Abstract
Objective
Vaginal cuff separation is a rare but serious complication following hysterectomy. The goal of our study was to determine the rate of vaginal cuff separation and associated risk factors in patients undergoing laparoscopic or robotic hysterectomy.
Methods
We retrospectively identified patients who underwent a minimally invasive simple or radical hysterectomy at one institution between January 2000 and 2009. Fisher's exact test, Wilcoxon rank sum test and multiple logistic regression was used to determine associations between variables and increased risk of separation.
Results
A total of 417 patients underwent laparoscopic (n=285) or robotic (n=132) hysterectomy during the study period. Three hundred and sixty-two underwent simple hysterectomy (249 laparoscopic, 113 robotic) and 57 underwent radical hysterectomy (36 laparoscopic, 19 robotic). Seven (1.7%) patients developed a cuff complication and all had a diagnosis of malignancy. Three (1.1%) patients in the laparoscopy group suffered a vaginal cuff evisceration (n=2) or separation (n=1). Four patients in the robotic group (3.0%) had a vaginal evisceration (n=1) or separation (n=3). There was no difference based on surgical approach (p=0.22). Vaginal cuff complications were 9.46-fold higher among patients who had a radical hysterectomy (p<0.01). Median time to presentation of vaginal cuff complication was 128 days (58–175) in the laparoscopy group and 37 days (range: 32–44) in the robotic group.
Conclusions
The overall risk of vaginal cuff complication was 1.7%. There appears to be no difference in cuff complication rates based on surgical approach. Radical hysterectomy, however, was associated with a 9-fold increase in vaginal cuff complications.
Introduction
Hysterectomy is one of the most common gynecologic surgeries performed in the United States annually. Although uncommon, a variety of complications can occur with hysterectomies, including bladder injury, bowel injury, ureteral injury, hemorrhage and postoperative infection. Less commonly, patients can develop vaginal cuff separation with or without small bowel evisceration. Hysterectomy can now be performed utilizing minimally invasive surgical techniques such as laparoscopy or robotic surgery. Previous studies suggest a higher rate of vaginal cuff separation in patients undergoing minimally invasive surgery compared with open procedures, and it has been hypothesized that the reason for the higher rate is thermal injury to the vagina at the time of colpotomy places these patients at higher risk for vaginal cuff separation or evisceration [1, 2]. Furthermore, with the advent of robotic-assisted surgery, it is the surgeon's responsibility to define inequalities between the surgical approaches and to ensure proper patient counseling in the perioperative period with regard to the risks, benefits, and complication rates associated with the different surgical approaches. At the University of Texas, MD Anderson Cancer Center both laparoscopy and robotic surgery are becoming common practice for a variety of benign and malignant gynecologic conditions, and although reports characterizing the rates of vaginal cuff separation among patients undergoing minimally invasive hysterectomy exist, reports comparing laparoscopy to robotic surgery are lacking, thus leading us to examine the rate of vaginal cuff separation among patients undergoing robotic hysterectomy compared to laparoscopic hysterectomy.
Materials and Methods
After approval was granted by the University of Texas M. D. Anderson Cancer Center Investigational Review Board, data were collected retrospectively on all patients who had undergone laparoscopic or robotic hysterectomy for either benign or malignant disease from January 2000 to January 2009. Patients who underwent conversion to an open procedure or laparoscopic-assisted vaginal hysterectomy were excluded from the analysis because these patients did not undergo colpotomy incision with a minimally invasive approach. Hysterectomy was defined as laparoscopic-assisted vaginal hysterectomy if the colpotomy incision was performed from a vaginal approach. Similarly, patients that underwent minimally invasive surgery without colpotomy incision were excluded from the analysis. The first gynecologic procedure utilizing the da Vinci surgical system (Intuitive Surgery, Sunnyvale, CA) at our institution was performed in 2006. Colpotomy at the time of hysterectomy was performed using either monopolar coagulation or the Harmonic Ace (Ethicon-Endosurgery, Inc., Cincinnati, OH). Techniques for cuff closure were based on surgeon preference. Cuff closure for laparoscopic hysterectomy was performed using interrupted sutures of polyglactin 0 secured with a laparoscopic knot pusher or sutures of polyglactin 2-0 on a CT-1 needle secured with titanium clips (Ti-knot; LSI Solutions, Victor, NY). The vaginal cuff closure during robotic procedures was either interrupted figure of eight sutures of polyglactin (Vicryl; Novartis, Basel Switzerland) 0 on a CT-1 needle, or nonlocking, running sutures of polyglactin 2-0 on a CT-1 needle with absorbable polydioxanone clips (Lapra-ty; Ethicon-Endosurgery, Inc., Cincinnati, OH) secured at each end based on surgeon preference.
Vaginal cuff separation was defined as partial or total full thickness opening of the anterior and posterior edges of the vaginal cuff without protruding bowel. Vaginal cuff evisceration was defined as separation with protruding bowel. Data extracted from the medical record included patient's age, body mass index, smoking history and relevant comorbid conditions including obesity, diabetes, hypertension, hyperlipidemia, and coronary artery disease. Operative reports were reviewed and operative times, estimated blood loss and technique of vaginal cuff closure were recorded. Patients diagnosed with vaginal cuff separation with or without small bowel evisceration were recorded. For patients who developed vaginal cuff complications, presenting symptoms, triggering event, and time from initial surgery to vaginal cuff complication, method of repair, patient outcomes and date of last contact were documented.
Statistical analyses were performed using STATA 10.0 (StataCorp, College Station, TX) and SPSS 17.0 (Chicago, IL). The incidence of vaginal cuff separation with or without small bowel evisceration was calculated using the binomial exact method. Associations between categorical variables and vaginal cuff separation and evisceration were determined using Fisher's exact test. Continuous variables were summarized and compared using the Wilcoxon rank sum test. Univariate logistic regression was used to determine independent variables associated with increased odds of vaginal cuff separation. Multivariate logistic regression was also performed. To avoid inadvertently eliminating potential confounding factors affecting cuff complications, stepwise backwards regression analyses included covariates with p-values ≤ 0.15. All tests were two-sided, and a p-value < 0.05 was considered statistically significant.
Results
Review of medical records from January 2000 to January 2009 revealed 417 women who met study criteria. Of these, 285 women underwent total laparoscopic hysterectomy (simple in 249 [87%] and radical in 36 [13%]), and 132 underwent a robotic hysterectomy (simple in 113 [86%] and radical in 19 [14%]). The median follow-up time across the entire cohort of patients was 4.7 months (range, 0.03 to 50.4 months). For patients undergoing robotic surgery, the median time to follow-up was 3.58 months (range, 0.23 to 26.3 months). For patients undergoing laparoscopic surgery, the median time to follow-up was 5.75 months (range, 0.03 to 50.5 months). Of the 285 patients in the laparoscopy group, three developed vaginal cuff separation with (n=2) or without (n=1) bowel evisceration (incidence, 1.1%: 95% confidence interval, 0.22% to 3.07%). Of the 132 patients in the robotic surgery group, four developed vaginal cuff separation with (n=1) or without (n=3) bowel evisceration (incidence, 3.0%: 95% confidence interval, 0.83% to 7.52%) [Figure 1]. Although the incidence of vaginal cuff complications was increased in patients undergoing robotic hysterectomy, this difference was not statistically significant (p=0.22). There was a significant increase in the incidence of vaginal cuff separation or evisceration among patients undergoing radical hysterectomy compared to simple hysterectomy (4/55 or 7.3% vs. 3/362 or 0.83%, p=0.007).
Figure 1.
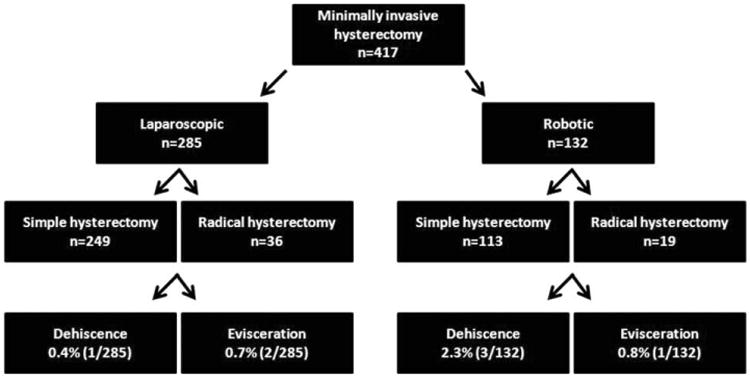
Schema of minimally invasive surgery among patients undergoing hysterectomy.
Demographic and clinical characteristics of the seven patients with vaginal cuff separation are presented in Table 1. All seven patients had a diagnosis of malignancy. Two patients underwent total laparoscopic radical hysterectomy and two patients had robotic radical hysterectomy. Of the three patients who had simple hysterectomies, one was laparoscopic and two were robotic. In the patients with separation after laparoscopic procedures, 2 of the vaginal colpotomies were closed with polyglactin 0 interrupted sutures and one was closed with polyglactin 2-0 interrupted sutures secured with titanium clips. In the patients with separation after robotic procedures, all of the vaginal colpotomies were closed with running nonlocking polyglactin 0 suture secured with absorbable polydioxanone clips. The colpotomy incision was performed using monopolar cautery for all but one patient who developed a vaginal cuff separation.
Table 1. Demographic and clinical characteristics of patients with vaginal cuff separation.
| Pt | Age | Procedure | Indication for Surgery | Postoperative Adjuvant therapy | Presenting Symptom | Trigger Event | POD | Pt Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 48 | TLRHa, pelvic LNDb | Cervical cancer | None | Postcoital spotting/pelvic pain | Coitus | 57 | Vaginal repair |
| 2 | 31 | TLRH, pelvic LND | Cervical cancer | IMRTc to pelvis + vaginal cuff boosts | Lower abdominal pain/vaginal discharge | Spontaneous | 128 | Allowed to heal by secondary intention |
| 3 | 50 | TLHd, BSOe Pelvic and paraaortic LND, omenectomy | Bilateral adnexal masses & elevated ca-125 | 6 cycles of carboplatin/paclitaxel | Vaginal discharge/mass protruding from vagina | Coitus | 175 | Vaginal repair |
| 4 | 66 | Robotic hysterectomy, BSO, pelvic and paraaortic LND, LOAf | Endometrial cancer | Vaginal cuff brachytherapy | Asymptomatic | Spontaneous | 44 | Allowed to heal by secondary intention |
| 5 | 47 | Robotic radical hysterectomy, BSO, pelvic LND | Cervical cancer | Whole pelvic XRTg + vaginal cuff brachytherapy with concurrent weekly cisplatin | Asymptomatic | Spontaneous | 41 | Allowed to heal by secondary intention |
| 6 | 66 | Robotic hysterectomy, BSO | Endometrial cancer | None | Pelvic pain and pressure/dysuria/vaginal discharge | Spontaneous | 37 | Allowed to heal by secondary intention |
| 7 | 64 | Robotic radical hysterectomy, BSO, pelvic LND | Cervical cancer | Whole pelvic XRT with concurrent weekly cisplatin | Vaginal discharge/dysuria | Spontaneous | 32 | Vaginal repair |
TLRH (total laparoscopic radical hysterectomy),
LND (lymph node dissection),
IMRT (intensity modulated radiation therapy),
TLH (total laparoscopic hysterectomy),
BSO (bilateral salpingooophorectomy),
LOA (lysis of adhesions),
XRT (external radiation therapy).
Common presenting symptoms at the time of cuff separation included vaginal discharge, lower abdominal or pelvic pain and dysuria. In two patients, separation was triggered by coitus; in the other five patients, separation was spontaneous. Three out the 7 (43%) patients that developed vaginal cuff separation required surgical intervention to repair the vagina. The median time to detection of vaginal cuff separation was 128 days (range, 57 to 175) in the laparoscopy group and 39 days (range, 32 to 44) in the robotic surgery group (p=0.06). Of the three patients with separation after laparoscopic hysterectomy, two had begun postoperative adjuvant therapy before separation was detected; the other patient received no postoperative adjuvant therapy. Of the four patients with separation after robotic hysterectomy, none had begun postoperative adjuvant therapy at the time separation was detected; although three eventually went on to receive adjuvant therapy.
Median age was 50 years (range: 31 to 66 years) in patients who had vaginal cuff separation versus 53 years (range: 19 to 88 years) in those patients who did not (p=0.93). There was no significant difference in the BMI of patients who had vaginal cuff separation versus those that did not (median 28.3 kg/m2 vs. 28.0 kg/m2, p=0.84), though three patients (43%) met BMI criteria for obesity. Median operative times among patients who developed vaginal cuff complications were significantly longer compared to those who did not (322 minutes vs. 210 minutes, p=0.02). At the time of vaginal cuff separation, 2 out of the total 7 patients were active smokers. Only one patient was diabetic and 2 patients had risks factors for peripheral vascular disease. There was no significant difference in the prevalence of active tobacco abuse (2/7 or 29% vs. 41/409 or 10%; p=0.16), diabetes (1/7 or 14% vs. 33/409 or 8.1%; p=0.45), and risk factors for peripheral vascular disease (2/7 or 29% vs. 153/409 or 37%; p=1.00) between patients with cuff separations and those without. [Table 2]. Differences in technique of vaginal cuff closure were not compared between patients with and without cuff separations because information regarding the method of cuff closure for patients that did not experience cuff separation was not collected. Obesity, active smoking, diabetes, risk factors for peripheral disease, surgical modality (robotic vs. laparoscopic surgery) and type of hysterectomy (radical vs. simple) were subsequently assessed using logistic regression analysis [Table 3]. After adjusting for all other covariates, only radical hysterectomy was associated with an independent increased odds of vaginal cuff complications (OR 9.46 [95% CI 2.06-43.54]; p=0.004).
Table 2. Risk factors for vaginal cuff separation.
| Variable | Vaginal cuff dehiscence/evisceration (n=7) | No vaginal cuff dehiscence/evisceration (n=409) | p valuea |
|---|---|---|---|
| Current smoker, no. of patients (%) | 2 (29) | 41 (11) | 0.16 |
| Diabetes, no of patients (%) | 1 (14) | 33 (8) | 0.45 |
| Risk factors for peripheral vascular disease, no of patients (%) | 2 (29) | 153 (37) | 1.00 |
| Radical hysterectomy, no of patients (%) | 4 (57) | 51 (12) | 0.007 |
p value from Fischer's exact test.
Table 3.
Logistic regression analyses of risk factors for vaginal cuff separation. Univariate logistic regression was used to determine independent variables associated with increased odds of vaginal cuff separation. To avoid eliminating potential confounding factors affecting cuff complications, stepwise backwards regression analyses included covariates with p-values ≤ 0.15. All tests were two-sided, and a p-value < 0.05 was considered statistically significant
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
| ||||
| Variable | OR (95% CI) | p | OR (95% CI) | p |
|
| ||||
| Age | 0.99 (0.94-1.05) | 0.97 | ||
|
| ||||
| BMI (kg/m2) | ||||
| ≤30 | 1.00 | |||
| > 30 | 1.17 (0.26-5.37) | 0.83 | ||
|
| ||||
| Smoking | ||||
| Nonsmokers | 1.00 | |||
| Current smokers | 3.66 (0.69-19.48) | 0.13 | ||
|
| ||||
| Diabetes | ||||
| No | 1.00 | |||
| Yes | 1.88 (0.22-16.16) | 0.56 | ||
|
| ||||
| Risk factors for peripheral vascular disease | ||||
| No | 1.00 | 0.63 | ||
| Yes | 0.67 (0.12-3.48) | |||
|
| ||||
| Type of hysterectomy | ||||
| Simple | 1.00 | |||
| Radical | 9.57 (2.08-43.91) | <0.01 | 9.46 (2.06-4.54) | <0.01 |
|
| ||||
| Modality of surgery | ||||
| Laparoscopic | 1.00 | |||
| Robotic | 2.92 (0.64-13.22) | 0.17 | ||
Discussion
Vaginal cuff separation with or without small bowel evisceration is a rare complication of hysterectomy; the reported estimated cumulative incidence among patients undergoing abdominal, vaginal or laparoscopic hysterectomy is 0.14% [2]. Reported rates of vaginal cuff separation following laparoscopic hysterectomy are much higher ranging from 0.79% to 4.93% [2, 3]. More recently, reports on the incidence of vaginal cuff separation following robotic hysterectomy have been come available [1, 4]. However, specific comparisons of robotic hysterectomy to laparoscopic hysterectomies are lacking, and consequently this was the focus of the current work. The unique findings of our study are comparable rates of vaginal cuff complications between patients undergoing laparoscopic or robotic hysterectomy, and the identification of potential risk factors for development of vaginal cuff separation among patients undergoing minimally invasive surgery.
In the largest reported cohort of patients, total laparoscopic hysterectomy was associated with a 21% increased relative risk of vaginal cuff complications compared to total vaginal hysterectomy and a more than 53.2% increased risk of vaginal cuff complications compared to total abdominal hysterectomy [2]. Predominant characteristics of patients with vaginal cuff separation in this study included premenopausal status and first postoperative coitus as the triggering event. Although the authors commented on risk factors associated with increased risk of separation, they were unable to perform a formal risk-factor analysis because they did not collect data on the presence of risk factors among patients who did not develop separation. Furthermore, they did not include the rate of vaginal cuff complications following robotic hysterectomy or among patients with a diagnosis of gynecologic malignancy.
In a retrospective review, Iaco et al., found that the incidence of vaginal cuff separation was 0.26% after abdominal hysterectomy, 0.25% after vaginal hysterectomy, and 0.79% after laparoscopic hysterectomy with no significant differences in separation rates between the various surgical approaches [3]. Although the authors included patients with gynecologic malignancies, their study was limited by the small number of laparoscopic hysterectomies performed (n=127) over the entire study period.
The incidence of vaginal cuff complications after robotic hysterectomy was described in two recent simultaneous publications. In a case series, Robinson et al., described two patients with vaginal cuff separation in a series of 205 patients who underwent robotic hysterectomy (incidence of 1%) and hypothesized that the etiology and risk factors for vaginal cuff separation are multifactorial with possible etiologies including initiation of sexual intercourse prior to complete healing of the vaignal cuff, increased intraabdominal pressure from frequent straining or Valsalva, and smoking [4]. Kho et al. report a 4.1% incidence of vaginal cuff complications following robotic hysterectomy, and although they speculated on potential risks factors for vaginal cuff separation following robotic hysterectomy, they did not perform formal comparative analyses between patients with and without separations, nor was there a comparison to laparoscopic hysterectomy.
In the current study, we found no significant differences in the prevalence of smoking, diabetes, obesity and risk factors for peripheral vascular disease among patients who had vaginal cuff separation after minimally invasive hysterectomy and those that did not. Surgical modality (laparoscopy vs. robotic surgery) was not associated with the risk of vaginal cuff separation. However, minimally invasive radical hysterectomy was associated with an increased risk. This finding suggests that changes in the vaginal support and/or foreshortening of the vagina may play a role in development of vaginal cuff complications. Further explanations include the increased tissue damage noted during minimally invasive surgery related to use of electrocautery for the colpotomy, initiation of postoperative radiation therapy, which further damages the existing capillary blood supply of the vaginal apex, and patient noncompliance with recommendations against resuming sexual intercourse early in the postoperative period.
The patients in the current study were all treated at a tertiary referral center predominantly responsible for cancer care; consequently, all patients who experienced vaginal cuff separation had a diagnosis of malignancy. Many of these patients received postoperative adjuvant chemotherapy or radiation therapy. The median time to presentation of vaginal cuff separation was longer in patients who underwent laparoscopic hysterectomy (median, 128 days) than in patients who underwent robotic hysterectomy (median, 39 days), and two of the three cases of separation in patients who underwent laparoscopic hysterectomy occurred after the initiation of adjuvant postoperative therapy. We speculate that these vaginal cuff separations could be attributed to postoperative rather than operative factors such as thermal injury, mode of hysterectomy or length of surgery. In contrast, all cases of vaginal cuff separation in patients who had robotic hysterectomy occurred before initiation of any postoperative adjuvant therapy, implicating surgical technique and patient risk factors as possible causes of the vaginal cuff separation.
Existing reports in the literature have identified potential risk factors for vaginal cuff separation and subsequent small bowel evisceration [5-8]. Historically, risk factors have been stratified into pre- and postmenopausal populations. Coitus and trauma causing vaginal cuff separation are more likely the precipitating events in premenopausal women [5, 6]. Other risk factors include thinning and scarring of the vaginal epithelium, foreshortening of the vagina, and diminished vascularity of the vaginal apex, all of which are more common in postmenopausal women [9]. The presence or absence of an enterocele and/or increased abdominal pressure further increases the risks of vaginal cuff separation in postmenopausal women [10].
As new surgical techniques are introduced, one must focus attention on differences in surgical approaches to minimize untoward side effects. The use of electrocautery for cutting and hemostasis during laparoscopic hysterectomies results in greater tissue necrosis and devascularization of the vaginal cuff than what is seen after colpotomies performed with sharp dissection during vaginal or abdominal hysterectomies [2, 5]. The extent of thermal tissue damage during robotic hysterectomies is proposed to be even greater than with laparoscopic hysterectomy because the colpotomy is performed with monopolar cautery set at 50 watts in the coagulation mode [1]. Furthermore, during laparoscopic suturing of the vaginal apex, the possibility weakening of the suture secondary to fraying with use of laparoscopic knot pushers or uncertain integrity of laparoscopic knots must be considered [2]. In addition, shallow suture placement incorporating minimal vaginal epithelium—secondary to the degree of magnification noted with minimally invasive surgery—theoretically increases the risk of postoperative vaginal cuff separation. Our findings have led us to modify our technique for closure of the vaginal apex during minimally invasive hysterectomy, particularly in robotic hysterectomy. We now incorporate interrupted sutures to reinforce the vaginal apex, are considering an alternate suture material (either delayed absorbable suture or barbed suture), and take great care to ensure a large purchase of vaginal cuff (≥ 5mm) when reapproximating the vaginal apex.
Patients undergoing minimally invasive surgery often report a faster recovery time and less pain than patients undergoing abdominal or vaginal hysterectomy, and they may resume sexual intercourse earlier postoperatively, contributing to the increased rate of vaginal cuff separation following laparoscopic or robotic hysterectomy. At our institution, patients undergoing minimally invasive hysterectomy are counseled to maintain pelvic rest for a total of 8 weeks particularly if they will be undergoing adjuvant treatment for malignancy, which may further impair their postoperative healing potential.
The current study is limited by its retrospective nature. Although the incidence of vaginal cuff complications did not differ significantly between laparoscopic and robotic hysterectomy, the lack of significance may have been due to the lower number of hysterectomies in the robotic surgery group. One must consider that as the number of robotic hysterectomies increases, if the rate of vaginal cuff complications remains constant, the increased rate of vaginal cuff separation or evisceration in the robotic surgery group may become statistical significant. One possible criticism is that we are overestimating the incidence of vaginal cuff complications by including patients that did not require operative intervention. However, we believe that any diagnosed separation impacts the postoperative course of the patient and should be considered clinically significant. In addition, the fact that all the patients were seen at a major tertiary referral center introduces the possibility of selection bias by reporting only on patients with available outcome data.
In summary, vaginal cuff separations with or without small bowel evisceration occurs in only a small proportion of patients undergoing laparoscopic or robotic radical or simple hysterectomy. Furthermore, we demonstrate that the incidence of vaginal cuff separation did not differ significantly between laparoscopic and robotic hysterectomy. Patients undergoing a minimally invasive radical hysterectomy are at increased risk of vaginal cuff complications in the postoperative period compared to patients undergoing either laparoscopic or robotic simple hysterectomy. We believe patients undergoing minimally invasive hysterectomy should be appropriately counseled of the slight increased risk of vaginal cuff complications so they can appropriately modify their postoperative activities. Further investigation into the precise etiology of vaginal cuff separation with or without small bowel evisceration after minimally invasive hysterectomy is warranted and will help minimize the incidence of this potentially devastating complication.
Acknowledgments
The authors thank Ms. Stephanie Deming for her assistance in preparation of this manuscript.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kho RM, et al. Incidence and characteristics of patients with vaginal cuff dehiscence after robotic procedures. Obstet Gynecol. 2009;114(2 Pt 1):231–5. doi: 10.1097/AOG.0b013e3181af36e3. [DOI] [PubMed] [Google Scholar]
- 2.Hur HC, et al. Incidence and patient characteristics of vaginal cuff dehiscence after different modes of hysterectomies. J Minim Invasive Gynecol. 2007;14(3):311–7. doi: 10.1016/j.jmig.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Iaco PD, et al. Transvaginal evisceration after hysterectomy: is vaginal cuff closure associated with a reduced risk? Eur J Obstet Gynecol Reprod Biol. 2006;125(1):134–8. doi: 10.1016/j.ejogrb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Robinson BL, et al. Vaginal cuff dehiscence after robotic total laparoscopic hysterectomy. Obstet Gynecol. 2009;114(2 Pt 1):369–71. doi: 10.1097/AOG.0b013e3181af68c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramirez PT, Klemer DP. Vaginal evisceration after hysterectomy: a literature review. Obstet Gynecol Surv. 2002;57(7):462–7. doi: 10.1097/00006254-200207000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Rolf BB. Vaginal evisceration. Am J Obstet Gynecol. 1970;107(3):369–75. doi: 10.1016/0002-9378(70)90559-4. [DOI] [PubMed] [Google Scholar]
- 7.Friedel W, Kaiser IH. Vaginal evisceration. Obstet Gynecol. 1975;45(3):315–9. [PubMed] [Google Scholar]
- 8.Choo YC, Lindenauer SM. Vaginal evisceration. Int J Gynaecol Obstet. 1981;19(4):313–7. doi: 10.1016/0020-7292(81)90081-3. [DOI] [PubMed] [Google Scholar]
- 9.Guttman A, Afilalo M. Vaginal evisceration. Am J Emerg Med. 1990;8(2):127–8. doi: 10.1016/0735-6757(90)90199-a. [DOI] [PubMed] [Google Scholar]
- 10.Kambouris AA, Drukker BH, Barron J. Vaginal evisceration. A case report and brief review of the literature. Arch Surg. 1981;116(7):949–51. doi: 10.1001/archsurg.1981.01380190077018. [DOI] [PubMed] [Google Scholar]


