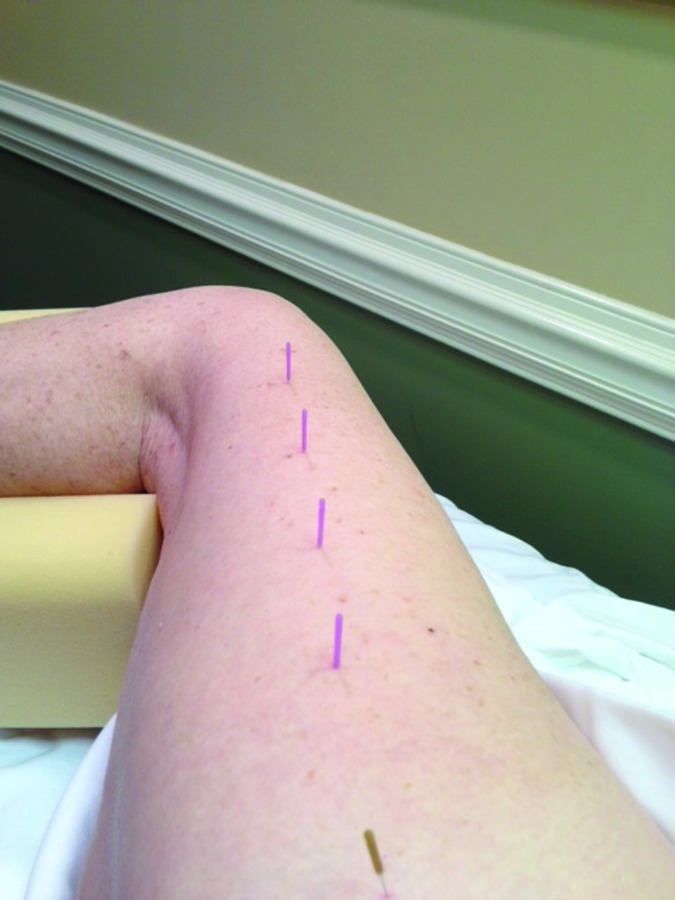
DN of the vastus lateralis/ ITB region (Figure 5) was performed with four 30 mm needles using flat palpation to identify multiple tender points throughout the midline of the lateral thigh. Once the initial needle was inserted, three more needles were inserted four fingerbreadths distal to the prior needle insertion location. The needles were then rotated clockwise to attain needle grasp, then left in‐situ for 15 minutes.
Figure 5.
Needle placements for the vastus lateralis and iliotibial band
OUTCOMES
The efficacy of DN intervention was measured by reduction of pain and disability levels, objective hip strength, subjective reports of improvement in the subject’s overall functional ability, and quality of life. Initially and eight weeks after the initial treatment session, pain and disability was assessed via the LEFS and QVAS outcome measures. Hip strength was assessed via MMT in sitting, as previously described. The results of these outcome measures are shown in Table 1. The LEFS showed improvement from 24/ 80 initially to 54/ 80 at completion of treatment, which is well above the MDC/ MDIC indicating clinically meaningful improvement. The QVAS (average) improved from 35 mm to 20 mm and QVAS (best) improved from 12 mm to 6 mm. The QVAS (current) significantly improved from 28 mm. to 13 mm., and the QVAS (worst) improved from 90 mm to 82 mm. The QVAS (average) and QVAS (current) both met the clinically meaningful change threshold, but the QVAS (best) and QVAS (worst) did not meet the clinically meaningful change threshold.
Table 2 shows objective results including BLE strength. The primary intent of this case report was not to attempt to directly address strength; rather it was to focus on reduction of pain. The improvements in strength that were noted with the use of DN were not expected, however, strength improvements were demonstrated, including hip flexion MMT improvement from 4‐/5 to 4/5 bilaterally, hip abduction MMT improvement from 4/5 to 5/5 bilaterally, and knee flexion MMT improvement from 4/5 to 5/5 bilaterally. The subject, upon completion of the eight‐week intervention period, also subjectively reported improved ambulation tolerance, sleep, and improved ability to sit and stand throughout the day.
Table 2:
Manual Muscle Scores
| Time period | Hip FLEX MMT R/L | Hip ABD MMT R/L | Hip ADD MMT R/L | Knee FLEX MMT R/L | Knee EXT MMT R/L | Ankle DF MMT R/L |
|---|---|---|---|---|---|---|
| Initial | 4‐/ 4‐ | 4/ 4 | 5/ 5 | 4/ 4 | 5/ 5 | 5/ 5 |
| Final | 4/ 4 | 5/ 5 | 5/ 5 | 5/ 5 | 5/ 5 | 5/ 5 |
DISCUSSION
The subject reported significant improvement of the initial hip and thigh regional pain she came to have addressed. The LEFS and QVAS sub groups for average and current pain showed clinically significant improvements, though her “best” and “worst” pain did not show clinically meaningful improvement per the 11‐point threshold of the VAS. She subjectively reported being able to sleep, walk without limping, and sit and stand for extended periods, which she could not tolerate prior to the intervention. She continued to have pain, and DN did not eliminate her pain symptoms, but clinical meaningful improvements were demonstrated. Strength in the hip flexors, abductors, and knee flexors improved bilaterally. Although this case report was not specifically intended to assess improvement in strength as an outcome, it is hypothesized that the improvements noted were likely due to reduced pain causing reduction of poor gait mechanics, improving her ability to tolerate walking. This in turn, may have allowed her strength to normalize. Again, this is a clinical hypothesis without evidence of support. The findings of this case report preliminarily support the use of DN as an initial intervention strategy for reduction of pain related to chronic lateral hip and thigh pain in order to improve functional disability. This initial intervention strategy may then allow the therapist to employ other intervention strategies focused on strength, posture, home exercise programming/ subject education for longer term relief of this condition.
This case report uses only a single subject, as is typical of a case report. This is an inherent limitation offering only results that relate to this single subject that cannot be generalized to larger populations. Larger randomized control studies looking at DN interventions need to be performed in order to fully assess the effectiveness of DN as a primary intervention strategy for GTPS and/ or ITB etiologies. Longer assessment periods looking at long‐term benefit versus immediate or short‐term benefit also need to be assessed, as this case report showed immediate and short‐term (two month) improvements in pain and disability, but did not assess longer‐term outcomes. Further research is recommended to determine if DN is clinically beneficial independent of other therapeutic interventions, such as general or specific exercises targeting the affected musculature, or other manual therapy techniques and massage or non‐thrust mobilization.
CONCLUSIONS
DN of the lateral hip and thigh was tolerated well by this subject, who demonstrated improvements in pain and function without adverse effects. Given her reduction in pain and improvements in reported function, the use of DN for chronic lateral hip and thigh pain etiologies shows promise. Future research is needed to determine the full effectiveness of DN for lateral hip and thigh pain, as well as, to determine longer‐ term outcomes.
REFERENCES
- 1.Pfirrmann CWA Chung CB Theumann NH Trudell DJ Resnick D Greater trochanter of the hip: Attachment of the abductor mechanism and a complex of three bursae—MR Imaging and MR bursography in cadavers and MR imaging in asymptomatic volunteers. Radiology. 2001;221(2):469‐477. [DOI] [PubMed] [Google Scholar]
- 2.Sayegh F Potoupnis M Kapetanos G Greater trochanter bursitis pain syndrome in females with chronic low back pain and sciatica. Acta orthopaedica Belgica. 2004;70(5):423‐428. [PubMed] [Google Scholar]
- 3.Alvarez‐Nemegyei J Canoso JJ Evidence‐Based Soft Tissue Rheumatology: III: Trochanteric Bursitis. JCR: J of Clin Rheum. 2004;10(3):123‐124. [DOI] [PubMed] [Google Scholar]
- 4.Ellis R Hing W Reid D Iliotibial band friction syndrome—A systematic review. Man Ther. 2007;12(3):200‐208. [DOI] [PubMed] [Google Scholar]
- 5.Fairclough J Hayashi K Toumi H, et al. Is iliotibial band syndrome really a friction syndrome? J Sci Med Sport. 2007;10(2):74‐76. [DOI] [PubMed] [Google Scholar]
- 6.Grau S Krauss I Maiwald C Best R Horstmann T Hip abductor weakness is not the cause for iliotibial band syndrome. Int J Sports Med. 2008;29(7):579‐583. [DOI] [PubMed] [Google Scholar]
- 7.Lavine R Iliotibial band friction syndrome. Curr Rev Musculoskelet Med. 2010;3(1‐4):18‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niemuth PE Johnson RJ Myers MJ Thieman TJ Hip Muscle Weakness and Overuse Injuries in Recreational Runners. Clin J Sport Med. 2005;15(1):14‐21. [DOI] [PubMed] [Google Scholar]
- 9.Noehren B Davis I Hamill J ASB Clinical Biomechanics Award Winner 2006: Prospective study of the biomechanical factors associated with iliotibial band syndrome. Clin Biomech. 2007;22(9):951‐956. [DOI] [PubMed] [Google Scholar]
- 10.Segal NA Felson DT Torner JC, et al. Greater trochanteric pain syndrome: Epidemiology and associated factors. Arch Phys Med and Rehab. 2007;88(8):988‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shbeeb MI Matteson EL Trochanteric Bursitis (Greater Trochanter Pain Syndrome). Mayo Clinic Proceedings. 1996;71(6):565‐569. [DOI] [PubMed] [Google Scholar]
- 12.Sher I Umans H Downie S Tobin K Arora R Olson T Proximal iliotibial band syndrome: What is it and where is it? Skeletal Radiol. 2011;40(12):1553‐1556. [DOI] [PubMed] [Google Scholar]
- 13.Silva F Adams T Feinstein J Arroyo RA Trochanteric bursitis: Refuting the myth of inflammation. J Clin Rheum. 2008;14(2):82‐86 10.1097/RHU.1090b1013e31816b34471. [DOI] [PubMed] [Google Scholar]
- 14.Strauss EJ Nho SJ Kelly BT Greater trochanteric pain syndrome. Sports Med and Arthroscop Rev. 2010;18(2):113‐119 110.1097/JSA.1090b1013e3181e1090b1092ff. [DOI] [PubMed] [Google Scholar]
- 15.Williams BS Cohen SP Greater trochanteric pain syndrome: A review of anatomy, diagnosis and treatment. Anesth & Analges. 2009;108(5):1662‐1670 1610.1213/ane.1660b1013e31819d36562. [DOI] [PubMed] [Google Scholar]
- 16.Fryer G Hodgson L The effect of manual pressure release on myofascial trigger points in the upper trapezius muscle. J Bodywork and Mov Ther. 2005;9(4):248‐255. [Google Scholar]
- 17.Gemmell H Miller P Nordstrom H Immediate effect of ischaemic compression and trigger point pressure release on neck pain and upper trapezius trigger points: A randomised controlled trial. Clin Chiro. 2008;11(1):30‐36. [Google Scholar]
- 18.Gerwin RD Shannon S Hong C‐Z Hubbard D Gevirtz R Interrater reliability in myofascial trigger point examination. Pain. 1997;69(1–2):65‐73. [DOI] [PubMed] [Google Scholar]
- 19.Hong CZ Pathophysiology of myofascial trigger point. J Formos Med Assoc. 1996;95(2):93‐104. [PubMed] [Google Scholar]
- 20.Lucas N Macaskill P Irwig L Moran R Bogduk N Reliability of physical examination for diagnosis of myofascial trigger points: A Systematic review of the literature. Clin J of Pain. 2009;25(1):80‐89 10.1097/AJP.1090b1013e31817e31813b31816. [DOI] [PubMed] [Google Scholar]
- 21.Myburgh C Larsen AH Hartvigsen J A systematic, critical review of manual palpation for identifying myofascial trigger points: Evidence and clinical significance. Arch of Phys Med and Rehab. 2008;89(6):1169‐1176. [DOI] [PubMed] [Google Scholar]
- 22.Myburgh C Lauridsen HH Larsen AH Hartvigsen J Standardized manual palpation of myofascial trigger points in relation to neck/shoulder pain; the influence of clinical experience on inter‐examiner reproducibility. Man Ther. 2011;16(2):136‐140. [DOI] [PubMed] [Google Scholar]
- 23.Sciotti VM Mittak VL DiMarco L, et al. Clinical precision of myofascial trigger point location in the trapezius muscle. Pain. 2001;93(3):259‐266. [DOI] [PubMed] [Google Scholar]
- 24.Simons DG Clinical and etiological update of myofascial pain from trigger points. J Musculoskel Pain. 1996;4(1‐2):93‐122. [Google Scholar]
- 25.Simons DG Diagnostic criteria of myofascial pain caused by trigger points. J Musculoskel Pain. 1999;7(1‐2):111‐120. [Google Scholar]
- 26.Simons DG Travell J Myofascial trigger points, a possible explanation. Pain. 1981;10(1):106‐109. [DOI] [PubMed] [Google Scholar]
- 27.Simons DG Travell JG Myofascial origins of low back pain. 1. Principles of diagnosis and treatment. Postgrad Med. 1983;73(2):66, 68‐70, 73 passim. [DOI] [PubMed] [Google Scholar]
- 28.Tough EA White AR Richards S Campbell J Variability of criteria used to diagnose myofascial trigger point pain syndrome—Evidence from a review of the literature. The Clin J Pain. 2007;23(3):278‐286 210.1097/AJP.1090b1013e31802fda31807c. [DOI] [PubMed] [Google Scholar]
- 29.Hong C‐Z Lidocaine injection versus dry needling to myofascial trigger point: The importance of the local twitch response. Amer J Phys Med Rehab. 1994;73(4):256‐263. [DOI] [PubMed] [Google Scholar]
- 30.Boonstra AM Schiphorst Preuper HR Reneman MF Posthumus JB Stewart RE Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Internat’l J of Rehab Resear. Internationale Zeitschrift fur Rehabilitationsforschung. Revue internationale de recherches de readaptation. 2008;31(2):165‐169. [DOI] [PubMed] [Google Scholar]
- 31.Bijur PE Silver W Gallagher EJ Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8(12):1153‐1157. [DOI] [PubMed] [Google Scholar]
- 32.Hawker GA Mian S Kendzerska T French M Measures of adult pain: Visual analog scale for pain (VAS Pain), numeric rating scale for pain (NRS Pain), McGill pain questionnaire (MPQ), short‐form McGill pain questionnaire (SF‐MPQ), chronic pain grade scale (CPGS), short form‐36 bodily pain scale (SF‐36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care & Research. 2011;63(S11):S240‐S252. [DOI] [PubMed] [Google Scholar]
- 33.Binkley JM Stratford PW Lott SA Riddle DL Network TNAORR The lower extremity functional scale (LEFS): Scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371‐383. [PubMed] [Google Scholar]
- 34.Cynthia J Watson MP Jennifer Ratner David L. Zeigler Patricia Horton Susan S. Smith Reliability and responsiveness of the lower extremity functional scale and the anterior knee pain scale in patients with anterior knee pain. J Orthop Sports Phys Ther. 2005;35(3):136‐146. [DOI] [PubMed] [Google Scholar]
- 35.Teresa S.M. Yeung JW Paul Stratford Joy MacDermid Reliability, validity, and responsiveness of the lower extremity functional scale for inpatients of an orthopaedic rehabilitation ward. J Orthop Sports Phys Ther. 2009;39(6):468‐477. [DOI] [PubMed] [Google Scholar]
- 36.Holmgren U Waling K Inter‐examiner reliability of four static palpation tests used for assessing pelvic dysfunction. Man Ther.13(1):50‐56. [DOI] [PubMed] [Google Scholar]
- 37.Riddle DL Freburger JK Network NAORR Evaluation of the presence of sacroiliac joint region dysfunction using a combination of tests: A multicenter intertester reliability study. Phys Ther. 2002;82(8):772‐781. [PubMed] [Google Scholar]
- 38.Robinson HS Brox JI Robinson R Bjelland E Solem S Telje T The reliability of selected motion‐ and pain provocation tests for the sacroiliac joint. Man Ther.12(1):72‐79. [DOI] [PubMed] [Google Scholar]
- 39.Majlesi J Togay H Ünalan H Toprak S The sensitivity and specificity of the slump and the straight leg raising tests in patients with lumbar disc herniation. J Clin Rheum. 2008;14(2):87‐91 10.1097/RHU.1090b1013e31816b31812f31899. [DOI] [PubMed] [Google Scholar]
- 40.Laslett M Evidence‐based diagnosis and treatment of the painful sacroiliac joint. J Man & Manip Ther. 2008;16(3):142‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Wurff P Buijs EJ Groen GJ A multitest regimen of pain provocation tests as an aid to reduce unnecessary minimally invasive sacroiliac joint procedures. Arch Phys Med Rehab.87(1):10‐14. [DOI] [PubMed] [Google Scholar]
- 42.Mens JMA Vleeming A Snijders CJ Stam HJ Ginai AZ The active straight leg raising test and mobility of the pelvic joints. Eur Spine J. 1999;8(6):468‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunning JD DN‐1: Dry needling for craniofacial, cervicothoracic & upper extremity conditions: An evidence‐based approach. Montgomery, AL: Dry Needling Institute of the American Academy of Manipulative Therapy; 2012:256.
- 44.Langevin HM Churchill DL Cipolla MJ Mechanical signaling through connective tissue: A mechanism for the therapeutic effect of acupuncture. The FASEB Journal. 2001;15(12):2275‐2282. [DOI] [PubMed] [Google Scholar]
- 45.Langevin HM Churchill DL Fox JR Badger GJ Garra BS Krag MH Biomechanical response to acupuncture needling in humans. Vol 912001. [DOI] [PubMed] [Google Scholar]
- 46.Langevin HM Bouffard NA Badger GJ Iatridis JC Howe AK Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. Vol 2882005. [DOI] [PubMed] [Google Scholar]
- 47.Langevin HM Bouffard NA Badger GJ Churchill DL Howe AK Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: Evidence for a mechanotransduction‐based mechanism. J Cell Physiol. 2006;207(3):767‐774. [DOI] [PubMed] [Google Scholar]