Abstract
Purpose/Background:
Despite recent advances in anterior cruciate ligament reconstruction (ACL) surgical techniques, an improved understanding of the ACL’s biomechanical role, and expanding research on optimal rehabilitation practices in ACL‐reconstructed (ACLR) patients, the re‐tear rate remains alarmingly high and athletic performance deficits persist after completion of the rehabilitation course in a large percentage of patients. Significant deficits may persist in strength, muscular activation, power, postural stability, lower extremity mechanics, and psychological preparedness. Many patients may continue to demonstrate altered movement mechanics associated with increased injury risk. The purpose of this clinical commentary and literature review is to provide a summary of current evidence to assist the rehabilitation professional in recognizing, assessing, and addressing factors which may have been previously underappreciated or unrecognized as having significant influence on ACLR rehabilitation outcomes.
Methods:
A literature review was completed using PubMed, Medline, and Cochrane Database with results limited to peer‐reviewed articles published in English. 136 articles were reviewed and included in this commentary.
Conclusions:
Barriers to successful return to previous level of activity following ACLR are multifactorial.Recent research suggests that changes to the neuromuscular system, movement mechanics, psychological preparedness, and motor learning deficits may be important considerations during late stage rehabilitation.
Level of evidence:
Level 5‐ Clinical Commentary
Keywords: Anterior Cruciate Ligament (ACL), biomechanics, exercise, injury prevention, knee
INTRODUCTION
ACL injuries account for up to 50% of all sustained knee injuries with an estimated frequency of 6.5 ACL injuries per 10,000 athletic exposures and an estimated one billion dollars spent annually on ACLR in the United States.1,2 Approximately 90% of patients who seek treatment for an ACL tear undergo surgical reconstruction.3 For many of these patients, the goal is return to sport or recreational activities at their pre‐injury level. Recent evidence suggests that the outcome rates for return to sport and continued participation after return are lower than desired.4‐8 Ardern et al reported return to sport rates at 12 months after ACLR ranged from 33% to 92%.4 Multiple authors report rates of re‐tear or secondary injury to the uninvolved lower extremity following return to high level activity ranging from four to thirty three percent.9‐15 Paterno et al reported results indicating that roughly one‐third of female high school athletes suffer an injury to the contralateral lower extremity within two years of return to sport following ACLR.9 Myer et al demonstrated that resolution of specific functional deficits following ACLR was not associated with time from surgery.10 This suggests that athletes may require longer time frames to restore acceptable levels of functional performance than currently recommended. These results may also suggest shortcomings in commonly utilized postoperative rehabilitation protocols and return to sport testing criteria. Many of these protocols have focused on biomechanical and musculoskeletal factors such as knee range of motion, lower extremity strength, measures of ability to produce power as compared to the uninvolved side, and graft laxity to determine readiness for rehabilitation advancement and return to previous level of activity. The purpose of this clinical commentary and literature review is to provide a summary of current evidence to assist the rehabilitation professional in recognizing, assessing, and addressing factors which may have been previously underappreciated or unrecognized as having significant influence on ACLR rehabilitation outcomes. Evidence for the potential influence of pain, psychological variables, and neurological impact of ACL injury and ACL reconstruction will be examined. Optimal strategies to enhance motor learning, restore movement quality and minimize secondary injury risk will be discussed. The current criteria for return to sport decision making and traditional guidelines will be addressed,10 and updated considerations and emerging evidence for newer methods of assessment and interventions in late stage ACLR rehabilitation will be reviewed.
MOVEMENT ALTERATIONS AND INJURY RISK
Actual reported re‐injury rates range from 1 in 4 to 1 in 17 in ACLR patients,11‐14 with a higher incidence reported in the first two years post‐injury.15 Multiple authors have identified altered biomechanics and movement patterns in male and female ACL‐reconstructed patients when comparing the involved limb to the uninvolved.16‐25 Altered lower extremity biomechanics have also been discovered when comparing patients with ACL injuries to uninjured control subjects.26 These movement alterations have been identified during multiple sport‐specific maneuvers including single leg jumping,16,17 sagittal double leg jumping and landings,18‐21 lateral hopping,22 sidestep cutting,23,24 and jogging.25 Movement alterations may persist for time frames ranging from six months to beyond two years post‐operatively, even in some athletes cleared to return to full participation in sport.18,21 Paterno et al examined factors that predicted second ACL injury risk in ACLR patients prospectively.11 These authors identified four factors including increased knee valgus, asymmetry in internal knee extensor moment at initial contact, single leg postural stability, and opposite hip rotation moment as significant predictors of re‐injury risk. It has also been demonstrated that specific targeted neuromuscular training can improve one or more of these identified risk factors.27,28 Recently, Goerger et al were able to examine dominant limb biomechanics in a group of subjects both pre‐ACL injury and after subsequent surgical reconstruction.26 Their findings indicated that ACL injury resulted in altered movement patterns in both the involved and uninvolved lower extremities, similar to those demonstrated to be predictive of lower extremity injury. These altered movement patterns did not resolve following ACLR and subsequent rehabilitation. This suggests that changes to the traditional ACLR rehabilitation paradigm may be necessary, particularly with return to sport training and timeframes for sport clearance. It is imperative that the rehabilitation professional appreciate the biomechanical and musculoskeletal issues that may result from ACL injury as well as the potential effects on higher levels of the neuromuscular system. Targeted interventions to improve movement quality during high level sport specific tasks may need to be further explored and refined in order to lower re‐tear and secondary injury rates.
NEUROLOGICAL EFFECTS OF ACL INJURY
The effects of ACL injury on joint stability, lower extremity biomechanics, and isolated muscle group performance have been documented in the medical literature.29 Research examining the potentially detrimental neuroplastic effects of ACL injury and subsequent surgical reconstruction is much less plentiful in spite of the potential effect on function and preparedness for return to sport.
Disruption of the native ACL leads to mechanical instability of the knee, but also can alter neuromuscular control due to disruption of mechanoreceptors within the ligament.30 Disruption of these mechanoreceptors alters somatosensory signals and decreases the afferent input to the central nervous system (CNS). The resultant decrease in joint position sense and kinesthesia, along with increased nociceptor activity associated with pain and effusion potentially impairs motor control.31 Kapreli et al concluded that ACL injury can cause reorganization of the CNS and result in changes in activation patterns of sensorimotor cortical areas as compared to matched controls with intact ACL.32 These changes in neurophysiologic function are not corrected with ACLR, as the afferent pathway from the mechanoreceptors present in the native ACL cannot be reliably restored.30 This concept is further supported by the work of Baumeister et al33 in a study comparing cortical activity during knee joint angle reproduction tasks in ACLR patients and matched controls. The authors found significantly higher levels of cortical activity during movement tasks in knees status post ACLR as compared to non‐injured knees. Grooms et al34 suggested that the decreased somatosensory input available following ACL injury requires the patient to rely on visual feedback and increased conscious cortical involvement in order to effectively regulate neuromuscular control. Grooms et al34 further suggested that the visual feedback and conscious motor planning mechanisms may be efficient during simple or predictable tasks but may become overwhelmed and less efficient in the complex athletic environment leading to increased injury risk. This information suggests that the traditional rehabilitation model focused on restoration of range of motion, muscle strength and endurance, and enhanced biomechanical function during varying levels of dynamic tasks may fall short when attempting to minimize re‐injury risk. Further investigation of rehabilitation techniques that could be utilized to impact the changes in neuroplasticity and motor control may be necessary to improve outcomes following ACLR.
PAIN
Recent research also appears to support the idea that pain may alter neuromuscular function and trigger adaptations that could result in detrimental effects on long term health and physical performance.35 Hodges and Tucker35 proposed that pain may trigger neuromuscular changes due to the intent to protect the injured region of the body and minimize the experience of pain. They proposed that these adaptations may include: redistribution of activity within or between muscles and changes in mechanical behavior including stiffness or modified movement patterns. They suggested that these changes occur at multiple levels of the nervous system and may be additive, complementary, or competitive.35 While these changes may provide the intended benefit of short term relief of pain, they may also result in decreased movement range, decreased movement variability, and increased load in specific regions of the knee joint. This may have long term implications on knee health, re‐injury risk, and athletic performance. It is not currently clear whether these adaptations are a result of pain suffered at the time of initial ACL injury, post‐operative pain, or the summative effects of both.
In support of this theory, Tucker et al36 found that motor unit discharge of the quadriceps was negatively affected not only by the presence of pain, but also the anticipation of pain. More importantly, changes in motor unit discharge continued regardless of whether pain was present or not. Hug et al found inter‐muscular changes in response to pain within the quadriceps muscle group suggesting adaptive differences that could be attributable to changes in role or function of individual muscles or neurophysiological differences or constraints.37 These changes may have significant rehabilitation implications in an ACLR population.
ALTERATIONS IN SPECIFIC MUSCLE FUNCTION
Pain and other factors may hinder optimal knee and lower extremity muscular performance in an ACLR population. Thomas et al found that residual weakness persisted post‐operatively in the knee extensors and knee flexors of ALCR subjects, while hip extensors, hip adductors, and ankle plantar flexors fully recovered to preoperative levels.38 These authors did not evaluate the effect of ACLR on strength in hip abductors and hip external rotators.
Multiple authors have identified weakness in the hip and core muscle groups as a predictor of lower extremity injury risk.39‐42 Proximal lower extremity muscle function has demonstrated a significant effect on lower extremity mechanics39,43 and weakness in these groups have been identified in other common lower extremity pathologies including patellofemoral pain syndrome,44‐46 iliotibial band syndrome,47 and ankle sprain.48 The link between proximal hip weakness and lower extremity pathology supports the concept of regional interdependence.
Core musculature strength has not been well studied in regards to an ACLR population, however, deficits in core proprioception and neuromuscular control have been found to be predictive of knee injury risk.49,50 Noehren et al examined female athletes who had undergone ACLR and did not find differences in hip abduction or external rotation strength, but did find significant differences in trunk neuromuscular control when compared to healthy, uninjured subjects.51 There are few current studies that have examined changes in hip and core muscle activation patterns in the presence of ACL injury or resultant surgical reconstruction. It is possible that selective proximal hip and core weakness or activation differences may be present pre and/or post‐ACLR and may have an influence on movement mechanics and function.52
Return of hamstring strength and torque following ACLR has been largely studied with respect to hamstring graft harvest and subsequent tendon regeneration and morphology. Multiple studies have identified hamstring weakness following ACLR, specifically at higher knee flexion angles.53‐57 However, few studies have examined potential neuromotor influence on hamstring weakness and activation following ACLR. Ristanis et al identified an electromechanical delay in hamstring activation following ACLR with hamstring graft harvest.58 Briem et al demonstrated altered inter‐limb hamstring activation patterns that also differed from healthy controls.59 ACLR subjects who had a hamstring graft demonstrated increased lateral hamstring activity versus medial hamstring activity.59 Arnason et al also found significant alterations in lateral and medial hamstring activation between lower extremities in ACLR subjects with hamstring autograft during hamstring exercise.60 Increased medial hamstring activation may limit knee valgus and subsequent ACL loading.61,62 Zebis et al demonstrated that decreased semitendinosus pre‐activation with cutting was a predictor for increased risk of noncontact ACL injury.63 The alterations in hamstrings neuromotor activity have been shown to contribute to modified lower extremity mechanics in ACLR subjects.64‐66 Targeted neuromuscular training is able to modify medial hamstring activity, and subsequently may impact knee valgus positioning during sport activity.67
Extensive research has examined influence of ACLR on quadriceps function. Multiple studies have identified persistent knee extensor or quadriceps muscle weakness and/or activation deficits in early and late postoperative periods.68‐73 In the early postoperative period, increased knee effusion is often present and has been shown to cause quadriceps inhibition along with changes in afferent feedback.74‐77 Lynch et al recently determined that knee effusion did not directly mediate quadriceps inhibition after initial ACL injury.78 They determined that arthrogenic muscle inhibition was present at the quadriceps bilaterally after ACL injury and theorized that pain, inflammation, and or inactivity may contribute to these deficits. These bilateral activation deficits are suggestive of more complex central nervous system involvement versus locally mediated neurologic responses at the knee.
Recently several authors have suggested that neuromotor deficits occur at higher central nervous system levels in ACLR patients.79‐81 Changes in quadriceps muscle mechanics and subsequent weakness were observed after ACLR versus the uninvolved lower extremity suggesting changes at a local quadriceps muscular level, but possibly also at a neuromotor level.79 These changes included decreased strength at more lengthened positions in higher knee flexion angles and at slower speeds with isokinetic and isometric testing.79 Kuenze et al found that ACLR patients demonstrated significant deficits in cortical excitability, quadriceps strength, and quadriceps activation in the surgical limb when compared to both the uninvolved limb and matched healthy controls.80 These cortical excitability deficits persisted beyond six months post‐operatively and extended into the return to recreational activity. In a similar study, Lepley et al determined that decreased spinal‐reflexive and corticospinal excitability was present preoperatively, two weeks post‐operatively, and six months post‐operatively.72 These studies documented bilateral quadriceps weakness and activation deficits, including decreased central activation ratio.80 Bilateral deficits in central activation ratio in ACLR subjects has also been linked to poor return of quadriceps activation and strength.81 It appears that current published ACLR rehabilitation model paradigms are not adequately addressing these neuromotor deficits based on current research. Tracking values such as central activation ratio may provide a more valuable future estimation of return of strength and activation to assist in return to sports decision‐making and rehabilitation progressions for ACLR athletes. Recently, Kuenze et al studied these values and determined that quadriceps central activation ratio above 89.3% was the strongest unilateral indicator at the involved leg of healthy‐knee related outcomes determined by pain, knee‐related function, and physical activity level.82 Schmitt et al demonstrated quadriceps index (QI) of less than 85% in comparison to the uninvolved lower extremity in ACLR subjects was predictive of poor functional hop test performance while scores greater than 90% were comparable to uninjured subjects.83 Similarly Schmitt et al found that quadriceps weakness (QI < 85%) was related to altered lower extremity landing mechanics and forces, while ACLR subjects with QI > 90% demonstrated mechanics similar to uninjured subjects.84 The group with QI < 85% demonstrated increased peak vertical ground reaction force and peak loading rate at the uninvolved limb.84 The profound effect on landing mechanics is significant given the evidence linking alterations in movement mechanics in ACLR patients to re‐injury risk, performance deficits, and increases in joint reactive forces.26
Rate of force development has been characterized as a measure of explosive muscle action and neural drive.85 Reduced rate of force development of specific muscles following ACLR may have similar effects on athletic performance as muscle weakness. Angelozzi et al found that at six months after ACLR maximum voluntary isometric contraction levels in the involved leg had returned to 97% of preinjury values.85 However, decreased rate of force development persisted at the affected lower extremity and did not near preinjury levels until twelve months post‐operatively. Knezevic et al also found deficits in rate of force development and maximal strength at the quadriceps and hamstrings between the involved and the uninvolved lower extremity in ACLR subjects at six months post‐operatively.86
The rate of force development is an important factor in athletics due to the need to accelerate, decelerate, and change direction. For this reason, the results of these studies have potentially significant implications in return to sport time frames and overall athletic performance. The authors of these studies did not offer a hypothesis for the continued deficits in rate of force development, but their results suggest that neuromuscular function of the involved lower extremity may remain impaired well into the late post‐operative rehabilitation course.
FATIGUE
In addition to changes in neuromuscular activation, multiple studies have identified the detrimental effects of fatigue on the involved and/or uninvolved lower extremity.87‐91 Fatigue has been reported to have a negative effect on postural stability, neuromuscular control, and lower extremity mechanics during sport activity or components of sport performance in ACLR subjects.91‐94 Deficits in postural stability, increased knee valgus, and increased opposite hip internal rotation moment of the lower extremity are correlated to increased ACL re‐injury risk.11 McLean and Samorezov noted a crossover effect on lower extremity mechanics from the involved lower extremity to uninvolved contralateral lower extremity after a fatigue protocol.87 Decreased knee flexion angle at intial contact was observed, as well as increased knee abduction and hip internal rotation at peak stance during single leg jumps.87 The crossover effect on the uninvolved lower extremity may have added significance given the results of a recent study which found a twenty percent injury rate in females who had undergone ACLR the contralateral lower extremity.9 This crossover effect also reinforces the concept of higher level central nervous system control and processing of lower extremity mechanics, which as noted previously appear to be impacted by ACL injury and/or reconstruction. The effects of fatigue may be more pronounced in ACLR subjects. Fatigue should be considered in late stage rehabilitation program design to ensure that ACLR subjects are able to maintain consistent movement mechanics under fatigued conditions. Exercise dosage and training intensity must be sufficient to reach fatigue thresholds encountered under sport conditions. Augustsson et al performed a study on functional hop testing in ACLR subjects and found that two thirds of subjects who had initially passed with greater than 90% limb symmetry index scores were unable to pass following a fatigue protocol for each lower extremity.75 Additionally, in this study lower extremity mechanics during testing were significantly negatively impacted by the fatigue protocol. This suggests that return to sport testing in a fatigued state may be beneficial to ensure that athletes are not cleared for return to play prematurely. The ability to maintain consistent movement quality and mechanics to avoid at‐risk postures for ACL re‐injury in the presence of fatigue is critical in rehabilitation planning and limiting injury risk in ACLR patients.
PSYCHOLOGY AND INFLUENCE ON RETURN TO SPORT FOLLOWING ACLR
Recent research has highlighted an enhanced understanding of psychological influence on the ability of ACLR patients to fully return to sport and restore performance to preinjury levels. Ardern et al showed that preoperative psychological responses were associated with likelihood of returning to preinjury levels 12 months after reconstruction.4 This may suggest that the role of psychology in the rehabilitation process has been underappreciated and further research may be warranted in this area.4 The most common reason cited in failure to return to sport is fear of reinjury.95 This fear of reinjury may manifest in the form of negative behaviors impacting sport performance including hesitation, giving less than maximal effort, and excessive protection of the affected body part during competition.96 Fear of reinjury or pain may also alter optimal motor function in the form of alterations in muscle tone, firing patterns, or sequential activation.97‐99 There is evidence that motor control alterations in response to pain or musculoskeletal injury may persist despite the resolution of pain and symptoms.99 Acclimating the ACLR patient to the anticipated sport or recreational demands under controlled conditions may improve comfort level and confidence with these tasks in order to reduce or limit the negative impact of fear behaviors. Ardern et al reported that the prospective judgment ACLR patients made about their ability to return to sport included their own experience and attitudes as well as advice of health care professionals.4 This suggests the ability of rehabilitation providers to have a profound effect on the psychological attitudes of these patients through education, patient interactions, and customized neuromuscular training interventions that incorporate functional specificity during rehabilitation. Authors report significant efficacy and improvements in pain and fear of reinjury using education and in vivo exposure therapy for musculoskeletal conditions.99‐104 While these studies are not specific to ACLR patients, they may be useful in developing rehabilitation strategies to address fear beliefs and kinesiophobia. Abbott et al also demonstrated superior effectiveness of post‐operative rehabilitation incorporating psychomotor therapy consisting of cognition, behavior, and motor relearning versus exercise alone in patients recovering from lumbar fusion.105 De Jong et al hypothesized that graded in vivo exposure may also activate cortical networks and reconcile motor output and sensory feedback that may be altered due to pain and fear of reinjury in patients with Complex Regional Pain Syndrome (CRPS).106 Providers working with ACLR patients may provide exposure to a variety of advanced sport‐specific movements during late stage rehabilitation to potentially improve psychological and neuromotor benefits. Early recognition of ACLR patients demonstrating evidence of the psychological variables linked to less than optimal outcomes appears critical to improving overall rehabilitation success. Patients exhibiting these behaviors may benefit from consultation with a sports psychologist to remediate limiting factors and minimize re‐injury risk. Many rehabilitation professionals lack the necessary training and psychology background to accurately identify patients demonstrating behaviors that are potentially detrimental to outcomes. Additionally, few peer‐reviewed and researched post‐operative ACLR protocols include screening tools to assist rehabilitation professionals in identifying these behaviors. Ardern et al recently utilized the ACL‐Return to Sport after Injury (ACL‐RSI) as a screening tool to determine psychological readiness to return to sport and recreational activity.107 The authors correlated higher ACL‐RSI scores with return to sports participation at pre‐injury level. Chmielewski et al also demonstrated an association between scores on the Tampa Scale of Kinesiophobia (TSK) and function in the late stage rehabilitation period following ACLR from 6‐12 months postoperatively.108 Higher TSK scores indicate greater pain‐related fear of movement or reinjury. Pain‐avoidance and fear‐avoidance psychological factors have been demonstrated in an ACLR postoperative population as well. Lentz et al found that those who did not return to preinjury level of sports participation following ACLR because of fear of reinjury or lack of confidence demonstrated higher pain‐related fear of movement.109 This supports the possibility that pain may alter neuromuscular function and pain avoidance behavior may have psychological implications that negatively impact rehabilitation. Rehabilitation professionals may find the above mentioned questionnaires or similar assessment tools useful to gauge psychological preparedness and aid in return to sport decision making, as well as to identify ACLR patients that may require additional interventions to enhance outcomes.
INTERVENTIONS
It has been well documented that deficits in range of motion110‐112 and strength69,71,83 can negatively affect lower extremity performance and functional outcome following ACLR. For this reason, restoration of acceptable levels of ROM, strength, and biomechanics are essential components of a well designed rehabilitation program. Unfortunately, inclusion of these key components does not always correlate with successful return to previous level of activity. Traditional rehabilitation programs have produced variable results with respect to restoring symmetrical lower extremity muscle strength and activation, postural stability, and symmetrical movement mechanics. The traditional rehabilitation model has produced less than optimal success rates for return to athletic performance at preinjury levels. This suggests changes may be needed to the traditional ACLR rehabilitation paradigm to ensure improved patient outcomes. The resultant neuromotor effects of ACL injury and reconstruction are increasingly recognized but may not be properly resolved with traditional ACLR rehabilitation protocols, necessitating a change and willingness to adapt current rehabilitation practices. Emerging evidence‐based rehabilitation strategies and concepts should be considered to remediate neuromotor changes.
Plyometric training is a mainstay in the mid and late rehabilitation phases of traditional ACLR rehabilitation protocols. Implementation of plyometrics is critical because early rehabilitation may not adequately simulate the forces required by full athletic participation and competition.113 ACLR patients who are not adequately prepared to accept and tolerate these forces and avoid at‐risk postures and mechanics are more susceptible to re‐injury.11 Plyometric training is often incorporated in conjunction with neuromuscular retraining with feedback to allow integration of improved lower extremity mechanics and simulate components of sports specific maneuvers.114 Plyometrics are an area where type of cuing and adequate supervision of movement mechanics may take on added importance due to the increased neuromuscular demand and higher loads and forces placed on the lower extremities.113
A growing body of evidence has emerged regarding the types of cues and feedback employed during plyometric exercise and sport specific movement training. Recent studies115‐117 have suggested that specific feedback methods may enhance motor learning and may be more efficacious in restoration of safe movement patterns than the methods employed during traditional rehabilitation activities. Feedback application of varying types and volume may significantly influence motor control and neuromuscular re‐education.115 The use of oral and video feedback has been shown to improve frontal plane lower extremity biomechanics during jumping tasks, improve strength, and decrease vertical ground reaction force.117 The use of combined verbal, visual, and tactile feedback has also allowed functional carryover of lower extremity biomechanics across multiple functional weight bearing tasks.114
Recent research has focused on the effects of externally and internally directed cues and their respective impact on movement mechanics and motor learning. The type and method of cuing and feedback offered to ACLR subjects may have a significant effect on landing mechanics, lower extremity symmetry, and postural stability. Physical therapists provide feedback inducing internally directed focus up to 95% of the time.116 Examples of internally directed cues may include instructing patients to land with flexed knees or to land with feet shoulder width apart.116 Recent evidence indicates that while use of internally directed cues may be more prevalent, it may actually limit potential for motor learning and full recovery following ACLR because it causes the patient to rely on more conscious versus automatic control at a central nervous system level.116 It has been suggested that cues involving externally directed focus may promote use of more unconscious or automatic mechanisms that may improve motor learning efficacy.116 Using external cues and targets such as cones, bars, or foot markers may allow patients to direct focus externally to improve quality of squatting, jumping, and sport‐specific movements (Figures 1A and 1B).116 Feedback wording and supplied images with external cues describing technique such as land “light as a feather”, “like a spring” and “shock absorber” were also found to improve landing mechanics in both healthy and ACLR subjects.116 Improvements in jump distance and jump height during plyometric activities and training were observed using external attentional focus compared to internal attentional focus (Figures 2A and 2B).118‐120 Multiple authors support improvements in force and athletic performance using externally directed attentional focus versus internally directed focus.119‐121 Augmented verbal feedback coupled with plyometric training has also been demonstrated to increase power output in trained athletes during jumping tasks.118‐120 Although it has not been studied specifically in an ACLR population, the enhanced benefits of external attentional focus during plyometric training may allow improved athletic performance and improved overall outcomes in ACLR subjects based on existing information from healthy subjects. Gokeler et al also found improvements in movement mechanics during single leg hopping in ACLR subjects using external attentional focus versus internal focus.121 Real time feedback is another means of incorporating external attentional focus. Real time visual feedback training utilizing mirrors and virtual reality images have recently been studied as a means of effectively altering neuromotor function and processing during both training and testing.117,122‐124 Investigators used real time feedback training and found improvements in knee abduction load, knee flexion angle, and trunk postures found to correlate with ACL injury risk.117,121,122,124 However, a recent study comparing real time feedback training with traditional post‐session visual or verbal feedback showed no added benefit to real time biofeedback versus traditional means in improving sagittal or frontal plane kinematics.123 The lack of consensus on the effectiveness of real time feedback training coupled with the cost and limited availability of the equipment needed to implement it are areas of concern for practical use in the clinical setting.
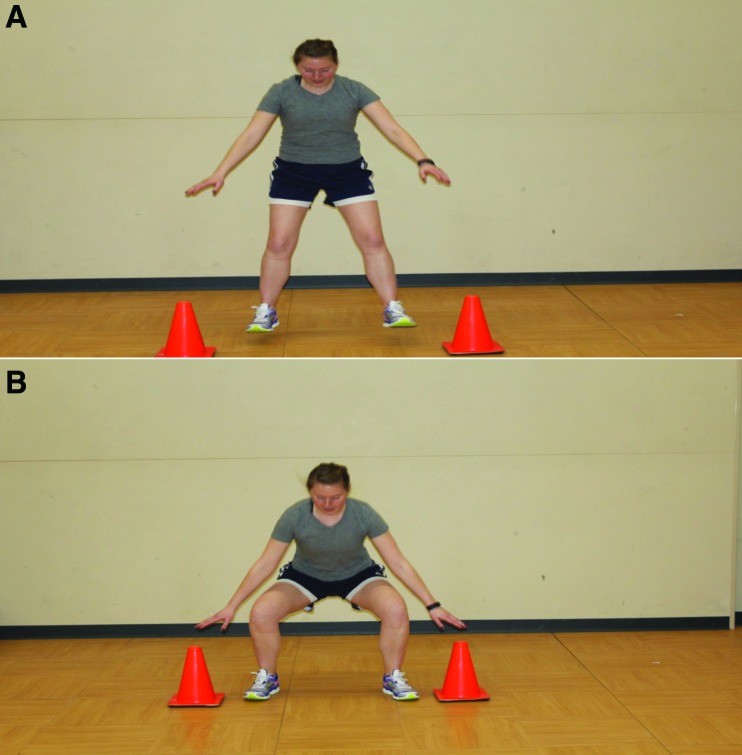
Figures 1A and 1B.
Cones or similar objects may be utilized to facilitate improved lower extremity mechanics with jumping (Figure 1A) and landing/squatting (Figure 1B). Use of these external cues in conjunction with visual and verbal feedback will facilitate increased hip and knee flexion to encourage improved force absorption at the lower extremities and increased hamstring activation.
Figures 2A and 2B.
CImprovements in jump height and jump distance may be observed during plyometric training using external attentional focus on objects such as cones, targets, etc. placed at increased distance.
One recently developed theoretical construct was proposed by Grooms et al34 in order to incorporate specific visual motor rehabilitation approaches in conjunction with neuromuscular training to affect some of the documented neuromotor changes that occur following ACL injury and ACLR as discussed in this clinical commentary. This theoretical construct proposed that the detrimental neuroplastic changes that occur following ACL injury and ACLR to efferent and afferent input, lead to a compensatory overreliance on the visual motor systems to provide adequate neuromuscular control and function at the involved knee.34 They proposed that this over reliance on visual motor input may actually lead to reinforcement of the detrimental top‐down cortical pathways discussed previously, focused on internal cuing and more conscious nervous system regulation versus externally focused pathways that utilize more optimal autonomic regulation of neuromotor feedback and control.34 This is an interesting contrast to the theory employed during real time feedback training, where visual input was used as a means to enhance neuromuscular function. Grooms et al34 proposed that limiting visual input through the use of stroboscopic eyewear, computer or web‐based applications to address visual processing, adding competing environmental stimuli (i.e. targets, reaction balls, etc.) (Figures 3A and 3B), or simply closing eyes during functional tasks and neuromuscular training in rehabilitation may help de‐emphasize this over reliance on visual motor input. The authors of this approach emphasized that this is a hypothetical construct which currently lacks substantial supporting evidence. However, there is some evidence to support this premise. Swanik et al found that athletes that suffered non‐contact ACL injuries had slower visual processing scores on neurocognitive testing versus healthy controls.126
Figures 3A and 3B.
The addition of competing environmental stimuli such as targets, balls, etc. during functional neuromuscular training may limit the overreliance on visual motor input for dynamic knee stability during sport‐specific tasks.
CONCLUSIONS
Successful return to sport following ACLR is likely affected by multiple factors. The biomechanical risk factors for ACL injury have well been studied and documented.127 Strategies to avoid ACL injury128 and re‐injury29 have been developed and examined. The disturbing rate of re‐injury and unsatisfactory outcomes reported by a significant number of patients indicates that there may be barriers to optimal performance that need to be better recognized and addressed.
Research has shown that strength training in isolation does not guarantee improvements in hip and knee kinematics and that improved lower extremity strength does not guarantee improved landing technique.129 This suggests that while deficits in strength and activation ratios are serious factors that contribute to re‐injury risk and sub‐optimal performance, there are likely other contributing factors that may not be remediated with strength training alone. Additional neuromuscular retraining to simulate the specific movement patterns and environmental stimuli the athlete will encounter during sport may be required in conjunction with traditional strength training in order to achieve optimal movement quality and biomechanics. The evolution of targeted neuromuscular training programs to address biomechanical deficits in at risk athletes has been a significant development in functional rehabilitation over the last several years.28 In spite of this, the likelihood that up to one‐third of athletes will re‐tear the surgically reconstructed ACL or tear the ACL on the contralateral side implies that the improvements in biomechanics demonstrated in the rehabilitation setting do not reliably carry over to the playing field.9,14,15 For this reason, it is imperative that the rehabilitation professional continue to explore other factors that may negatively affect motor control and develop strategies to enhance outcomes.
Recent research examining the correlation between psychological preparedness and successful return to sport4,107‐109 indicate that this is an area that may need to be addressed to improve outcomes. Based on the current evidence, it may be of significant benefit to the patient to utilize questionnaires or other measures to gauge psychological preparedness for discharge in the same manner that knee‐specific patient reported outcome tools (like the International Knee Documentation Committee, Lysholm, Tegner, and Lower Extremity Functional Scale) are utilized to assess knee function and outcomes. Athletes who present with concerns in the psychological realm must be referred to a qualified provider to address these issues.
Testing to assess movement quality in both basic and sport specific patterns is an essential component of the return to sport decision making process. Assessment tools like the Functional Movement ScreenTM and Y Balance TestTM have recently been validated in the literature as effective methods to assess injury risk.130‐133 Metrics like these that assess competency in gross movement patterns may be valuable in determining readiness to return to the field. The FMSTM and lower quarter version of the Y Balance TestTM were recently studied in an adolescent population after an ACLR, and have been implemented to determine prospective injury risk and movement quality for safe return to sport.134 It is also essential that movement quality is assessed during dynamic activities that closely resemble sport requirement with regards to speed and force development. Significant work has been done to identify and target movement patterns and biomechanical flaws that increase injury risk during sport specific tasks like jumping, hopping, landing, and changing direction.128,135,136 Neuromuscular training philosophy has evolved and improved based on the work of these authors. These techniques will likely be further enhanced with greater understanding of optimal strategies to improve motor learning. This, coupled with a better understanding of the neuroplastic effect of ACL injury and the impact both the injury and subsequent surgery have on higher cortical areas will hopefully provide clinicians with valuable insight and more effective means to improve motor control and minimize injury risk. Finally, existing research regarding muscle activation and the effects of fatigue on function and performance suggest that it is essential that movement training and movement testing in a fatigued state be included in the rehabilitation program.67,71,85,91,92 It is important that the rehabilitation professional assess the athlete’s ability to perform in situations that most closely simulate the competition environment. This includes varying levels of stress, fatigue, and external stimuli. Rehabilitation programs have continuously improved over the past several years as evidence has emerged regarding biomechanical and neuromuscular risk factors for injury or reinjury. These programs will likely continue to evolve and become more efficient at minimizing injury risk as rehabilitation professionals continue to examine the intricate relationships between the various systems of the human body. Further research is necessary to optimally target deficits in neuromuscular control, neuromotor status, and psychological readiness to best prepare athletes for a return to the playing field following ACL injury.
REFERENCES
- 1.Risberg M A Lewek M Synder‐Mackler L A systematic review of evidence for anterior cruciate ligament rehabilitation: how much and what type? Phys Ther in Sport. 2004; 5(3): 125‐145. [Google Scholar]
- 2.Joseph AM Collins CL Henke NM, et al. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J Athl Train. 2013; 48(6):810‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linko E Harilainen A Malmivaara A, et al. Surgical versus conservative interventions for anterior cruciate ligament ruptures in adults. Cochrane Database Syst Rev. 2005; 18;(2):CD001356. [DOI] [PubMed] [Google Scholar]
- 4.Ardern CL Taylor NF Feller JA, et al. Psychological responses matter in returning to preinjury level of sport after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2013; 41(7):1549‐58. [DOI] [PubMed] [Google Scholar]
- 5.Brophy RH Schmitz L Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012; 40(11):2517‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langford JL Webster KE Feller JA A prospective longitudinal study to assess psychological changes following anterior cruciate ligament reconstruction surgery. Br J Sports Med 2009; 43:377‐378. [DOI] [PubMed] [Google Scholar]
- 7.McCullough KA Phelps KD Spindler K et al. Return to high school‐ and college‐level football after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Am J Sports Med. 2012; 40(11):2523‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakayama Y Shirai Y Narita T, et al. Knee functions and a return to sports activity in competitive athletes following anterior cruciate ligament reconstruction. J Nippon Med Sch. 2000; 67(3):172‐6. [DOI] [PubMed] [Google Scholar]
- 9.Paterno MV Rauh MJ Schmitt LC, et al. Incidence of Second ACL Injuries 2 Years After Primary ACL Reconstruction and Return to Sport. Am J Sports Med. 2014; 42(7):1567‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myer GD Martin L Jr Ford KR, et al. No association of time from surgery with functional deficits in athletes after anterior cruciate ligament reconstruction: evidence for objective return‐to‐sport criteria. Am J Sports Med. 2012; 40(10):2256‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paterno MV Schmitt LC Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010; 38(10):1968‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinczewski LA Lyman J Salmon LJ, et al. A 10‐year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007; 35(4):564‐74. [DOI] [PubMed] [Google Scholar]
- 13.Salmon L Russell V Musgrove T, et al. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005; 21(8):948‐57. [DOI] [PubMed] [Google Scholar]
- 14.Shelbourne KD Gray T Haro M Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009; 37(2):246‐51. [DOI] [PubMed] [Google Scholar]
- 15.Wright RW Dunn WR Amendola A, et al. Risk of tearing the intact anterior cruciate ligament in the contralateral knee and rupturing the anterior cruciate ligament graft during the first 2 years after anterior cruciate ligament reconstruction: a prospective MOON cohort study. Am J Sports Med. 2007; 35(7):1131‐4. [DOI] [PubMed] [Google Scholar]
- 16.de Fontenay BP Argaud S Blache Y, et al. Motion alterations after anterior cruciate ligament reconstruction: comparison of the injured and uninjured lower limbs during a single‐legged jump. J Athl Train. 2014; 49(3):311‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orishimo KF Kremenic IJ Mullaney MJ, et al. Adaptations in single‐leg hop biomechanics following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010; 18(11):1587‐93. [DOI] [PubMed] [Google Scholar]
- 18.Castanharo R da Luz BS Bitar AC, et al. Males still have limb asymmetries in multijoint movement tasks more than 2 years following anterior cruciate ligament reconstruction. J Orthop Sci. 2011; 6(5):531‐5. [DOI] [PubMed] [Google Scholar]
- 19.Decker MJ Torry MR Noonan TJ, et al. Landing adaptations after ACL reconstruction. Med Sci Sports Exerc. 2002;34(9):1408‐13. [DOI] [PubMed] [Google Scholar]
- 20.Ernst GP Saliba E Diduch DR, et al. Lower extremity compensations following anterior cruciate ligament reconstruction. Phys Ther. 2000; 80(3):251‐60. [PubMed] [Google Scholar]
- 21.Paterno MV Ford KR Myer GD, et al. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007; 17(4):258‐62. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz A Olson S Trudelle‐Jackson E, et al. Landing mechanics during side hopping and crossover hopping maneuvers in noninjured women and women with anterior cruciate ligament reconstruction. PM R. 2011; 3(1):13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SP Chow JW Tillman MD Persons with reconstructed ACL exhibit altered knee mechanics during high‐speed maneuvers. Int J Sports Med. 2014; 35(6):528‐33. [DOI] [PubMed] [Google Scholar]
- 24.Stearns KM Pollard CD Abnormal frontal plane knee mechanics during sidestep cutting in female soccer athletes after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2013; 41(4):918‐23. [DOI] [PubMed] [Google Scholar]
- 25.Kuenze C Hertel J Weltman A, et al. Jogging biomechanics after exercise in individuals with ACL‐reconstructed knees. Med Sci Sports Exerc. 2014; 46(6):1067‐76. [DOI] [PubMed] [Google Scholar]
- 26.Goerger BM Marshall SW Beutler AI, et al. Anterior cruciate ligament injury alters preinjury lower extremity biomechanics in the injured and uninjured leg: the JUMP‐ACL study. Br J Sports Med. 2014; Feb 21. doi: 10.1136/bjsports‐2013092982. [DOI] [PubMed] [Google Scholar]
- 27.Myer GD Ford KR McLean SG, et al. The effects of plyometric versus dynamic stabilization and balance training on lower extremity biomechanics. Am J Sports Med. 2006; 34(3):445‐55. [DOI] [PubMed] [Google Scholar]
- 28.Myer GD Ford KR Palumbo JP, et al. Neuromuscular training improves performance and lower‐extremity biomechanics in female athletes. J Strength Cond Res. 2005; 19(1):51‐60. [DOI] [PubMed] [Google Scholar]
- 29.Di Stasi S Myer GD Hewett TE Neuromuscular training to target deficits associated with second anterior cruciate ligament injury. J Ortho Sports Phys Ther. 2013; 43(11):777‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhillon MS Kamal B Sharad P Differences among mechanoreceptors in healthy anterior cruciate ligaments and their clinical importance. Muscles Ligaments Tendons J. 2012; 2(1):38‐43. [PMC free article] [PubMed] [Google Scholar]
- 31.Kapreli E Athanasopoulos S The anterior cruciate ligament deficiency as a model of brain plasticity. Med Hypotheses. 2006;67(3):645‐650. [DOI] [PubMed] [Google Scholar]
- 32.Kapreli E Athanasopoulos S Gliatis J, et al. Anterior cruciate ligament deficiency causes brain plasticity: a functional MRI study. Am J Sports Med. 2009;37(12):2419‐2426. [DOI] [PubMed] [Google Scholar]
- 33.Baumeister J Reinecke K Schubert M, et al. Altered Electrocortical Brain Activity after ACL Reconstruction during Force Control. Journal of Orthopaedic Research. 2011;29(9):1383‐1389. [DOI] [PubMed] [Google Scholar]
- 34.Grooms D Appelbaum G Onate J Neuroplasticity following anterior cruciate ligament injury: a framework for visual‐motor training approaches in rehabilitation. J Orthop Sports Phys Ther. 2015;10(1):1‐33. [DOI] [PubMed] [Google Scholar]
- 35.Hodges PW Tucker K Moving differently in pain: a new theory to explain the adaptation to pain. Pain. 2011; 152(3 Suppl):S90‐8. [DOI] [PubMed] [Google Scholar]
- 36.Tucker K Larsson AK Oknelid S, et al. Similar alteration of motor unit recruitment strategies during the anticipation and experience of pain. Pain 2012; 153(3):636‐43. [DOI] [PubMed] [Google Scholar]
- 37.Hug F Hodges PW van den Hoorn W, et al. Between muscle differences in the adaptation of experimental pain. J Appl Physiol. 2014; 117(10):1132‐40. [DOI] [PubMed] [Google Scholar]
- 38.Thomas AC Villwock M Wojtys EM, et al. Lower extremity muscle strength after anterior cruciate ligament injury and reconstruction. J Athl Train. 2013; 48(5):610‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powers CM The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010; 40(2):42‐51. [DOI] [PubMed] [Google Scholar]
- 40.Davis IS Powers CM Patellofemoral pain syndrome: proximal distal, and local factors, an international retreat, April 30‐May 2, 2009, Fells Point, Baltimore, MD. J Orthop Sports Phys Ther. 2010; 40(3):A1‐16. [DOI] [PubMed] [Google Scholar]
- 41.Leetun DT Ireland ML Willson JD, et al. Core stability measures as risk factors for lower extremity injury in athletes. Med Sci Sports Exerc. 2004; 36(6):926‐34. [DOI] [PubMed] [Google Scholar]
- 42.Ireland ML Willson JD Ballantyne BT et al. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther. 2003; 33(11):671‐6. [DOI] [PubMed] [Google Scholar]
- 43.Reiman MP Bolgla LA Lorenz D Hip functions influence on knee dysfunction: a proximal link to a distal problem. J Sport Rehabil. 2009; 8(1):33‐46. [DOI] [PubMed] [Google Scholar]
- 44.Finnoff JT Hall MM Kyle K, et al. Hip strength and knee pain in high school runners: a prospective study. PM R. 2011; 3(9):792‐801. [DOI] [PubMed] [Google Scholar]
- 45.Prins MR van der Wurff P Females with patellofemoral pain syndrome have weak hip muscles: a systematic review. Aust J Physiother. 2009; 55(1):9‐15. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa TH Moriya ET Maciel CD, et al. Trunk, pelvis, hip, and knee kinematics, hip strength, and gluteal muscle activation during a single‐leg squat in males and females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2012; 42(6):491‐501. [DOI] [PubMed] [Google Scholar]
- 47.Fredericson M Cookingham CL Chaudhari AM, et al. Hip abductor weakness in distance runners with iliotibial band syndrome. Clin J Sport Med. 2000; 10(3):169‐75. [DOI] [PubMed] [Google Scholar]
- 48.Friel K McLean N Myers C, et al. Ipsilateral hip abductor weakness after inversion ankle sprain. J Athl Train. 2006; 41(1):74‐8. [PMC free article] [PubMed] [Google Scholar]
- 49.Zazulak BT Hewett TE Reeves NP, et al. Deficits in neuromuscular control of the trunk predict knee injury risk: a prospective biomechanical‐epidemiologic study. Am J Sports Med. 2007; 35(7):1123‐30. [DOI] [PubMed] [Google Scholar]
- 50.Zazulak BT Hewett TE Reeves NP, et al. The effects of core proprioception on knee injury: a prospective biomechanical‐epidemiological study. Am J Sports Med. 2007; 35(3):368‐73. [DOI] [PubMed] [Google Scholar]
- 51.Noehren B Abraham A Curry M, et al. Evaluation of proximal joint kinematics and muscle strength following ACL reconstruction surgery in female athletes. J Orthop Res. 2014; 32(10):1305‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank B Bell DR Norcross MF, et al. Trunk and hip biomechanics influence anterior cruciate loading mechanisms in physically active participants. Am J Sports Med. 2013; 41(11):2676‐83. [DOI] [PubMed] [Google Scholar]
- 53.Nomura Y Kuramochi R Fukubayashi T Evaluation of hamstring muscle strength and morphology after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2014. Mar 20. doi: 10.1111/sms.12205. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Ardern CL Webster KE Taylor NF, et al. Hamstring strength recovery after hamstring tendon harvest for anterior cruciate ligament reconstruction: a comparison between graft types. Arthroscopy. 2010; 26(4):462‐9. [DOI] [PubMed] [Google Scholar]
- 55.Makihara Y Nishino A Fukubayashi T, et al. Decrease of knee flexion torque in patients with ACL reconstruction: combined analysis of the architecture and function of the knee flexor muscles. Knee Surg Sports Traumatol Arthrosc. 2006; 14(4):310‐7. [DOI] [PubMed] [Google Scholar]
- 56.Vairo GL Knee flexor strength and endurance profiles after ipsilateral hamstring tendons anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. 2014; 95(3):552‐61. [DOI] [PubMed] [Google Scholar]
- 57.Emami Meybodi MK Jannesari M Rahim Nia A, et al. Knee Flexion Strength Before and After ACL Reconstruction Using Hamstring Tendon Autografts. Trauma Mon. 2013; 18(3):130‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ristanis S Tsepis E Giotis D, et al. Electromechanical delay of the knee flexor muscles is impaired after harvesting hamstring tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 2009; 37(11):2179‐86. [DOI] [PubMed] [Google Scholar]
- 59.Briem K Ragnarsdóttir AM Arnason SI, et al. Altered medial versus lateral hamstring muscle activity during hop testing in female athletes 1‐6 years after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014 Sep 24. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Arnason SM Birnir B Guðmundsson TE, et al. Medial hamstring muscle activation patterns are affected 1‐6 years after ACL reconstruction using hamstring autograft. Knee Surg Sports Traumatol Arthrosc. 2014; 22(5):1024‐9. [DOI] [PubMed] [Google Scholar]
- 61.Lloyd DG Buchanan TS Besier TF Neuromuscular biomechanical modeling to understand knee ligament loading. Med Sci Sports Exerc. 2005; 37(11):1939‐47. [DOI] [PubMed] [Google Scholar]
- 62.Palmieri‐Smith RM Wojtys EM Ashton‐Miller JA Association between preparatory muscle activation and peak valgus knee angle. J Electromyogr Kinesiol. 2008; 18(6):973‐9. [DOI] [PubMed] [Google Scholar]
- 63.Zebis MK Andersen LL Bencke J, et al. Identification of athletes at future risk of anterior cruciate ligament ruptures by neuromuscular screening. Am J Sports Med. 2009; 37(10):1967‐73. [DOI] [PubMed] [Google Scholar]
- 64.Wild CY Steele JR Munro BJ Insufficient hamstring strength compromises landing technique in adolescent girls. Med Sci Sports Exerc. 2013; 45(3):497‐505. [DOI] [PubMed] [Google Scholar]
- 65.Walsh M Boling MC McGrath M, et al. Lower extremity muscle activation and knee flexion during a jump‐landing task. J Athl Train. 2012; 47(4):406‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ortiz A Capo‐Lugo CE Venegas‐Rios HL Biomechanical Deficiencies in Women with Semitendinosus‐Gracilis Anterior Cruciate Ligament Reconstruction During Drop Jumps. PM R. 2014; 6(12):1097‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zebis MK Bencke J Andersen LL, et al. The effects of neuromuscular training on knee joint motor control during side cutting in female elite soccer and handball players. Clin J Sport Med 2008; 18(4):329‐37. [DOI] [PubMed] [Google Scholar]
- 68.Gokeler A Bisschop M Benjaminse A, et al. Quadriceps function following ACL reconstruction and rehabilitation: implications for optimisation of current practices. Knee Surg Sports Traumatol Arthrosc. 2014; 22(5):1163‐74. [DOI] [PubMed] [Google Scholar]
- 69.Snyder‐Mackler L Delitto A Bailey SL, et al. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am. 1995; 77(8):1166‐73. [DOI] [PubMed] [Google Scholar]
- 70.Hart JM Pietrosimone B Hertel J, et al. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010; 45(1):87‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pietrosimone BG Lepley AS Ericksen HM, et al. Quadriceps strength and corticospinal excitability as predictors of disability after anterior cruciate ligament reconstruction. J Sport Rehabil. 2013; 22(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 72.Lepley AS Gribble PA Thomas AC, et al. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: A 6‐month longitudinal investigation. Scand J Med Sci Sports. 2015 Feb 18. doi: 10.1111/sms.12435. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 73.Palmieri‐Smith RM Thomas AC Wojtys EM Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008; 27(3):405‐24 vii‐ix. [DOI] [PubMed] [Google Scholar]
- 74.Spencer JD Hayes KC Alexander IJ Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984; 65(4):171‐7. [PubMed] [Google Scholar]
- 75.Kennedy JC Alexander IJ Hayes KC Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982; 10(6):329‐35. [DOI] [PubMed] [Google Scholar]
- 76.Hopkins JT Ingersoll CD Krause BA, et al. Effect of knee joint effusion on quadriceps and soleus motoneuron pool excitability. Med Sci Sports Exerc. 2001; 33(1):123‐6. [DOI] [PubMed] [Google Scholar]
- 77.Palmieri‐Smith RM Villwock M Downie B, et al. Pain and effusion and quadriceps activation and strength. J Athl Train. 2013; 48(2):186‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lynch AD Logerstedt DS Axe MJ, et al. Quadriceps activation failure after anterior cruciate ligament rupture is not mediated by knee joint effusion. J Orthop Sports Phys Ther. 2012; 42(6):502‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsiao SF Chou PH Hsu HC, et al. Changes of muscle mechanics associated with anterior cruciate ligament deficiency and reconstruction. J Strength Cond Res. 2014; 28(2):390‐400. [DOI] [PubMed] [Google Scholar]
- 80.Kuenze CM Hertel J Weltman A, et al. Persistent Neuromuscular and Corticomotor Quadriceps Asymmetry After Anterior Cruciate Ligament Reconstruction. J Athl Train. 2015 Jan 26. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lepley AS Ericksen HM Sohn DH, et al. Contributions of neural excitability and voluntary activation to quadriceps muscle strength following anterior cruciate ligament reconstruction. Knee. 2014; 21(3):736‐42. [DOI] [PubMed] [Google Scholar]
- 82.Kuenze C Hertel J Saliba S, et al. Clinical thresholds for quadriceps assessment after anterior cruciate ligament reconstruction. J Sport Rehabil. 2015;24(1):36‐46. [DOI] [PubMed] [Google Scholar]
- 83.Schmitt LC Paterno MV Hewett TE The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012; 42(9):750‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schmitt LC Paterno MV Ford KR, et al. Strength Asymmetry and Landing Mechanics at Return to Sport after ACL Reconstruction. Med Sci Sports Exerc. 2014 Nov 4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Angelozzi M Madama M Corsica C, et al. Rate of force development as an adjunctive outcome measure for return‐to‐sport decisions after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012; 42(9):772‐80. [DOI] [PubMed] [Google Scholar]
- 86.Knezevic OM Mirkov DM Kadija M, et al. Asymmetries in explosive strength following anterior cruciate ligament reconstruction. Knee. 2014; 21(6):1039‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McLean SG Samorezov JE Fatigue‐induced ACL injury risk stems from a degradation in central control. Med Sci Sports Exerc. 2009; 41(8):1661‐72. [DOI] [PubMed] [Google Scholar]
- 88.Borotikar BS Newcomer R Koppes R, et al. Combined effects of fatigue and decision making on female lower limb landing postures: central and peripheral contributions to ACL injury risk. Clin Biomech (Bristol, Avon). 2008; 23(1):81‐92. [DOI] [PubMed] [Google Scholar]
- 89.Sugimoto D Alentorn‐Geli E Mendiguchía J, et al. Biomechanical and Neuromuscular Characteristics of Male Athletes: Implications for the Development of Anterior Cruciate Ligament Injury Prevention Programs. Sports Med. 2015 Feb 7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 90.Khalid AJ Ian Harris S Michael L, et al. Effects of neuromuscular fatigue on perceptual‐cognitive skills between genders in the contribution to the knee joint loading during side‐stepping tasks. J Sports Sci. 2015; 6:1‐10. [DOI] [PubMed] [Google Scholar]
- 91.Gokeler A Eppinga P Dijkstra PU, et al. Effect of fatigue on landing performance assessed with the landing error scoring system (less) in patients after acl reconstruction. A pilot study. Int J Sports Phys Ther. 2014; 9(3):302‐11. [PMC free article] [PubMed] [Google Scholar]
- 92.Frank BS Gilsdorf CM Goerger BM, et al. Neuromuscular fatigue alters postural control and sagittal plane hip biomechanics in active females with anterior cruciate ligament reconstruction. Sports Health. 2014; 6(4):301‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Ste Croix MB Priestley AM Lloyd RS, et al. ACL injury risk in elite female youth soccer: Changes in neuromuscular control of the knee following soccer‐specific fatigue. Scand J Med Sci Sports. 2014 Dec 30. doi: 10.1111/sms.12355. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 94.Webster KE Santamaria LJ McClelland JA, et al. Effect of fatigue on landing biomechanics after anterior cruciate ligament reconstruction surgery. Med Sci Sports Exerc. 2012; 44(5):910‐6. [DOI] [PubMed] [Google Scholar]
- 95.Kvist J Ek A Sporrstedt K Good L Fear of re‐injury: a hindrance for returning to sports after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 2005; 13(5):393‐7. [DOI] [PubMed] [Google Scholar]
- 96.Johnston LH Carroll DJ The context of emotional responses to athletic injury: a qualitiative analysis. J Sport Rehabil 1998; 7:206‐220. [Google Scholar]
- 97.Trost Z France CR Sullivan MJ, et al. Pain‐related fear predicts reduced spinal motion following experimental back injury. Pain. 2012; 153(5):1015‐21. [DOI] [PubMed] [Google Scholar]
- 98.Nederhand MJ Hermens HJ Ijzerman MJ, et al. The effect of fear of movement on muscle activation in posttraumatic neck pain disability. Clin J Pain. 2006; 22(6):519‐25. [DOI] [PubMed] [Google Scholar]
- 99.Sterling M Jull G Vicenzino B, et al. Development of motor system dysfunction following whiplash injury. Pain. 2003; 103(1‐2): 65‐73. [DOI] [PubMed] [Google Scholar]
- 100.Robinson JP Theodore BR Dansie EJ, et al. The role of fear of movement in subacute whiplash‐associated disorders grades I and II. Pain. 2013; 154(3):393‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brox JI Storheim K Grotle M, et al. Systematic review of back schools, brief education, and fear‐avoidance training for chronic low back pain. Spine J. 2008; 8(6):948‐58. [DOI] [PubMed] [Google Scholar]
- 102.Woods MP Asmundson GJ Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: a randomized controlled clinical trial. Pain. 2008; 136(3):271‐80. [DOI] [PubMed] [Google Scholar]
- 103.de Jong JR Vlaeyen JW Onghena P, et al. Fear of movement/(re)injury in chronic low back pain: education or exposure in vivo as mediator to fear reduction? Clin J Pain. 2005; 21(1):9‐17. [DOI] [PubMed] [Google Scholar]
- 104.Boersma K Linton S Overmeer T, et al. Lowering fear‐avoidance and enhancing function through exposure in vivo. A multiple baseline study across six patients with back pain. Pain. 2004; 108(1‐2):8‐16. [DOI] [PubMed] [Google Scholar]
- 105.Abbott AD Tyni‐Lenné R Hedlund R Early rehabilitation targeting cognition #behavior #and motor function after lumbar fusion: a randomized controlled trial. Spine (Phila Pa 1976). 2010. ; 35(8):848‐57. [DOI] [PubMed] [Google Scholar]
- 106.de Jong JR Vlaeyen JW Onghena P, et al. Reduction of pain‐related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. Pain. 2005; 116(3):264‐75. [DOI] [PubMed] [Google Scholar]
- 107.Ardern CL Österberg A Tagesson S, et al. The impact of psychological readiness to return to sport and recreational activities after anterior cruciate ligament reconstruction. Br J Sports Med. 2014 Dec;. 48(22):1613‐9. [DOI] [PubMed] [Google Scholar]
- 108.Chmielewski TL Jones D Day T, et al. The association of pain and fear of movement/reinjury with function during anterior cruciate ligament reconstruction rehabilitation. J Orthop Sports Phys Ther. 2008; 38(12):746‐53. [DOI] [PubMed] [Google Scholar]
- 109.Lentz TA Zeppieri G Jr George SZ, et al. Comparison of Physical Impairment, Functional, and Psychosocial Measures Based on Fear of Reinjury/Lack of Confidence and Return‐to‐Sport Status After ACL Reconstruction. Am J Sports Med. 2015; 43(2):345‐53. [DOI] [PubMed] [Google Scholar]
- 110.Shelbourne KD Urch SE Gray T et al. Loss of normal motion after anterior cruciate ligament reconstruction is associated with radiographic arthritic changes after surgery. Am J Sports Med. 2012; 40(1):108‐14. [DOI] [PubMed] [Google Scholar]
- 111.Shelbourne KD Freeman H Gray T Osteoarthritis after anterior cruciate ligament reconstruction: the importance of regaining and maintaining full range of motion. Sports Health. 2012; 4(1):79‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shelbourne KD Range of motion loss will cause osteoarthritis. Arthroscopy. 2011; 27(4):451‐2. [DOI] [PubMed] [Google Scholar]
- 113.Chmielewski TL Myer GD Kauffman D et al. Plyometric exercise in the rehabilitation of athletes: physiological responses and clinical application. J Orthop Sports Phys Ther. 2006; 36(5):308‐19. [DOI] [PubMed] [Google Scholar]
- 114.Myer GD Stroube BW DiCesare CA, et al. Augmented feedback supports skill transfer and reduces high‐risk injury landing mechanics: a double‐blind, randomized controlled laboratory study. Am J Sports Med. 2013; 41(3):669‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Benjaminse A Gokeler A Dowling AV et al. Optimization of the Anterior Cruciate Ligament Injury Prevention Paradigm: Novel Feedback Techniques to Enhance Motor Learning and Reduce Injury Risk. J Orthop Sports Phys Ther. 2015;27:1‐46 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 116.Gokeler A Benjaminse A Hewett TE, et al. Feedback techniques to target functional deficits following anterior cruciate ligament reconstruction: implications for motor control and reduction of second injury risk. Sports Med 2013; 43(11):1065‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ford KR DiCesare CA Myer GD, et al. Real‐Time Biofeedback to Target Risk of Anterior Cruciate Ligament Injury: A Technical Report for Injury Prevention and Rehabilitation. J Sport Rehabil. 2014 Jun 23. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 118.Wu WF Porter JM Brown LE Effect of attentional focus strategies on peak force and performance in the standing long jump. J Strength Cond Res. 2012; 26(5):1226‐31. [DOI] [PubMed] [Google Scholar]
- 119.Porter JM Anton PM Wikoff NM et al. Instructing skilled athletes to focus their attention externally at greater distances enhances jumping performance.. J Strength Cond Res. 2013; 27(8):2073‐8. [DOI] [PubMed] [Google Scholar]
- 120.Makaruk H Porter JM Czaplicki A et al. The role of attentional focus in plyometric training. J Sports Med Phys Fitness. 2012; 52(3):319‐27. [PubMed] [Google Scholar]
- 121.Gokeler A Benjaminse A Welling W et al. The effects of attentional focus on jump performance and knee joint kinematics in patients after ACL reconstruction. Phys Ther Sport. 2014; 2 [Epub ahead of print]. pii: S1466‐853X(14)00039‐X. [DOI] [PubMed] [Google Scholar]
- 122.Ericksen HM Thomas AC Gribble PA et al. Immediate effects of real‐time feedback on jump‐landing kinematics. J Orthop Sports Phys Ther. 2015; 45(2):112‐8. [DOI] [PubMed] [Google Scholar]
- 123.Beaulieu ML Palmieri‐Smith RM Real‐time feedback on knee abduction moment does not improve frontal‐plane knee mechanics during jump landings. Scand J Med Sci Sports. 2013; 24 doi: 10.1111/sms.12051. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 124.Dowling AV Favre J Andriacchi TP Inertial sensor‐based feedback can reduce key risk metrics for anterior cruciate ligament injury during jump landings. Am J Sports Med. 2012; 40(5):1075‐83. [DOI] [PubMed] [Google Scholar]
- 125.Myer GD Ford KR Khoury J et al. Development and validation of a clinic‐based prediction tool to identify female athletes at high risk for anterior cruciate ligament injury. Am J Sports Med. 2010; 38(10):2025‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Swanik CB Covassin T Stearne DJ et al. The relationship between neurocognitive function and non contact anterior cruciate ligament injuries. Am J Sports Med. 2007; 35(6):943‐8. [DOI] [PubMed] [Google Scholar]
- 127.Myer GD Ford KR Brent JL et al. An integrated approach to change the outcome part I: neuromuscular screening methods to identify high ACL injury risk athletes. J Strength Cond Res. 2012; 26(8):2265‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Myer GD Ford KR Brent JL et al. An integrated approach to change the outcome part II: targeted neuromuscular training techniques to reduce identified ACL injury risk factors. J Strength Cond Res. 2012; 26(8):2272‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Herman DC Weinhold PS Guskiewicz KM, et al. The effects of strength training on the lower extremity biomechanics of female recreational athletes during a stop‐jump task. Am J Sports Med. 2008; 36(4):733‐40. [DOI] [PubMed] [Google Scholar]
- 130.Garrison M Westrick R Johnson MR et al. Association between the functional movement screen and injury development in college athletes. Int J Sports Phys Ther. 2015; 10(1):21‐8. [PMC free article] [PubMed] [Google Scholar]
- 131.Kraus K Schütz E Taylor WR et al. Efficacy of the functional movement screen: a review. J Strength Cond Res. 2014; 28(12):3571‐84. [DOI] [PubMed] [Google Scholar]
- 132.Plisky PJ Gorman PP Butler RJ, et al. The reliability of an instrumented device for measuring components of the star excursion balance test. N Am J Sports Phys Ther. 2009; 4(2):92‐9. [PMC free article] [PubMed] [Google Scholar]
- 133.Plisky PJ Rauh MJ Kaminski TW, et al. Star Excursion Balance Test as a predictor of lower extremity injury in high school basketball players. J Orthop Sports Phys Ther. 2006; 36(12):911‐9. [DOI] [PubMed] [Google Scholar]
- 134.Boyle MJ Butler RJ Queen RM Functional Movement Competency and Dynamic Balance After Anterior Cruciate Ligament Reconstruction in Adolescent Patients. J Pediatr Orthop. 2015 Jan 28. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 135.Hewett TE Myer GD Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005; 33(4):492‐501. [DOI] [PubMed] [Google Scholar]
- 136.Myer GD Ford KR Palumbo JP, et al. Neuromuscular training improves performance and lower‐extremity biomechanics in female athletes. J Strength Cond Res. 2005; 19(1):51‐60. [DOI] [PubMed] [Google Scholar]