Abstract
Humans are increasingly and consistently exposed to a variety of endocrine disrupting chemicals (EDCs), chemicals that have been linked to neurobehavioral disorders such as ADHD and autism. Many of such EDCs have been shown to adversely influence brain mesocorticolimbic systems raising the potential for cumulative toxicity. As such, understanding the effects of developmental exposure to mixtures of EDCs is critical to public health protection. Consequently, this study compared the effects of a mixture of four EDCs to their effects alone to examine potential for enhanced toxicity, using behavioral domains and paradigms known to be mediated by mesocorticolimbic circuits (Fixed Interval (FI) schedule controlled behavior, novel object recognition memory and locomotor activity) in offspring of pregnant mice that had been exposed to vehicle or relatively low doses of four EDCs, Atrazine (ATR – 10mg/kg), Perfluorooctanoic acid (PFOA – 0.1 mg/kg), Bisphenol-A (BPA - 50μg/kg), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD – 0.25μg/kg) alone or combined in a mixture (MIX), from gestational day 7 until weaning. EDC-treated males maintained significantly higher horizontal activity levels across 3 testing sessions, indicative of delayed habituation, whereas no effects were found in females. Statistically significant effects of MIX were seen in males, but not females, in the form of increased FI response rates, in contrast to reductions in response rate with ATR, BPA and TCDD, and reduced short term memory in the novel object recognition paradigm. MIX also reversed the typically lower neophobia levels of males compared to females. With respect to individual EDCs, TCDD produced notable increases in FI response rates in females, and PFOA significantly increased ambulatory locomotor activity in males. Collectively, these findings show the potential for enhanced behavioral effects of EDC mixtures in males and underscore the need for animal studies to more fully investigate mixtures, including chemicals that converge on common physiological substrates to examine potential mechanisms of toxicity with full dose effect curves to assist in interpretations of relevant mechanisms.
Introduction
Multiple classes of chemicals, e.g. fertilizers and herbicides, plastics, organic pollutants, metals, flame retardants and heat stabilizers, have been shown to have endocrine disrupting characteristics. Given the chemical heterogeneity of EDCs, a broad range of physiological targets have been identified. EDCs may interfere with the production, secretion, transportation, metabolism, binding action and/or excretion of natural hormones (Diamanti-Kandarakis et al., 2009). There is mounting evidence that developmental exposures to EDCs impact neurochemical pathways leading to lifelong disease susceptibility and behavioral deficits into adulthood (de Cock et al., 2012, Schantz and Widholm, 2001, Schug et al., 2011). Developmental EDC exposures can cause physiological reprogramming of hormonal homeostasis with impacts on peripheral and neurological hormone relationships, e.g., glucocorticoids and glutamate, estrogen and dopamine function (Patisaul and Adewale, 2009, Vandenberg et al., 2012). EDCs have been implicated in the etiopathogenesis of ADHD, autism, and other neurodevelopmental and behavioral disorders (de Cock, Maas, 2012, Schug, Janesick, 2011); thus, understanding the consequences of developmental exposure to low dose EDC mixtures for neurological disease etiology is vital (Colborn, 2004b, de Cock, Maas, 2012).
Studies in animal models indicate that monoaminergic neural pathways are specifically altered as a result of developmental exposure to EDCs, particularly mesocorticolimbic dopaminergic systems (Palanza et al., 2008). For instance, in vivo and in vitro studies indicate atrazine (ATR) causes a reduction in striatal dopamine (Coban and Filipov, 2007, Hossain and Filipov, 2008). In rats, prenatal exposure to ATR decreased striatal dopamine and decreased locomotor activity (Bardullas et al., 2011, Lin et al., 2013a, Rodríguez et al., 2012). Prenatal exposures to perfluorooctanoic acids (PFOAs), as fire retardants, increased home-cage activity in male mice, and enhanced astrogliosis and pro-inflammatory cytokines in the hippocampus and cortex of rats (Onishchenko et al., 2011, Zeng et al., 2011). Mice exposed to low dose TCDD in the perinatal period exhibited hypo-activation of the prefrontal cortex, increased brain monoamines and increased social behavior abnormalities (Ahmed, 2011, Endo et al., 2012). In mice, prenatal bisphenol A (BPA) exposure altered the development of central dopaminergic systems and resulted in hyperactivity and increased reward-seeking behavior (Mizuo et al., 2004a, Mizuo et al., 2004b, Narita et al., 2006, Suzuki et al., 2003). Also, prenatal and developmental exposure to BPA has been shown to elicit multiple sex-specific behavioral deficits including increase impulsivity, neophobia and exploratory behavior, altered maternal behavior and adult social behavior (Adriani et al., 2003, Gioiosa et al., 2013, Palanza et al., 2002, Patisaul et al., 2012, Spulber et al., 2014, Wolstenholme et al., 2011).
This is notable given that mesocorticolimbic dopamine systems mediate multiple behavioral domains particularly related to cognitive and executive functions as well as memory consolidation, temporal discrimination, exploratory and food-reinforced reward behaviors (Cory-Slechta et al., 1997, Rossato et al., 2013, Sy et al., 2010). Further, there is growing appreciation that maternal and early life exposure to environmental toxicants can potentially have a profound lifelong impact on the central nervous system (CNS). Evidence is accumulating that these alterations occur at low doses, as many EDCs show non-monotonic dose-response relationships (Vandenberg, Colborn, 2012). However, most of this information has been obtained on a chemical-by-chemical basis. The neuroendocrine and behavioral deficits associated with EDC mixtures, which is more representative of human exposures, have not been evaluated (Diamanti-Kandarakis, Bourguignon, 2009).
There has been significant controversy as to whether low levels of EDCs can act together, particularly when present at lower than threshold concentrations, particularly if they have different mechanisms of action. However, as we have previously pointed out, multiple insults occurring concurrently at multiple sites within the e.g., dopamine system, may constrict the range and flexibility of compensatory mechanisms, thereby compromising integrity and viability of the system, and ultimately be more damaging than multiple insults at the same molecular target sites (Cory-Slechta, 2005). Indeed, recent studies of low dose mixtures of chemicals reducing androgens via different mechanisms resulted in additive male reproductive dysfunction effects (Howdeshell et al., 2008, Rider et al., 2009).
Based on this presumption, this study sought to determine whether the impact of multiple EDCs, all known to impact brain mesocorticolimbic systems but by different mechanisms, would yield enhanced effects in combination, as manifest in behaviors known to be mediated by these dopamine/glutamate circuits and correspondingly whether observed effects would, as expected, differ by sex. To this end, we assessed performance under the fixed-interval schedule of reinforcement, object exploration, novel object recognition, and spontaneous locomotor activity, all behaviors in which mesocorticolimbic system function is important (Cory-Slechta, Pazmino, 1997, Rossato, Radiske, 2013, Sequeira-Cordero et al., 2013), in offspring exposed developmentally to four EDCs alone and combined in a mixture. As a first such study pursuing this hypothesis, it did not include full concentration-effect curves for all EDCs but was intended to provide critical information that could be used to more specifically formulate subsequent studies.
Methods
Developmental Exposure
C57BL/6 mice (age 9 weeks) were obtained from the Jackson Laboratory (Bar Harbor, ME). Nulliparous females were housed with males, and checked daily for presence of a vaginal plug. The day a vaginal plug was found was designated as gestational day (GD) 0. Pregnant mice were then individually housed for the remainder of the study. Pregnant mice were exposed orally to either the single EDC dose or the combination of all four doses: Atrazine (ATR – 10mg/kg), Perfluorooctanoic acid (PFOA – 0.1 mg/kg), Bisphenol-A (BPA - 50μg/kg), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD – – 0.25μg/kg) and their mixture (MIX), from GD 7, a time point chosen because it represents the period shortly after embryonic implantation, until weaning (Wang and Dey, 2006). Vehicle (VEH) control dams were gavaged with peanut oil containing an equivalent concentration of anisole and given control treats daily. These compounds have all been shown to influence mesocorticolimbic and neuroendocrine systems (see above), and doses were defined as relatively low doses either based on current human reference doses or were below levels typically shown to cause effects in animal studies (Fenton et al., 2009, Rowe et al., 2008, Vom Saal and Hughes, 2005, Vorderstrasse et al., 2006). ATR and BPA (Sigma Aldrich, St. Louis, MO) were dissolved in peanut oil and administered to pregnant mice daily via puffed wheat cereal. PFOA (Sigma Aldrich, St. Louis, MO) was dissolved in water and administered daily via puffed wheat cereal. TCDD (Cambridge Isotopes, Cambridge, MA) was dissolved in anisole and diluted in peanut oil. Female mice were administered TCDD via oral gavage on GD 7 and 14, and postnatal day (PND) 2, as TCDD has a relatively longer half-life (11 days), this schedule facilitates an even dosing throughout the pregnancy (Gasiewicz et al., 1983). MIX exposed mice were given puffed wheat cereal containing the same dose of each EDC and gavaged on the same schedule as each singly treated group. Random observations of dams indicated total consumption of the treated puffed wheat cereal used for dosing.
Offspring were weaned at PND 21. In order to minimize litter specific effects, no more than 2 mice per sex per dam were used. All mice were pair-housed in microisolator cages after weaning in a specific pathogen-free facility under a 12hr light-dark cycle maintained at 22 ± 2 °C at the University of Rochester Medical Center. All animal treatments were conducted with approval of the Institutional Animal Care and Use Committees at the University of Rochester.
Maternal Health and Weight
Pregnancies were monitored daily to evaluate whether there were differences in time to parturition, litter size, or sex ratio of all the offspring born to each dam. As adults, all offspring were weighed to ensure that there were no significant body weight differences before behavioral testing began. In preparation for behavioral testing, mice were food-restricted to 85% free feed weight. For this purpose, mice were fed individualized amounts of food and weighed five times a week to ensure maintenance of individual 85% free feeding weights throughout behavioral testing.
Behavioral Battery
Behavioral testing of offspring began at 60 days of age, and consisted of locomotor activity, followed by novel object recognition performance, and finally performance on a fixed interval schedule of food reward, all behaviors under control of brain mesocorticolimbic systems. These specific behaviors were chosen to provide information on potential changes in cognitive and motor related functions. Since performance on the FI schedule has been demonstrated to influence subsequent behavioral performances on reinforcement schedules, it was tested last in the sequence.
Locomotor activity
Locomotor activity was assessed in photobeam chambers to determine any treatment-related motor deficits that might also impact performance on the novel object recognition paradigm or Fixed Interval performance. Spontaneous locomotor activity was measured in chambers (27.3 cm × 27.3 cm × 20.3 cm) equipped with 48-channel infrared photobeams (Med Associates Inc., St. Albans, Vermont). Photobeam breaks were recorded every 5 minutes for an hour to assess horizontal, vertical, and ambulatory movements. Mice were initially habituated to the locomotor activity chambers in two 60 min sessions occurring on consecutive days and locomotor behavior assessed during the third session. Ambulatory counts were defined as the number of beam breaks while in ambulatory movement. Vertical activity was defined as total time spent breaking any photobeam in the Z-axis, with the z-axis measured by photobeams mounted 7 cm above the floor of the locomotor box. Horizontal counts were defined as the number of beam breaks in a 2×2 inch photobeam box that were non-ambulatory (i.e. movement that occurred while the animal remained within the 2×2 inch defined photobeam box). Resting time was defined as time spent with no new photobeam breaks.
Novel Object Exploration and Recognition
NOR testing consisted of two phases and was conducted in an open plexiglass arena (dimensions: 30.5 cm × 30.5 cm × 30.5 cm). In the first session, mice were placed in the test environment that contained two objects for 10 min, enabling individual assessments of response to novelty, decreased exploratory behavior or neophobia. During that time, side preference, exploration time, and patterns of exploration among treatment groups were assessed.
The second session of the NOR paradigm assessed short-term memory, premised on an animal’s awareness of novelty and its memory of already familiar objects. In the second session, occurring 24 hours after session 1, mice were returned for 5 min to the arena, in which a novel object now replaced one of the previous two objects. Placement of the novel object was counterbalanced across treatments to preclude bias. All sessions were videotaped and scored by a reviewer blinded to treatment group. Exploration was defined as a mouse oriented towards the object with head first entry into a pre-marked 2 cm circle surrounding the object. A recognition index was calculated based on the time spent with novel object compared to the familiar object (time spent with novel object/(time spent with novel object + time spent with familiar object). Time per approach was calculated by the average time spent per bout of investigation for either the novel or familiar object.
Fixed-interval schedule of reinforcement
The fixed interval (FI) schedule of reward is a behavioral paradigm that can be used to assess multiple behavioral dimensions, including learning, ability to inhibit responding and motivation to act for reward. FI testing occurred in sound-attenuated operant chambers (30.5 cm × 24.5 cm × 21 cm; Med Associates Inc., St. Albans, Vermont). Chambers were equipped with a grid floor, speaker, house light and three response levers connected to a feeder that delivered 20 mg food pellets. Only one lever was activated for reinforcement contingencies during the FI schedule, although responses on the other two levers were recorded. Mice were trained to depress the response levers for food reward using a previously described protocol (Cory-Slechta et al. 1985). The training schedule consisted of a 6 hour variable time 60 sec fixed ratio 1 (VT60FR1) schedule followed by 1 hour FR1 sessions across several days. Training was considered complete when subjects earned 50 rewards within a FR1 session. Subjects required between 1 and 4 FR1 sessions, and numbers of sessions to meet this criterion did not differ by treatment group.
A 60 second FI schedule was then imposed in the next behavioral test session. Sessions were initiated by the first response which initiated a 60 second fixed interval. The first lever press response after completion of the 60 sec interval produced food reward and initiated the next 60 second fixed interval. Sessions were 30 minutes in duration and carried out for 6 sessions to focus on early acquisition of characteristic FI performance. Standard FI performance was measured by overall response rate (total number of responses on the designated FI lever divided by total session time) evaluating the integrated performance on the schedule that includes both responding and pausing between rewards. Additionally, post-reinforcement pause (PRP) time and inter-response times (IRT) were measured during each FI session to assess behavioral mechanisms associated with any observed differences in overall response rate. PRP is defined as the amount of time between food reward delivery and the first lever press response in the next 60 sec interval. IRT is median time-lapsed between FI responses. These two measures allow separation between the time delay to begin lever pressing from the duration of time between lever presses once lever pressing has resumed.
Statistical Analysis
Breeding outcomes and NOR data were analyzed by ANOVA with treatment group as a between groups factor. Adult weight, FI and locomotor testing were analyzed separately using a repeated measures ANOVA with treatment group (ATR, TCDD, PFOA, BPA, MIX and VEH) as a between group factor and session number as a continuous within group factor. Post hoc testing was conducted contingent on ANOVA outcomes using Tukey-Kramer HSD to correct for multiple comparisons for one-way ANOVAs and contrast tests for repeated measures ANOVAs. Given that the two sexes characteristically maintain different hormone profiles and show sex-specific behavioral responses to EDC exposures (Bigsby et al., 1999, Gioiosa et al., 2007, Jašarević et al., 2013), and that our previous data indicates robust sex specific differences during these behavioral tests (Allen et al., 2014, Weston et al., 2014), all analyses were conducted separately by sex. Statistical analyses were conducted using JMP Pro 9.0 (SAS Institute Inc., Cary, N.C.).
Results
Breeding outcomes
There were no treatment related deficits observed during pregnancy or time to parturition. Litter sizes (Table 1, F(5, 32) = 0.16, p = 0.98) and sex ratios (F / M) were equal across treatment groups (Table 1, F(5, 32) = 0.57, p = 0.73). There were no significant weight differences in pups during the month before behavioral testing began (females: F(5, 40) = 1.44, p = 0.23; males: F(5, 65) = 1.31, p = 0.27).
Table 1.
Pregnancy Outcomes and Adult Weights
| Treatment | Total number of Dams |
Mean litter size ± S.E. |
Average sex ratio ± S.E. f/m |
Mean Adult Weight ± S.E. (F) |
Mean Adult Weight ± S.E. (M) |
|---|---|---|---|---|---|
|
| |||||
| ATR | 7 | 7.4 ± 0.24 | 0.81 ± 0.13 | 18.9 ± 0.32 | 23.8 ± 0.38 |
| BPA | 6 | 7.2 ± 0.71 | 1.12 ± 0.17 | 19.5 ± 0.31 | 23.5 ± 0.40 |
| MIX | 6 | 6.6 ± 1.02 | 0.98 ± 0.37 | 19.2 ± 0.36 | 24.8 ± 0.48 |
| PFOA | 6 | 7.5 ± 0.28 | 1.24 ± 0.28 | 18.6 ± 0.26 | 24.0 ± 0.43 |
| TCDD | 7 | 7.0 ± 0.19 | 0.65 ± 0.18 | 18.9 ± 0.36 | 24.2 ± 0.33 |
| VEH | 11 | 7.0 ± 0.76 | 1.16 ± 0.35 | 19.0 ± 0.20 | 23.7 ± 0.29 |
Behavioral Testing
Locomotor Behavior
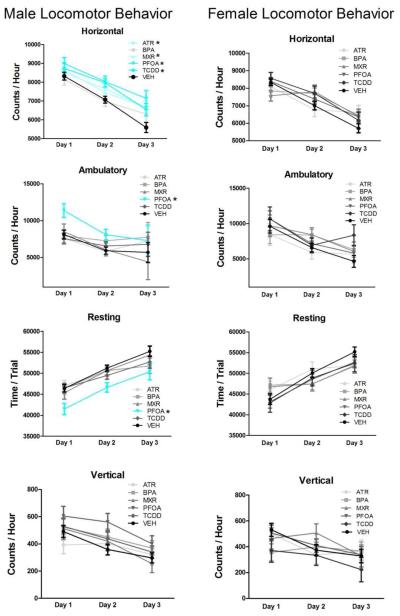
Significant EDC-induced changes in locomotor activity were seen only in males (Fig. 1). Males in nearly all EDC groups (ATR, TCDD, PFOA and MIX) showed significantly increased horizontal movement across the three days of locomotor assessment (F(5,68) = 3.93, p = 0.004) relative to vehicle control. Interestingly, these effects emerged only with repeated testing, with significant differences seen only during the second and third test sessions, findings consistent with a potential reduction in environmental habituation (Session 1: F(5,68) = 1.05, p = 0.39; Session 2: F(5,68) = 3.42, p = 0.008; Session 3: F(5,68) = 2.62, p = 0.03). In addition to the elevated horizontal activity, PFOA males also exhibited decreased resting time (Resting: F(5,68) = 2.38, p = 0.048) and increased ambulatory movements in session one of the locomotor assessment (F(5,68) = 2.71, p = 0.03). No significant treatment-related differences were found for vertical activity in males.
Figure 1a-h.
Locomotor behavior was significantly altered in the exposed male offspring but not females. Group mean ± S.E locomotor counts and time for males (left) and females (right) for treatment groups as indicated. Asterisks indicate significant difference from vehicle control. Males: MIX = 7, ATR = 11, BPA = 10, PFOA = 9, TCDD = 14, VEH = 22; Females: MIX = 6, ATR = 6, BPA = 9, PFOA = 12, TCDD = 5, VEH = 16.
Novel Object Exploration and Recognition
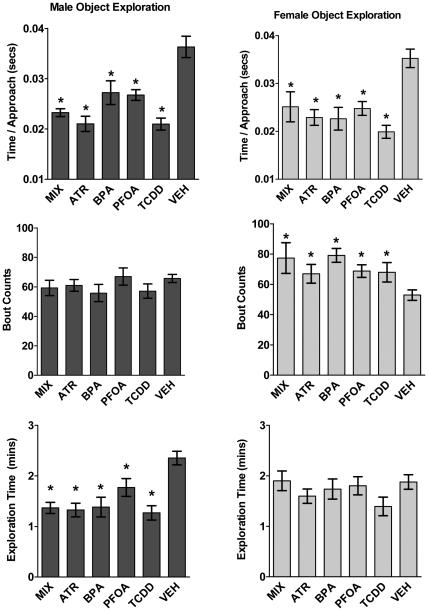
A robust decrease in the rate of exploration during the initial phase of NOR testing (session 1) occurred in all treatment groups in both sexes. Specifically, males and females exposed to BPA, PFOA, TCDD, ATR and MIX all showed reductions in initial exploration of the novel objects, as indicated by significant reductions in time spent per approach (Figure 2: male by treatment group: F(5, 62) = 13.13, p < 0.001, post hoc: Tukey-Kramer HSD: ATR, p = 0.001, BPA, p < 0.006, PFOA, p < 0.005, TCDD, p < 0.001, MIX, p < 0.001; female by treatment group: F(5, 40) = 8.22, p < 0.001, post hoc: Tukey-Kramer HSD: ATR, p = 0.002, BPA, p < 0.001, PFOA, p < 0.001, TCDD, p < 0.001, MIX, p = 0.03). However, while EDC-treated females compensated by increasing the number of times they approached the novel objects, EDC-treated males did not (Figure 2: male by treatment group: F(5, 62) = 1.00, p = 0.43; female by treatment group: F(5, 40) = 4.31, p < 0.01). Consequently, only EDC-treated males showed significantly decreased overall exploration time, suggesting the different treatments resulted in deficits in exploratory behavior in males (Figure 2: male: F(5, 62) = 9.60 p < 0.001, post hoc: Tukey-Kramer HSD: ATR, p < 0.001, BPA, p < 0.001, PFOA, p = 0.09, TCDD, p < 0.001, MIX, p < 0.001; female: F(5, 40) = 0.95, p = 0.45).
Figure 2a-f.
NOR exploratory behavior was disrupted by EDC exposure. Group mean ± S.E measures of NOR performance in the first testing session, including time spent per each approach to the object replaced in session 1 by a novel object, number of bouts of approach to that object, and time spent exploring the object for males (left column) and females (right column) for treatment groups as labeled. Asterisks signify significant difference from vehicle control. Males: MIX = 7, ATR = 11, BPA = 10, PFOA = 8, TCDD = 14, VEH = 17; Females: MIX = 5, ATR = 6, BPA = 7, PFOA = 9, TCDD = 5, VEH = 15.
In accordance with other studies using NOR testing (Aubele et al., 2008a, Frick and Gresack, 2003), control males had significantly longer exploration times than did control (vehicle) females (Figure 3: VEH male: VEH female, t = 2.41, p < 0.05). However, MIX exposed males actually showed significantly lower rates of exploration compared to MIX exposed females (Figure 3: MIX male: MIX female, t = −3.40, p < 0.05), suggesting a reversal of sex-associated differences in exploratory behavior by the MIX treatment. The reversal of this sex specific exploration bias suggests that MIX males experienced the greatest reduction of exploratory behavior, as female rates did not change significantly in response to MIX treatment (Figure 3: VEH male: MIX male, t = 4.42; p < 0.001; VEH female: MIX female, t = −0.08; p = 0.93). This enhanced reversal of sex specific exploratory behavior was only seen in the MIX treatment group (data not shown).
Figure 3.
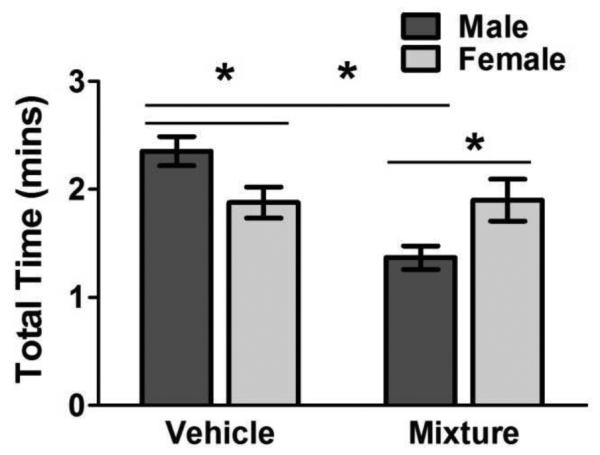
Reversal of sexually dimorphic exploratory behavior was seen in mixture group. Group mean ± S.E total time (min) exploring the objects in session 1 for male and female, vehicle and mixture-treated groups as indicated. Asterisks designate significant differences between groups indicated by horizontal lines. Males: MIX = 7, ATR = 11, BPA = 10, PFOA = 8, TCDD = 14, VEH = 17; Females: MIX = 5, ATR = 6, BPA = 7, PFOA = 9, TCDD = 5, VEH = 15.
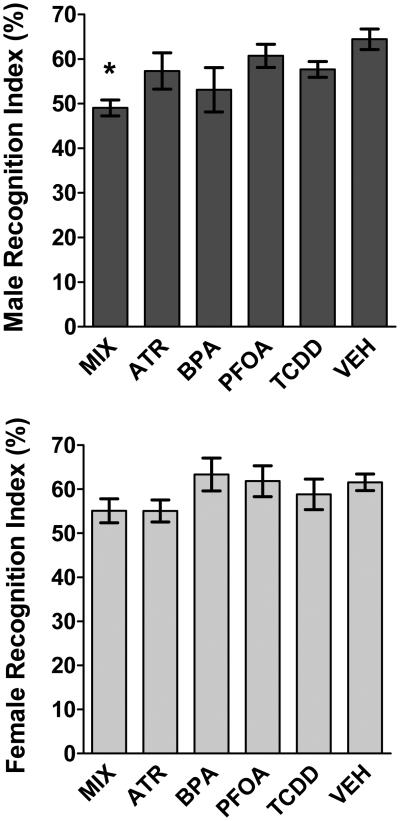
In the second session, enhanced deficits in short-term memory were observed in MIX exposed males, as reflected in the significantly reduced recognition indices based on overall exploration time (Figure 4, F(5, 61) = 2.92, p < 0.02; Tukey-Kramer HSD: p = 0.018). Males exposed to single EDCs did not show significant differences in recognition time compared to control (Figure 4, F(5, 61) = 2.92, p < 0.02; Tukey-Kramer HSD: ATR, p = 0.48; BPA, p = 0.10; TCDD, p = 0.47; PFOA, p = 0.26). For females, significant treatment-related deficits in short-term memory were not observed based on the overall exploration time recognition index (Figure 4: F(5, 40) = 0.84, p = 0.52). However, TCDD-treated females spent significantly less time per each approach of the novel object compared to VEH exposed females, suggesting slight memory deficits (t = 2.02, p = 0.02), as VEH females spent more time with the novel object during each exploration bout (data not shown).
Figure 4a and b.
Enhanced NOR recognition deficits in MIX exposed male mice. Group mean ± S.E recognition index values (percent) in session 2 for males (a, top) and females (b, bottom) for treatment groups as indicated. Asterisks indicate significant difference from vehicle control. Males: MIX = 7, ATR = 11, BPA = 10, PFOA = 8, TCDD = 14, VEH = 17; Females: MIX = 5, ATR = 6, BPA = 7, PFOA = 9, TCDD = 5, VEH = 15.
Fixed Interval Reinforcement Schedule-Controlled Behavior
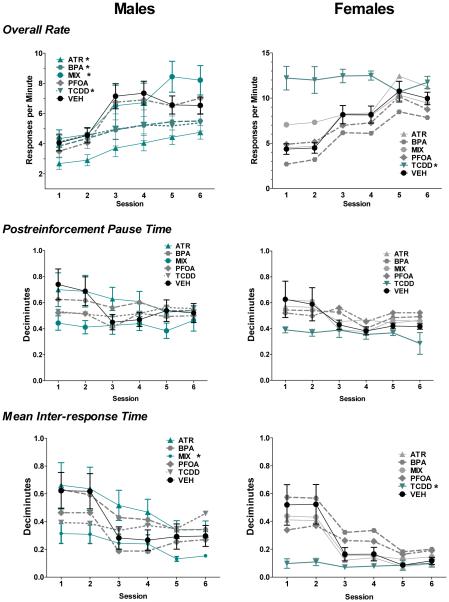
Enhanced behavioral deficits were seen in male MIX offspring during the acquisition of the FI reinforcement schedule (Figure 5). MIX males showed significantly increased FI response rates, suggesting an inability to inhibit responding or impulsive responding (treatment (TX) by session, F(25, 310) = 3.08, p < 0.001, post hoc: mixture vs. control, F(5, 310) = 4.43, p < 0.001). This behavioral response was unique to MIX exposed males, as increased rates of responding were not seen in any other male treated group. In fact, ATR, BPA and TCDD males exhibited significant reductions in response rates relative to controls, suggestive of reduced attention to task or impaired motivation (post hoc: ATR vs. control, F(5, 310) = 3.86, p < 0.002; TCDD: F(5, 58) = 5.61, p < 0.001; BPA: F(5, 310) = 5.61, p < 0.001). Response rates of PFOA exposed males did not differ from control males (F(5, 310) = 0.68, p = 0.64).
Figure 5a-f.
Enhance deficits in FI behavior seen only in male mice exposed to the mixture. Group mean ± S.E. changes in overall response rate (top row), postreinforcement pause time (middle row) and mean inter-response times of male (left column) and female (right column) treatment groups as indicated across the sessions of FI testing. Asterisks next to the designation of a treatment group designate significant difference from control. Males: MIX = 7, ATR = 11, BPA = 10, PFOA = 9, TCDD = 14, VEH = 17; Females: MIX = 4, ATR = 6, BPA = 7, PFOA = 9, TCDD = 5, VEH = 15.
TCDD exposed females showed marked increases in FI response rates during early sessions, suggesting potential increased impulsivity or inability to acquire the temporal pattern of response (Figure 5; TX*session, F(5, 39) = 2.38, p < 0.001), post hoc: TCDD vs. control, F(5, 195) = 8.38, p < 0.001). MIX females did not show enhanced effects during the FI schedule (post hoc: MIX vs. control, F(5, 195) = 1.45, p = 0.21). In fact, the response rates of MIX females were significantly lower than TCDD exposed females (post hoc: MIX vs. TCDD, F(6, 195) = 2.91, p < 0.02).
PRP time was not significantly different between treatment groups or treatment groups over time, for either males or females (Figure 5: males: TX; F(5, 62) = 0.95, p = 0.45; TX by session, F(25, 310) = 1.19, p = 0.17; Figure 5: females: TX; F(5, 39) = 0.62, p = 0.68; TX by session, F(25, 195) = 0.57, p = 0.95). Consistent with the significantly increased overall response rates in MIX exposed males and TCDD exposed females, however, both also exhibited significantly reduced IRT times. Collectively with the absence of changes in PRP times, these findings suggest an inability to inhibit responding and not a more rapid initiation of responding within the interval, i.e., a temporal discrimination dysfunction, likely explained increased overall response rates of MIX males and TCDD females (Figure 5: males: TX by session, F(25, 310) = 2.05, p < 0.01), post hoc: MIX vs. controls, F(5, 310) = 19.41, p < 0.001; Figure 5: females: post hoc: TCDD vs. controls, F(5, 195) = 3.22, p < 0.01). However, male treatment groups that showed significant decreases in overall response rate, i.e., ATR, BPA and TCDD, did not show significant alterations in either PRP time or in IRT values, suggesting that modest but non-statistically significant delays to begin responding in the interval and lack of repeated responding once initiated may combine to create significantly lower overall response rates.
Discussion
Developmental exposure to EDCs alone and as a mixture led to multiple behavioral deficiencies in adulthood in behaviors that included a fixed interval reinforcement schedule (FI), novel object recognition (NOR) paradigm and spontaneous locomotor activity, all known to involve mesocorticolimbic mediation (Antunes and Biala, 2012, Cory-Slechta, Pazmino, 1997, Cory-Slechta et al., 1998, Ennaceur et al., 2005, Evans and Cory-Slechta, 2000) predicated on the fact that developmental exposures to these EDCs are known to alter monoaminergic neural pathways (De Coster and van Larebeke, 2012, Frye et al., 2012, Suzuki, Mizuo, 2003), particularly along the mesolimbic dopaminergic systems (Palanza et al. 2008). Specifically, behavioral deficits were found in response to all five EDC treatments, but were enhanced with exposure to a mixture of EDCs in a sex-specific manner, with only males showing unique behavioral deficits compared to the single EDC exposed males.
MIX exposed males had significantly elevated FI rates of responding, whereas components of the MIX, including ATR, TCDD and BPA significantly reduced overall rates of responding on the FI schedule, demonstrating that the combination produced a unique alteration of adult male FI performance. It is interesting to think about the possibility that these results reflect altered underlying mesocorticolimbic dopamine function, as we have previously reported a U-shaped curve relating dopamine to FI response rates (Cory-Slechta, 1998), wherein either increases or decreases in dopamine ultimately suppress FI response rates through their ability to suppress locomotor (low levels) and induce stereotypy (high levels). This will require future assessment of dose effect curves for dopamine-related changes in response to the single EDCs and the mixture. However, the dichotomous nature of the effects is notable given the accompanying disparate interpretations, i.e., that MIX could lead to increased impulsivity or an inability to inhibit responding and/or time discrimination deficits, whereas individual components induce a lack of attention to task and/or even impaired motivation or impaired temporal discrimination. Considerations of non-linearity of underlying neurotransmitter mediating functions will be critical to interpretations of behavioral outcomes in future studies of mixtures and may indicate that disparate behavioral changes can nevertheless reflect interactions in a common neurotransmitter system.
Male specific MIX effects likewise characterized novel object recognition performance. Evidence of neophobia for both sexes exposed developmentally to EDCs occurred during the initial session of the NOR task in that all EDC groups of both sexes showed decreased time per approach to objects relative to vehicles, but only males exhibited significantly decreased overall time spent exploring the objects, suggesting a more severe effect in males. These findings may be consistent with the EDC profile of locomotor activity and habituation failure changes in response to EDCs in males, who failed to exhibit the typical decreases in horizontal movement over repeated activity testing sessions in the locomotor chambers, suggesting an absence of habituation to the novelty of the chamber. These differences were not seen in female mice.
Novelty exploration and neophobia is typically a sexually dimorphic behavior. Male preference for novelty has been suggested to have adaptive functions (Wilson and Daly, 1985), with support from rodent studies indicating that males spend more time exploring novel objects than females (Cyrenne and Brown, 2011, Frick and Gresack, 2003). Even when females show increased exploratory behavior, developmental exposure to EDCs eliminates any sex specific differences in exploratory behavior and alters sex-specific neurochemistry (Gioiosa, Fissore, 2007, Rubin et al., 2006). The current findings add further support to those assertions that developmental exposure to EDCs creates sex-specific alterations in exploratory behavior (Patisaul, Sullivan, 2012). In this study, vehicle males showed significantly increased overall exploration time with objects compared to vehicle females, findings consistent with prior reports (Cummings et al., 2013, Cyrenne and Brown, 2011, Frick and Gresack, 2003, Wilson and Daly, 1985). In contrast, mice developmentally exposed to the mixture of EDCs showed the opposite relationship, as MIX exposed males showed significantly decreased exploration times compared to MIX exposed females. There is experimental evidence that such behavioral differences can be hormonally mediated, as rats exposed to a long-acting gonadotrophin-releasing hormone antagonist, Antide, demonstrated a similar behavioral reversal. Antide treated males showed reduced serum testosterone and diminished preference for novelty compared to females (Cyrenne and Brown, 2011). Additionally, gonadectomized males showed diminished exploratory behavior compared to control males, while gonadectomized males given testosterone increased novel object exploration (Aubele et al., 2008b). Future studies will assist in understanding whether these sex-dependent EDC effects relate to feminized hormone physiology and/or altered neurochemical function.
Novel object recognition is considered an assay of short-term memory. Memory deficits occur after exposure to EDCs, e.g., BPA, ATR and TCDD, and have been associated with spatial and object memory deficits in developmentally exposed male mice (Belloni et al., 2011, Brouillette and Quirion, 2008, Lin et al., 2013b, Palanza, Gioiosa, 2008, Tian et al., 2010, Xu et al., 2011). However, in the current study after controlling for multiple comparisons, only MIX males showed significant memory deficits with a reduced recognition index in the NOR assay based on overall exploration time of novel relative to familiar objects. The unique influence of the mixture of EDCs on male memory warrants further investigation as the reduced exploration patterns during the first NOR test session may be responsible for the memory deficits seen during the recognition phase of the NOR testings, as animals that have reduced experience with the objects during that habituation phase may have difficultly separating novel from familiar in the recognition phase. As such, it is critical to include information from the NOR training sessions in any analyses of short-term memory.
Of additional note in this study were the dramatic behavioral consequences of TCDD exposure in females. As TCDD is a selective AhR receptor, it is relevant that AhR genes, ARNT, and ARNT2, are sensitive to perinatal steroid hormone manipulations and expressed in sexually dimorphic brain regions (Petersen et al., 2006) such as the sexually dimorphic nuclei of the preoptic area, which contain estrogen receptors, and the anteroventral periventricular nucleus, which is a structure critical for sex-specific, estrogen-dependent, luteinizing hormone surge release (Petersen and Barraclough, 1989, Petersen et al., 1989, Petersen, Krishnan, 2006). Gene expression for AhR, ARNT, and ARNT2, mRNAs was also found in the sexually dimorphic medial preoptic regions and the ventromedial hypothalamus, brain regions critical to expression of male sexual behaviors (Gray and Brooks, 1984, Meisel and Sachs, 1994) and female (Kow and Pfaff, 1998). Of further note is the report that nearly all AhR gene expression in the brain of developing and adult rats is found in GABAergic neurons (i.e., those expressing the glutamic acid decarboxylase, the enzymes critical for GABA synthesis) suggesting GABA inhibitory function may be a target of TCDD exposure (Hays et al., 2002) which could modulate the dopamine/glutamate balance of mesocorticolimbic systems (Harte and O'Connor, 2005, Yamaguchi et al., 2011). Animal and human studies suggest that exposure to TCDD elicits memory deficits, with some suggestion that females are more susceptible than males (Barrett et al., 2001, Brouillette and Quirion, 2008).
PFOA exposed males also showed increased ambulatory movement and decreased resting time, suggesting significantly increased hyperactivity compared to controls. Multiple studies also identify a causal role of developmental exposure to PFOA with increased hyperactivity in rodents, and epidemiological studies support a role for PFOA in human ADHD etiology, particularly at low exposure levels (Hoffman et al., 2010, Johansson et al., 2008, Ode et al., 2014, Onishchenko, Fischer, 2011). Taken together, the role of low level EDC exposures, particularly to perfluorinated chemicals, in the etiology of attention-related behaviors warrants further investigation.
Some limitations to the current study should be noted. One is the fact that TCDD was administered differently (gavage) than BPA, ATR and PFOA. It has become increasingly clear that prenatal stress can act synergistically to enhance behavioral and neurochemical toxicity (Cory-Slechta et al., 2010, Weiss and Bellinger, 2006). Because TCDD has a longer half-life, TCDD is traditionally administered via oral gavage: as a result TCDD, mixture and control mice were all gavaged once every seven days, potentially exposing these groups to increased prenatal stress, as gavage has been long known to cause stress (Brown et al., 2000). Therefore, prenatal stress is a potential confounding variable for MIX, TCDD and control mice. Future research should identify alternative means of appropriate administration of TCDD to reduce stress or should specifically investigate the potential role prenatal stress may have on synergistically eliciting neurobehavioral deficits associated with EDC exposure. The fact that control mice did receive vehicle gavage, however, provides support for the enhanced behavioral toxicity of MIX relative to control values.
In summary, the sex dependent alterations observed in behavioral performance, e.g., impulsivity and neophobia, could correspond with sex-biased human behavioral disorders, like addiction and ADHD. Further, this male-biased susceptibility to EDC mixtures was observed in the absence of signs of overt toxicity, or changes in body weight, litter size or sex ratios, and despite the heterogeneity of the chemical classes and associated mechanisms. Collectively, these findings support the need to further evaluate relationships between developmental exposure to EDCs and sex dependent neurobehavioral toxicity. EDCs have been implicated in the increased incidence of neurobehavioral disorders with sex biased prevalence rates, such as ADHD and autism, where males are increasingly susceptible (Colborn, 2004a, b, Frye, Bo, 2012). Such research will should include the generation of full dose-effect curves for individual EDCs and the MIX, particularly given findings of non-monotonic dose effect curves and the recognition of the non-linearity of underlying neurobiological substrates, e.g., dopamine (Cory-Slechta, 2005, Cory-Slechta, O'Mara, 1998, Evans and Cory-Slechta, 2000). Further, the fundamental role of sex hormones in behavioral neurodevelopment, including time-course assessments of hormone profiles will be requisite to this understanding.
Supplementary Material
Highlights.
All males exposed to EDCs showed exploratory deficits during NOR habituation.
Only MIX exposed males showed a sex specific reversal in exploratory behavior.
MIX exposed males displayed reduced object recognition during NOR testing.
MIX exposed males showed uniquely elevated response rates during FI acquisition.
TCDD exposure produced notable increases in FI response rates in females.
Acknowledgements
This work was supported by P30 ES001247 and T32 ES007026-36.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Della Seta D, Dessí-Fulgheri F, Farabollini F, Laviola G. Altered profiles of spontaneous novelty seeking, impulsive behavior, and response to D-amphetamine in rats perinatally exposed to bisphenol A. Environmental health perspectives. 2003;111:395. doi: 10.1289/ehp.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R. Perinatal TCDD exposure alters developmental neuroendocrine system. Food and Chemical Toxicology. 2011;49:1276–84. doi: 10.1016/j.fct.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Allen JL, Liu X, Weston D, Prince L, Oberdörster G, Finkelstein JN, et al. Developmental Exposure to Concentrated Ambient Ultrafine Particulate Matter Air Pollution in Mice Results in Persistent and Sex-Dependent Behavioral Neurotoxicity and Glial Activation. Toxicological Sciences. 2014:kfu059. doi: 10.1093/toxsci/kfu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognitive processing. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Kaufman R, Montalmant F, Kritzer M. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Hormones and behavior. 2008;54:244–52. doi: 10.1016/j.yhbeh.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardullas U, Giordano M, Rodríguez VM. Chronic atrazine exposure causes disruption of the spontaneous locomotor activity and alters the striatal dopaminergic system of the male Sprague–Dawley rat. Neurotoxicology and teratology. 2011;33:263–72. doi: 10.1016/j.ntt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Barrett DH, Morris RD, Akhtar FZ, Michalek JE. Serum Dioxin and Cognitive Functioning among Veterans of Operation Ranch Hand. Neurotoxicology. 2001;22:491–502. doi: 10.1016/s0161-813x(01)00051-1. [DOI] [PubMed] [Google Scholar]
- Belloni V, Dessí-Fulgheri F, Zaccaroni M, Di Consiglio E, De Angelis G, Testai E, et al. Early exposure to low doses of atrazine affects behavior in juvenile and adult CD1 mice. Toxicology. 2011;279:19–26. doi: 10.1016/j.tox.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Bigsby R, Chapin RE, Daston GP, Davis BJ, Gorski J, Gray LE, et al. Evaluating the effects of endocrine disruptors on endocrine function during development. Environmental health perspectives. 1999;107(Suppl 4):613–8. doi: 10.1289/ehp.99107s4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillette J, Quirion R. The common environmental pollutant dioxin-induced memory deficits by altering estrogen pathways and a major route of retinol transport involving transthyretin. Neurotoxicology. 2008;29:318–27. doi: 10.1016/j.neuro.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Brown AP, Dinger N, Levine BS. Stress produced by gavage administration in the rat. Journal of the American Association for Laboratory Animal Science. 2000;39:17–21. [PubMed] [Google Scholar]
- Coban A, Filipov N. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. Journal of neurochemistry. 2007;100:1177–87. doi: 10.1111/j.1471-4159.2006.04294.x. [DOI] [PubMed] [Google Scholar]
- Colborn T. Endocrine disruption overview: are males at risk? Advances in experimental medicine and biology. 2004a;545:189–201. doi: 10.1007/978-1-4419-8995-6_12. [DOI] [PubMed] [Google Scholar]
- Colborn T. Neurodevelopment and endocrine disruption. Environmental health perspectives. 2004b;112:944–9. doi: 10.1289/ehp.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta D, Pazmino R, Bare C. The critical role of nucleus accumbens dopamine systems in the mediation of fixed interval schedule-controlled operant behavior. Brain research. 1997;764:253–6. doi: 10.1016/s0006-8993(97)00591-x. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA. Studying toxicants as single chemicals: does this strategy adequately identify neurotoxic risk? Neurotoxicology. 2005;26:491–510. doi: 10.1016/j.neuro.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, O'Mara DJ, Brockel BJ. Nucleus accumbens dopaminergic medication of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. The Journal of pharmacology and experimental therapeutics. 1998;286:794–805. [PubMed] [Google Scholar]
- Cory-Slechta DA, Stern S, Weston D, Allen JL, Liu S. Enhanced learning deficits in female rats following lifetime Pb exposure combined with prenatal stress. Toxicological Sciences. 2010;117:427–38. doi: 10.1093/toxsci/kfq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Clinton SM, Perry AN, Akil H, Becker JB. Male rats that differ in novelty exploration demonstrate distinct patterns of sexual behavior. Behavioral neuroscience. 2013;127:47. doi: 10.1037/a0031528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrenne D-LM, Brown GR. Effects of suppressing gonadal hormones on response to novel objects in adolescent rats. Hormones and behavior. 2011;60:625–31. doi: 10.1016/j.yhbeh.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cock M, Maas YGH, van de Bor M. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review. Acta Paediatrica. 2012;101:811–8. doi: 10.1111/j.1651-2227.2012.02693.x. [DOI] [PubMed] [Google Scholar]
- De Coster S, van Larebeke N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. Journal of environmental and public health. 2012 doi: 10.1155/2012/713696. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine reviews. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Kakeyama M, Uemura Y, Haijima A, Okuno H, Bito H, et al. Executive Function Deficits and Social-Behavioral Abnormality in Mice Exposed to a Low Dose of Dioxin In Utero and via Lactation. PloS one. 2012;7:e50741. doi: 10.1371/journal.pone.0050741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Bradford A, Ahmed S. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behavioural brain research. 2005;159:247–66. doi: 10.1016/j.bbr.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Evans S, Cory-Slechta D. Prefrontal cortical manipulations alter the effects of intra-ventral striatal dopamine antagonists on fixed-interval performance in the rat. Behavioural brain research. 2000;107:45–58. doi: 10.1016/s0166-4328(99)00108-4. [DOI] [PubMed] [Google Scholar]
- Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, et al. Analysis of PFOA in dosed CD-1 mice. Part 2: Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reproductive Toxicology. 2009;27:365–72. doi: 10.1016/j.reprotox.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behavioral neuroscience. 2003;117:1283. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Frye C, Bo E, Calamandrei G, Calzá L, Dessí-Fulgheri F, Fernández M, et al. Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. Journal of neuroendocrinology. 2012;24:144–59. doi: 10.1111/j.1365-2826.2011.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiewicz TA, Geiger LE, Rucci G, Neal RA. Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug metabolism and disposition: the biological fate of chemicals. 1983;11:397–403. [PubMed] [Google Scholar]
- Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Hormones and Behavior. 2007;52:307–16. doi: 10.1016/j.yhbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Parmigiani S, vom Saal FS, Palanza P. The effects of bisphenol A on emotional behavior depend upon the timing of exposure, age and gender in mice. Hormones and behavior. 2013;63:598–605. doi: 10.1016/j.yhbeh.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Gray P, Brooks PJ. Effect of lesion location within the medial preoptic-anterior hypothalamic continuum on maternal and male sexual behaviors in female rats. Behavioral neuroscience. 1984;98:703. doi: 10.1037//0735-7044.98.4.703. [DOI] [PubMed] [Google Scholar]
- Harte M, O'Connor W. Evidence for a selective prefrontal cortical gaba b receptor mediated inhibition of glutamate release in the ventral tegmental area: A dual probe microdialysis study in the awake rat. Neuroscience. 2005;130:215–22. doi: 10.1016/j.neuroscience.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Hays LE, Carpenter CD, Petersen SL. Evidence that GABAergic neurons in the preoptic area of the rat brain are targets of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin during development. Environmental health perspectives. 2002;110:369. doi: 10.1289/ehp.02110s3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Webster TF, Weinberg J, Vieira VM, Weisskopf MG. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in US children 12–15 years of age. 2010 doi: 10.1289/ehp.1001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, Filipov NM. Alteration of dopamine uptake into rat striatal vesicles and synaptosomes caused by an in vitro exposure to atrazine and some of its metabolites. Toxicology. 2008;248:52–8. doi: 10.1016/j.tox.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicological Sciences. 2008;105:153–65. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Jašarević E, Williams SA, Vandas GM, Ellersieck MR, Liao C, Kannan K, et al. Sex and dose-dependent effects of developmental exposure to bisphenol A on anxiety and spatial learning in deer mice (Peromyscus maniculatus bairdii) offspring. Hormones and Behavior. 2013;63:180–9. doi: 10.1016/j.yhbeh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson N, Fredriksson A, Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008;29:160–9. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Kow L-M, Pfaff DW. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behavioural brain research. 1998;92:169–80. doi: 10.1016/s0166-4328(97)00189-7. [DOI] [PubMed] [Google Scholar]
- Lin Z, Dodd CA, Filipov NM. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicology and Teratology. 2013a doi: 10.1016/j.ntt.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sachs BD. The physiology of male sexual behavior. The physiology of reproduction. 1994;2:3–105. [Google Scholar]
- Mizuo K, Narita M, Miyagawa K, Narita M, Okuno E, Suzuki T. Prenatal and neonatal exposure to bisphenol-A affects the morphine-induced rewarding effect and hyperlocomotion in mice. Neuroscience letters. 2004a;356:95–8. doi: 10.1016/j.neulet.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Mizuo K, Narita M, Yoshida T, Narita M, Suzuki T. Functional changes in dopamine D3 receptors by prenatal and neonatal exposure to an endocrine disruptor bisphenol-A in mice. Addiction biology. 2004b;9:19–25. doi: 10.1080/13556210410001674059. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyagawa K, Mizuo K, Yoshida T, Suzuki T. Prenatal and neonatal exposure to low-dose of bisphenol-A enhance the morphine-induced hyperlocomotion and rewarding effect. Neuroscience letters. 2006;402:249–52. doi: 10.1016/j.neulet.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Ode A, Källén K, Gustafsson P, Rylander L, Jönsson BA, Olofsson P, et al. Fetal Exposure to Perfluorinated Compounds and Attention Deficit Hyperactivity Disorder in Childhood. PloS one. 2014;9:e95891. doi: 10.1371/journal.pone.0095891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishchenko N, Fischer C, Ibrahim WNW, Negri S, Spulber S, Cottica D, et al. Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related manner. Neurotoxicity research. 2011;19:452–61. doi: 10.1007/s12640-010-9200-4. [DOI] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environmental research. 2008;108:150–7. doi: 10.1016/j.envres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environmental health perspectives. 2002;110(Suppl 3):415–22. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Frontiers in behavioral neuroscience. 2009:3. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Sullivan AW, Radford ME, Walker DM, Adewale HB, Winnik B, et al. Anxiogenic effects of developmental bisphenol a exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PloS one. 2012;7:e43890. doi: 10.1371/journal.pone.0043890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Barraclough CA. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain research. 1989;484:279–89. doi: 10.1016/0006-8993(89)90371-5. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Cheuk C, Hartman RD, Barraclough CA. Medial Preoptic Microimplants of the Antiestrogen, Keoxifene, Affect Luteinizing Hormone-Releasing Hormone mRNA Levels, Median Eminence Luteinizing Hormone-Releasing Hormone Concentrations and Luteinizing Hormone Release in Ovariectomized, Estrogen-Treated Rats. Journal of neuroendocrinology. 1989;1:279–83. doi: 10.1111/j.1365-2826.1989.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Krishnan S, Hudgens ED. The aryl hydrocarbon receptor pathway and sexual differentiation of neuroendocrine functions. Endocrinology. 2006;147:s33–s42. doi: 10.1210/en.2005-1157. [DOI] [PubMed] [Google Scholar]
- Rider CV, Wilson VS, Howdeshell KL, Hotchkiss AK, Furr JR, Lambright CR, et al. Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development. Toxicologic pathology. 2009;37:100–13. doi: 10.1177/0192623308329478. [DOI] [PubMed] [Google Scholar]
- Rodríguez VM, Limón-Pacheco JH, Mendoza-Trejo MS, González-Gallardo A, Hernández-Plata I, Giordano M. Repeated exposure to the herbicide atrazine alters locomotor activity and the nigrostriatal dopaminergic system of the albino rat. Neurotoxicology. 2012 doi: 10.1016/j.neuro.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Radiske A, Kohler CA, Gonzalez C, Bevilaqua LR, Medina JH, et al. Consolidation of object recognition memory requires simultaneous activation of dopamine D1/D5 receptors in the amygdala and medial prefrontal cortex but not in the hippocampus. Neurobiology of learning and memory. 2013;106:66–70. doi: 10.1016/j.nlm.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Rowe AM, Brundage KM, Barnett JB. Developmental immunotoxicity of atrazine in rodents. Basic & clinical pharmacology & toxicology. 2008;102:139–45. doi: 10.1111/j.1742-7843.2007.00175.x. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–91. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environmental health perspectives. 2001;109:1197. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. The Journal of steroid biochemistry and molecular biology. 2011;127:204–15. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira-Cordero A, Mora-Gallegos A, Cuenca-Berger P, Fornaguera-Trías J. Individual differences in the immobility behavior in juvenile and adult rats are associated with monoaminergic neurotransmission and with the expression of corticotropin-releasing factor receptor 1 in the nucleus accumbens. Behavioural brain research. 2013;252:77–87. doi: 10.1016/j.bbr.2013.05.046. [DOI] [PubMed] [Google Scholar]
- Spulber S, Kilian P, Ibrahim WNW, Onishchenko N, Ulhaq M, Norrgren L, et al. PFOS Induces Behavioral Alterations, Including Spontaneous Hyperactivity That Is Corrected by Dexamfetamine in Zebrafish Larvae. PloS one. 2014;9:e94227. doi: 10.1371/journal.pone.0094227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Mizuo K, Nakazawa H, Funae Y, Fushiki S, Fukushima S, et al. Prenatal and neonatal exposure to bisphenol-a enhances the central dopamine d1 receptor-mediated action in mice: enhancement of the methamphetamine-induced abuse state. Neuroscience. 2003;117:639–44. doi: 10.1016/s0306-4522(02)00935-1. [DOI] [PubMed] [Google Scholar]
- Sy H-N, Wu S-L, Wang W-F, Chen C-H, Huang Y-T, Liou Y-M, et al. MPTP-induced dopaminergic degeneration and deficits in object recognition in rats are accompanied by neuroinflammation in the hippocampus. Pharmacology Biochemistry and Behavior. 2010;95:158–65. doi: 10.1016/j.pbb.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Tian YH, Baek JH, Lee SY, Jang CG. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse. 2010;64:432–9. doi: 10.1002/syn.20746. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr., Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocrine reviews. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environmental health perspectives. 2005;113:926. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorderstrasse BA, Cundiff JA, Paige Lawrence B. A dose-response study of the effects of prenatal and lactational exposure to TCDD on the immune response to influenza a virus. Journal of Toxicology and Environmental Health, Part A. 2006;69:445–63. doi: 10.1080/15287390500246985. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nature reviews Genetics. 2006;7:185–99. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- Weiss B, Bellinger DC. Social ecology of children’s vulnerability to environmental pollutants. Environmental health perspectives. 2006;114:1479. doi: 10.1289/ehp.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston HI, Sobolewski ME, Allen JL, Weston D, Conrad K, Pelkowski S, et al. Sex-dependent and non-monotonic enhancement and unmasking of methylmercury neurotoxicity by prenatal stress. Neurotoxicology. 2014;41:123–40. doi: 10.1016/j.neuro.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Daly M. Competitiveness, risk taking, and violence: The young male syndrome. Ethology and sociobiology. 1985;6:59–73. [Google Scholar]
- Wolstenholme JT, Taylor JA, Shetty SR, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PloS one. 2011;6:e25448. doi: 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Tian D, Hong X, Chen L, Xie L. Sex-specific influence of exposure to bisphenol-A between adolescence and young adulthood on mouse behaviors. Neuropharmacology. 2011;61:565–73. doi: 10.1016/j.neuropharm.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Wang H-L, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. The Journal of neuroscience. 2011;31:8476–90. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H-c, Zhang L, Li Y-y, Wang Y-j, Xia W, Lin Y, et al. Inflammation-like glial response in rat brain induced by prenatal PFOS exposure. Neurotoxicology. 2011;32:130–9. doi: 10.1016/j.neuro.2010.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.