Abstract
Background
The use of herbal medicines as complements or alternatives to orthodox medicines has been on the increase. There has been the erroneous belief that these medicines are free from adverse effects. Schefflera barteri is popularly used in the West region of Cameroon for the treatment of various diseases such as diarrhea, spasm, pneumonia and animals bite. Considering the ethnopharmacological relevance of this plant, this study was designed to investigate the possible toxic effects of the stem bark extract of S. barteri.
Methods
The extract was prepared by maceration of stem bark dry powder in methylene chloride/methanol mixture. Phytochemical analysis was performed by chemical reaction method. Oral acute toxicity study was carried out by administering single geometric increasing doses (2 to 16 g/kg body weight) of plant extract to Swiss albino mice. For sub-acute toxicity study, repeated doses (100, 200, 400 and 800 mg/kg bw) of plant extract were given to Wistar albino rats for 28 consecutive days by oral route. At the end of the treatment period, hematological and biochemical parameters were assessed, as well as histopathological studies.
Results
Phytochemical analysis of stem bark extract of S. barteri revealed the presence of anthocyanins, anthraquinons and saponins. Acute toxicity results showed that the LD50 was greater than 16000 mg/kg. Sub-acute treatment significantly (P < 0.05) increased the level of serum transaminase, proteins and HDL cholesterol. On the other hand, the extract significantly (P < 0.05) reduced the level of leucocytes as well as neutrophils, basophils and monocytes in female. No significant variation of serum creatinine, LDL cholesterol, serum triglycerides as well as liver, spleen, testicles and ovaries proteins was noted. Histopathological analysis of organs showed vascular congestion, inflammation of peri-portal and vacuolization of hepatocytes at the level of the liver. Leucocytes infiltration of peri-portal veins were noticed on lungs and liver cells as well as inflammatory peri-bronchial and basal membranes seminar tube merely joined on lungs and testis respectively.
Conclusion
The results suggest that acute administration of the stem bark extract of S. barteri is associated with signs of toxicity, administration over a long duration provokes hepatotoxicity, testes and lungs toxicities.
Keywords: Schefflera barteri, Acute toxicity, Sub-acute toxicity, Histopathological analysis
Background
In recent times, focus on plant research has increased all over the world and a large body of evidence has collected to show immense potentials of medicinal plants used in various traditional systems [1-3]. The World Health Organization (WHO) estimates that 70 to 80% of the people in developing countries use traditional medicine as a major source of health care. However, many people underestimate the toxicity of natural products and do not realize that these agents could be as toxic or more than synthetic drugs. So far, many plants have been reported to be toxic to both human and animals [1,4]. It should therefore, be emphasized that the traditional use of any plant for medicinal purposes, by no means, warrants the safety of such plant. Plants in folk medicine should therefore, be evaluated for safety or toxicity and necessary recommendations made on their use.
Schefflera barteri locally called “Dehethe” in Dschang (Cameroon), “Rukiganame” or “Omwamira” in Uganda, is a shrub belonging to the Araliaceae family. It is distributed throughout Africa’s mountainous forests gallery (Guinea, Sierra Leone, Niger, Uganda…) [5]. In the highlands of the West Region of Cameroon, S. barteri is well known for medicinal purpose [6]. The stem bark is widely used for peg fence [7]. From ethnopharmacological data, the leaves or the stem bark are also used to treat diarrhea, spasm, pneumonia and bite from animals. In Uganda, S. barteri is reported to reduce dog insensitivity, tiredness and aggressiveness [8]. In spite of the use of S. barteri in traditional medicine, scientific data on the plant is limited. Also, systematic evaluation of its toxic effects is lacking. Therefore, this study was designed to investigate the acute and sub-acute toxicity of S. barteri stem bark extract.
Methods
Plant material
The stem bark of S. barteri was collected in Baleveng, Menoua Division, West Region of Cameroon, in March 2010. Identification of the plant was done at the National Herbarium, in Yaounde-Cameroon, using a voucher specimen registered under the reference HNC N° 26155/RSF-Cam.
Preparation of plant extract
S. barteri stem bark were air-dried at room temperature (23 ± 2°C) and milled to coarse particles. A 100 g sample of the powdered material was macerated three times at room temperature in 500 ml of a mixture of methylene chloride/methanol (1:1) for 48 hr, and then filtrated. The filtrate was concentrated using a rotary evaporator (Büchi R200) and the obtained volume was later dried at 50°C to yield 10.05 g of extract. The extract was kept in the freezer at 4°C for further studies. Phytochemical analysis of this extract was performed by standard chemical reaction methods [9].
Experimental animals
Fifty Swiss albino mice (25 males and 25 females, 8 - 10 weeks old) weighing 18-24 g, and 50 Wistar albino rats (25 males and 25 females, 8 - 10 weeks old), weighing 120-185 g were used for acute and sub-acute toxicity studies respectively. These animals were bred in the animal house of the University of Dschang and housed in plastic cages under normal laboratory conditions (12 hr light/dark cycle: 23 ± 2°C). They were fed with standard diet. Food and water were given ad libitum to all animals used for the experiments. They were handled according to standard protocols for the use of laboratory animals. The studies were conducted according to the ethical guidelines of the Committee for Control and Supervision of Experiments on Animals (Registration no. 173/CPCSEA, dated 28 January, 2000), Government of India, on the use of animals for scientific research.
Toxicological investigations
Acute toxicity study
Fifty mice were randomly allocated into five groups of ten animals each (5 females and 5 males. Group I (Control) was administered orally with vehicle (2.5% (v/v) DMSO/tween 80). Remaining groups (II, III, IV and V)) were administered with geometric increased doses of 2000, 4000, 8000 and 16000 mg/kg body weight of S. barteri extract respectively via gastric intubation. Those doses were chosen after several screenings on mice. They were prepared using 2.5% (v/v) DMSO/tween 80 and the administered volume was not more than 1 ml as a unique administration.
The experimental animals were deprived of food for 18 hr prior to extract administration. They were observed continuously for 3 hr thereafter for activity (locomotion), reaction to noise, reaction to pinch, state of excrements and mortality. After this period, the animals were given food and water ad libitum. Dead animals in each group were noticed within 48 hr following the administration of the extract. The surviving animals were monitored daily for 14 days for changes in body weight, food and water consumptions [10].
Sub-acute toxicity
Fifty albino rats of both sexes were used. They were grouped into five groups of ten animals each (5 males and 5 females). The control group (Group 1) received orally throughout the experiment a solution of 2.5% (v/v) DMSO/tween 80. The test group (2, 3, 4 and 5) received the plant extract at 100, 200, 400 and 800 mg/kg body weight. The administration of various doses of the extract was done by gastric intubation once a day, for 28 consecutive days [11].
Food intake and weight gain estimation
Food intake and weight gain was recorded every two days during the experimental time.
Sample collection
Rats were fasted overnight on the 28th day and urine was collected from individual metabolic cages, centrifuged and store at +4°C for 24 hours. Upon fasting, the blood samples were collected by cardiac puncture into heparinized and non-heparinized tubes from chloroform anaesthetized rats. Animals were further sacrificed and used for gross pathological examinations and relative organ indices determination.
Haematological analysis
The heparinized blood was used for hematological analysis (hematocrit, total red cell (RBCs), total white blood cell (WBCs), lymphocytes, neutrophils, monocytes, eosinophils and basophils) [12].
Biochemical analysis
The non-heparinized blood was allowed for complete clotting and then centrifuged at 3000 × g for 5 min. The supernatants (serum samples) were aspired and frozen at -15°C. The serum was assayed for creatinine, aspartate amino transferase (AST), alanine amino transferase (ALT), total cholesterol, high density lipoprotein (HDL), triglycerides and total protein using commercial kits (IMNESCO GmbH, Germany). Urine was assayed for total protein and creatinine using the same commercial kits.
Tissues proteins analysis
Immediately after blood collection, the liver, lungs, heart, kidneys, spleen, testis and ovaries were isolated, freed of blood, and weighed using an electronic balance (Mettler PE 160, France). A section of each organ was used for estimation of protein concentration. For this purpose, the homogenate of each organ was prepared in 0.9% NaCl solution at 10% (i.e. 10 g organ in 100 ml of solution). The protein concentrations were determined by the Biuret method [13].
Histopathological study
Immediately after collecting the blood samples, vascular perfusion was performed for the organ mentioned above and tissue section were further performed (5-micron thickness).
These tissues were further fixed in 10% formalin and then, embedded in paraffin for histopathological analysis. They were routinely stained with haematoxylin and eosin (H & E), and examined under a light microscope (Olympus CH02). Any alterations compared to the normal structures were registered [14].
Statistical analysis
Results were expressed as mean value ± standard deviation (S.E.M.). Within group, comparisons were performed by the analysis of variance using ANOVA test. Significant difference between control and experimental groups were assessed by Waller Duncan-test.
Results
Qualitative phytochemical screening
Phytochemical screening of the stem bark methanol/methylene chloride extract of S. barteri revealed the presence of saponins, anthraquinons and anthocyanins while alkaloids, phenols, sterols and triterpenes were not detected.
Acute oral toxicity
Mice behavior was affected in both sexes by acute treatment with S. barteri extract. From 4 000 mg/kg, a reduction of locomotion, reaction to noise and reaction to pinch were noticed. No death were recorded within 48 hours after administration of the extract in animals of both sexes at doses less than or equal to 16 000 mg/kg of body weight.
Food consumption recorded during the periods of observation following the administration of extract is presented in Table 1. A significant (P < 0.05) reduction of food consumption was noticed for all treated mice (i.e. from 2000 mg/kg). The decrease was more pronounced as the doses increased.
Table 1.
Effect of daily intake of the methanol/methylene chloride stem bark extract of S. barteri on food consumption in mice according to sex and dose
| Sexes | Dose (g/kg) | Food consumption (g) | |
|---|---|---|---|
| Week 1 | Week 2 | ||
| Male | 0 | 4.88 ± 0.16a | 5.38 ± 0.31a |
| 2 | 4.07 ± 0.13b | 4.76 ± 0.23b | |
| 4 | 4.08 ± 0.22b | 4.64 ± 0.12b | |
| 8 | 2.83 ± 0.49c | 3.58 ± 0.18c | |
| 16 | 2.54 ± 0.38c | 3.30 ± 0.08c | |
| Female | 0 | 5.41 ± 0.26a | 6.32 ± 0.13a |
| 2 | 4.50 ± 0.33b | 5.21 ± 0.44b | |
| 4 | 3.94 ± 0.46c | 4.70 ± 0.09c | |
| 8 | 3.75 ± 0.47c | 4.26 ± 0.15d | |
| 16 | 3.71 ± 0.52c | 4.00 ± 0.88d | |
Data are expressed as mean ± S.E.M. n = 5. Values for a given group in a line followed by same letter as superscript are not significantly different according to Waller Duncan’s multiple comparison test (P < 0.05).
The weights of the experimental animals recorded during the two weeks of observation are presented in Figure 1. Mice that received the extract at doses of 8000 and 16000 mg/kg showed a reduction in body weight all over the experimentation time in both sexes, with the reduction being more visible during the first week of experimentation.
Figure 1.
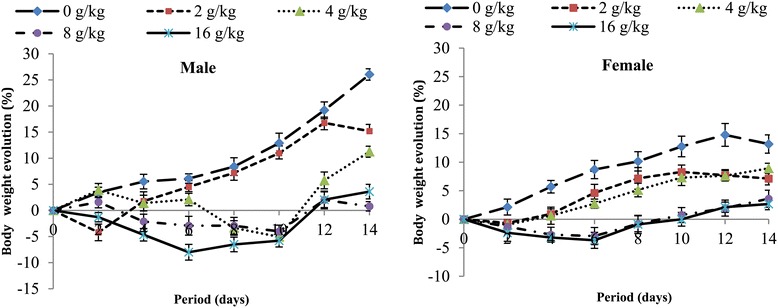
Body weight evolution of mice in acute toxicity of the methylene/chloride extract of S. barteri.
Sub-acute toxicity
General signs
No death or significant change in general behavior or other physiological activities were observed during the treatment period either in the controls group or in the extract treated groups.
Food intake, weight gain and organ indices
The extract did not affect food consumption of male rats but the females were negatively affected during the second week of treatment (Table 2). Throughout the experiment, weight gain decreased in female rats from 400 mg/kg but the males were not affected (Figure 2).
Table 2.
Effect of daily intake of the methylene chloride /methanol extract of S. barteri on food consumption in rats according to sex and dose
| Sexes | Doses (mg/kg) | Food consumption (g) | |||
|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | ||
| 0 | 23.28 ± 2.22a | 28.99 ± 1.82a | 28.65 ± 3.54a | 21.97 ± 1.94a | |
| 100 | 22.27 ± 2.21a | 30.64 ± 0.84a | 28.39 ± 0.67a | 24.35 ± 2.57a | |
| Male | 200 | 23.16 ± 1.98a | 31.40 ± 1.33a | 28.44 ± 2.17a | 22.05 ± 2.82a |
| 400 | 21.61 ± 1.99a | 30.07 ± 1.10a | 26.93 ± 2.18a | 22.24 ± 2.50a | |
| 800 | 24.18 ± 1.85a | 28.09 ± 2.40a | 26.58 ± 3.18a | 23.39 ± 2.93a | |
| 0 | 20.85 ± 0.58a | 26.31 ± 1.41ab | 23.92 ± 1.18a | 21.97 ± 2.41a | |
| 100 | 20.24 ± 1.25a | 27.59 ± 1.82a | 23.62 ± 1.85a | 23.35 ± 1.39a | |
| Female | 200 | 21.35 ± 1.13a | 27.64 ± 1.71a | 23.32 ± 1.61a | 22.05 ± 2.39a |
| 400 | 21.89 ± 1.20a | 24.49 ± 2.35b | 22.57 ± 3.56a | 22.24 ± 2.70a | |
| 800 | 21.82 ± 2.07a | 24.13 ± 1.42b | 22.15 ± 2.33a | 23.39 ± 2.75a | |
Data are expressed as mean ± S.E.M. n = 5. Values for a given group in a line followed by different letter as superscript are significantly different according to Waller Duncan’s multiple comparison test (P < 0.05).
Figure 2.
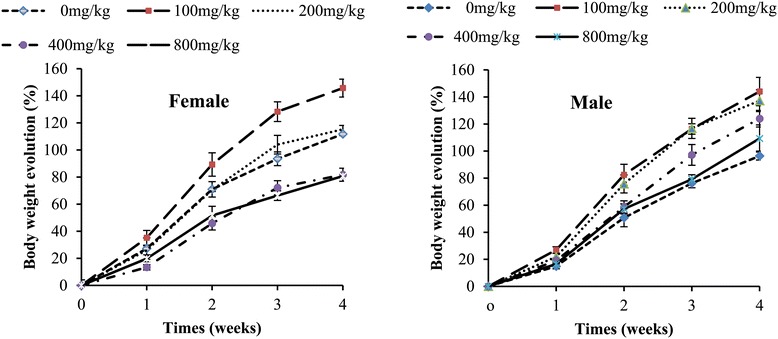
Body weight evolution of rats in sub-acute toxicity of the methylene/chloride extract of S. barteri.
The results of the effects of S. barteri extract on relative organ indices of both male and female rats are summarized in Table 3. There were no significant changes in the lung, kidney, and ovaries or testis to body weight ratios in both groups. However, the extract significantly increased heart and spleen to body weight in both male and female. The significant increase in liver to body weight was observed in male.
Table 3.
Relative organ weights indices (g/100g) of rats in sub-acute toxicity of the methylene Chloride/methanol extract of S. barteri
| Doses (mg/kg) | Liver | Spleen | Kidneys | Lungs | Heart | Testis/ovaries | |
|---|---|---|---|---|---|---|---|
| Male | Control | 3.68 ± 0.25b | 0.32 ± 0.06b | 0.82 ± 0.04a | 0.67 ± 0.03a | 0.34 ± 0.03c | 1.21 ± 0.06a |
| 100 | 3.84 ± 0.27b | 0.37 ± 0.03a | 0.79 ± 0.04a | 0.68 ± 0.07a | 0.37 ± 0.04b | 1.17 ± 0.07a | |
| 200 | 4.10 ± 0.65a | 0.37 ± 0.03a | 0.79 ± 0.08a | 0.68 ± 0.03a | 0.38 ± 0.02b | 1.16 ± 0.03a | |
| 400 | 4.11 ± 0.39a | 0.37 ± 0.05a | 0.79 ± 0.04a | 0.69 ± 0.13a | 0.40 ± 0.05a | 1.16 ± 0.07a | |
| 800 | 4.63 ± 1.35a | 0.37 ± 0.06a | 0.79 ± 0.26a | 0.70 ± 0.17a | 0.40 ± 0.06a | 1.16 ± 0.35a | |
| Female | Control | 4.19 ± 0.36a | 0.28 ± 0.08c | 0.89 ± 0.08a | 0.69 ± 0.14a | 0.34 ± 0.03c | 0.07 ± 0.02a |
| 100 | 4.20 ± 0.98a | 0.28 ± 0.06c | 0.89 ± 0.02a | 0.68 ± 0.14a | 0.37 ± 0.04b | 0.07 ± 0.02a | |
| 200 | 4.32 ± 0.33a | 0.31 ± 0.04b | 0.90 ± 0.07a | 0.68 ± 0.10a | 0.40 ± 0.02a | 0.07 ± 0.01a | |
| 400 | 4.30 ± 0.75a | 0.31 ± 0.02b | 0.90 ± 0.08a | 0.69 ± 0.04a | 0.41 ± 0.03a | 0.06 ± 0.01a | |
| 800 | 4.31 ± 0.50a | 0.34 ± 0.07a | 0.91 ± 0.06a | 0.69 ± 0.06a | 0.42 ± 0.05a | 0.06 ± 0.02a |
Data are expressed as mean ± S.E.M. n = 5. Values for a given group in a line followed by different letter as superscript are significantly different according to Waller Duncan’s multiple comparison test (P < 0.05).
Hematological parameters
Hematological analysis indicated that hematocrit, red blood cells count (RBCs), lymphocytes, eosinophils, basophils and monocytes were not affected in males (Table 4). However, total WBCs significantly decreased in both groups with females being more affected. Similarly, neutrophils, basophils and monocytes significantly decreased in female from 200 mg/kg b.w.
Table 4.
Hematological parameters of rats in sub-acute toxicity of the methylene chloride/methanol extract of S. barteri
| Sexes | Parameters studied | Control | 100 mg/kg | 200 mg/kg | 400 mg/kg | 800 mg/kg |
|---|---|---|---|---|---|---|
| Total RBC (x 106/mm3) | 3.90 ± 0.22a | 3.84 ± 0.56a | 3.80 ± 0.37a | 3.78 ± 0.55a | 3.73 ± 0.47a | |
| Total WBC (x 103/mm3) 1&1103/mm3) | 226.00 ± 19.50a | 222.00 ± 16.10a | 218.00 ± 12.07a | 214.00 ± 10.25a | 194.00 ± 16.46b | |
| Hematocrit (%) | 45.40 ± 5.63a | 50.00 ± 2.73a | 48.40 ± 1.81a | 49.20 ± 3.96a | 49.20 ± 3.56a | |
| Male | Eosinophils (%) | 0.80 ± 0.44a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.44a |
| Neutrophils (%) | 26.60 ± 4.77a | 27.00 ± 5.00a | 27.20 ± 4.14a | 27.00 ± 7.00a | 27.60 ± 3.03a | |
| Basophils (%) | 0.40 ± 0.54a | 0.40 ± 0.54a | 0.41 ± 0.44a | 0.40 ± 0.44a | 0.40 ± 0.30a | |
| Monocytes (%) | 6.20 ± 1.30a | 6.20 ± 1.30a | 5.60 ± 0.55a | 5.40 ± 0.07a | 5.40 ± 0.19a | |
| Lymphocytes (%) | 66.00 ± 5.00a | 65.60 ± 2.96a | 64.80 ± 4.55a | 61.80 ± 8.13a | 61.00 ± 2.34a | |
| Total RBC (x 106/mm3) | 3.39 ± 0.42a | 3.37 ± 0.24a | 3.35 ± 0.50a | 3.37 ± 0.36a | 3.30 ± 0.40a | |
| Total WBC (x 103/mm3) | 248.00 ± 13.82a | 246.00 ± 20.74a | 244.00 ± 11.40a | 168.00 ± 8.34b | 150.00 ± 20.00b | |
| Hematocrit (%) | 46.40 ± 2.79a | 48.60 ± 5.32a | 48.00 ± 5.43a | 49.40 ± 3.71a | 49.80 ± 2.59a | |
| Female | Eosinophils (%) | 1.40 ± 0.54a | 1.20 ± 0.44a | 1.20 ± 0.44a | 1.200 ± 0.00a | 1.17 ± 0.00a |
| Neutrophils (%) | 30.00 ± 5.33a | 29.20 ± 2.50a | 25.80 ± 1.10b | 24.60 ± 2.61b | 23.60 ± 3.03b | |
| Basophils (%) | 1.60 ± 0.54a | 1.20 ± 0.44bc | 1.00 ± 0.70bc | 0.80 ± 0.44c | 0.60 ± 0.54d | |
| Monocytes (%) | 6.20 ± 0.77a | 5.20 ± 0.83ab | 4.60 ± 1.51b | 4.20 ± 1.30b | 4.60 ± 1.51b | |
| Lymphocytes (%) | 61.80 ± 5.40a | 64.00 ± 1.58a | 65.40 ± 3.58a | 65.20 ± 2.58a | 65.20 ± 2.64a |
Data are expressed as mean ± S.E.M. n = 5. Values for a given group in a line followed by same letter as superscript are not significantly different according to Waller Duncan’s multiple comparison test (P < 0.05).WBCs = white blood cells, RBCs = red blood cells.
Biochemical parameters
Biochemical values of rats treated with the methylene chloride/methanol extract from S. barteri are shown in Table 5. This extract did not affected serum creatinine of animal of both sexes, although, a decrease in the urine creatinine level was noted. Total cholesterol and urinary proteins was not affected in both males and females. Triglycerides decreased significantly in females. HDL-cholesterol increased significantly only in females while LDL-cholesterol decreased significantly in males and females. ALT and AST levels significantly increased in both sexes. Serum proteins increased significantly while hepatic proteins, spleen proteins and testis/ovaries proteins decreased significantly.
Table 5.
Biochemical parameters of rats in sub-acute toxicity of the methylene/chloride extract of S. barteri
| Parameters studied | control | 100 mg/kg | 200 mg/kg | 400 mg/kg | 800 mg/kg | |
|---|---|---|---|---|---|---|
| Urinary creatinine (mg/dl) | 38.64 ± 3.80a | 37.76 ± 3.28a | 37.44 ± 3.14a | 30.96 ± 1.07b | 28.96 ± 3.40b | |
| Serum creatinine (mg/dl) | 21.04 ± 1.02a | 24.60 ± 2.47a | 24.80 ± 4.01a | 24.80 ± 4.01a | 24.84 ± 3.20a | |
| Total cholesterol (mg/dl) | 122.30 ± 3.37a | 122.80 ± 3.47a | 121.92 ± 5.14a | 121.84 ± 2.65a | 121.73 ± 1.78a | |
| Male | HDL cholesterol (mg/dl) | 90.42 ± 7.31a | 90.73 ± 7.18a | 90.95 ± 6.51a | 92.86 ± 4.39a | 93.73 ± 5.12a |
| LDL cholesterol (mg/dl) | 4.15 ± 0.60a | 4.31 ± 1.56a | 3.45 ± 1.23ab | 1.41 ± 0.29b | 1.40 ± 0.17b | |
| Triglycerides (mg/dl) | 141.62 ± 5.38a | 138.80 ± 8.20a | 137.60 ± 3.10a | 137.82 ± 0.35a | 133.00 ± 3.15a | |
| ALT(U/L) | 45.88 ± 1.56c | 52.00 ± 2.19b | 54.43 ± 7.36b | 66.07 ± 5.78a | 66.24 ± 5.29a | |
| AST (U/L) | 7.60 ± 1.10b | 9.65 ± 0.95ab | 9.77 ± 0.73a | 9.79 ± 2.50a | 9.91 ± 0.63a | |
| Serum protein (mg/dl) | 8.35 ± 0.32c | 8.68 ± 1.22c | 11.37 ± 1.83b | 13.73 ± 0.79a | 14.66 ± 1.76a | |
| Urinary protein (mg/dl) | 3.48 ± 0.81a | 3.42 ± 0.29a | 3.35 ± 0.45a | 3.09 ± 0.56a | 3.06 ± 0.83a | |
| Hepatic protein (mg/g) | 562.12 ± 71.22a | 436.16 ± 39.54b | 348.67 ± 24.80c | 273.41 ± 33.30d | 282.62 ± 32.76d | |
| Spleen protein (mg/g) | 332.00 ± 31.87a | 291.01 ± 27.49ab | 290.00 ± 32.62ab | 280.00 ± 26.53b | 280.00 ± 29.60b | |
| Testis protein (mg/g) | 87.10 ± 11.07a | 84.48 ± 5.43a | 63.74 ± 13.74b | 54.69 ± 8.33b | 53.49 ± 4.25b | |
| Urinary creatinine (mg/dl) | 64.32 ± 6.16a | 63.24 ± 5.06a | 54.52 ± 2.30b | 48.68 ± 3.30c | 48.40 ± 0.35c | |
| Serum creatinine (mg/dl) | 31.08 ± 3.00a | 31.44 ± 1.20a | 31.44 ± 2.79a | 31.04 ± 2.10a | 31.68 ± 1.24a | |
| Total cholesterol (mg/dl) | 119.60 ± 2.58a | 121.73 ± 5.15a | 121.73 ± 5.15a | 122.34 ± 3.01a | 122.59 ± 4.99a | |
| Female | HDL cholesterol (mg/dl) | 71.61 ± 3.35 b | 73.26 ± 1.18b | 77.41 ± 7.43b | 96.44 ± 2.72a | 94.44 ± 7.77a |
| LDL cholesterol (mg/dl) | 26.98 ± 5.44a | 30.98 ± 5.10a | 28.71 ± 4.39a | 13.05 ± 2.57b | 12.16 ± 2.48b | |
| Triglycerides (mg/dl) | 96.60 ± 8.90a | 87.40 ± 7.67a | 87.00 ± 9.78a | 71.80 ± 8.08b | 68.81 ± 4.00b | |
| ALT(U/L) | 42.58 ± 9.10b | 49.57 ± 2.82a | 49.60 ± 5.37a | 49.65 ± 8.94a | 49.91 ± 4.38a | |
| AST (U/L) | 9.10 ± 0.80c | 9.58 ± 0.76bc | 10.38 ± 1.36b | 11.96 ± 0.99a | 12.61 ± 0.99a | |
| Serum protein (mg/dl) | 7.13 ± 0.61b | 8.10 ± 0.20b | 12.56 ± 0.97a | 12.79 ± 1.0 a | 13.34 ± 0.83a | |
| Urinary protein (mg/dl) | 3.30 ± 10.59a | 3.23 ± 0.37a | 3.33 ± 0.14a | 3.35- ± 0.17a | 3.34 ± 0.56a | |
| Hepatic protein (mg/g) | 645.02 ± 19.55a | 527.62 ± 36.79b | 380.26 ± 26.11c | 359.04 ± 43.43c | 350.88 ± 27.47c | |
| Spleen protein (mg/g) | 335.20 ± 34.45a | 284.00 ± 26.08b | 288.00 ± 27.27b | 272.40 ± 37.64b | 268.00 ± 17.44b | |
| Ovaries protein (mg/g) | 546.00 ± 39.77a | 480.00 ± 84.85ab | 451.20 ± 31.29 b | 449.30 ± 44.18b | 416.68 ± 24.99b |
Data are expressed as mean ± S.E.M. n = 5. Values for a given group in a line followed by different letter as superscript are significantly different according to Waller Duncan’s multiple comparison test (P < 0.05). ALT = alanine transaminase; AST = Aspartate transaminase; HDL = high density lipoprotein; LDL = low density lipoprotein.
Histopathology analysis
Histopathological analysis of organs portions after treatment with S. barteri stem bark extract revealed varying effect (Figure 3). At the level of the liver, vascular congestion, leucocytes infiltration, periportal inflammation and vacuolization of hepatocytes were noted in both sexes. Leucocytes infiltration and inflammatory peri-bronchial were noticed on the lungs. Inflammatory peri-bronchial and merely joined basal membrane seminar tube were observed on lungs and testis respectively. At the level of the kidney, a congestion of glomeruli and a widening of the urinary space in the 800 mg/kg treated rats as compared to the control group were observed.
Figure 3.
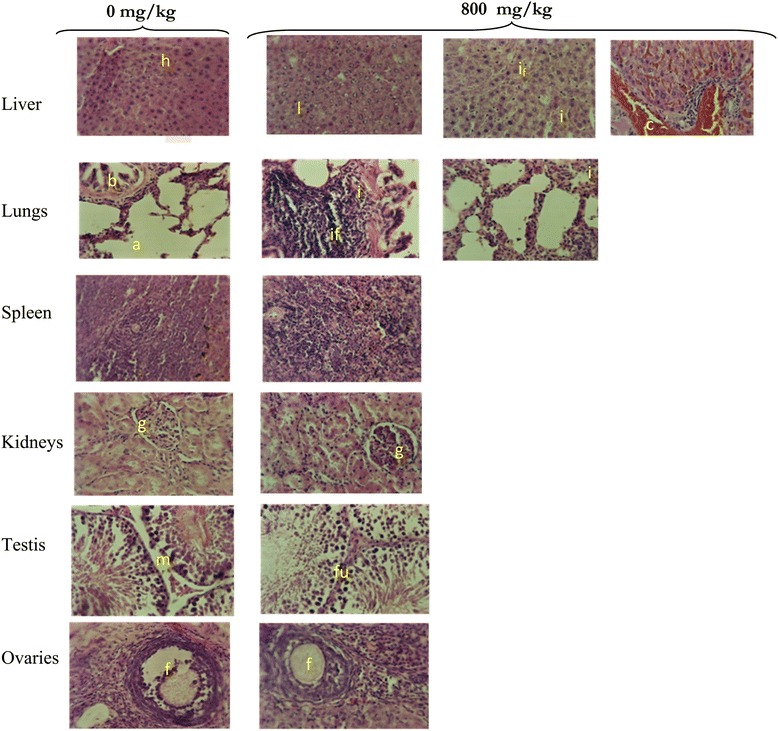
Histology of organs of the control rats and those exposed of Schefflera barteri for 28 days. The organs of the rats exposed S. barteri extract showed: Light vacuole-like space (l) and congestions (c) in liver, inflammations (i), peri-portal /peri-bronchial and leucocytes infiltration (i f ) in both the liver and lungs, fusion (f u ) of basal membranes of seminar tubes (m) in testis; congestion of glomeruli and widening of the urinary space, normal spleen and ovary. Histological analysis of the organs of the control rats showed normal structure: hepatocytes (h), alveols (a), bronchi (b), glomeruli (g), membrane, follicle (f).
Discussion
Although significant advances have been made in the development and application of in vitro toxicity assays, in vivo safety evaluation remains the most useful tool for identifying target organ toxicity [15]. The rat has been the species of choice for the vast majority of preclinical toxicology studies performed in the evaluation of pharmaceutical candidates. Recent finding revealed that mouse is a suitable model for very early safety assessment since earlier identification of preclinical toxicities are generally predictive of human toxicity and could save time, money, and effort spent [16].
The acute toxicity study showed no mortality at a dose limit of 16000 mg/kg b.w. by oral administration. The extract S. barteri is therefore relatively harmless based on Hodge and Sterner Scale [17]. However, the reduction in mice activity and reaction to noise may be due to depressant and sedative effect on the central nervous system [18]. The reduction of reaction to pinch and reactivity may be due to its inhibitory action on nocireptors or inhibition of the production of algogenic substances (prostaglandins, histamines), or inhibition of the pain signal transmission at the central level [19]. Phytochemical studies of stem bark of S. barteri revealed the presence of saponins and such substances may provoke anorexia and weight loss in animals [20]. Their presence in this plant could justify the decrease of both food consumption and weight loss observed during acute toxicity study in mice. Improvement of weight gain noted in the second week may be justified by the biotransformation and elimination of the responsible compounds contained in absorbed extract.
The daily oral administration of the CH2Cl2/MeOH extract of S. barteri stem bark for 28 days did not affect red blood cells, suggesting that oral administration of this extract has no oxygenation and anaemia risk [21,22]. However the decrease in white blood cells indicates that the 28- day’s administration of this extract resulted in the weakening of the immune system [20]. The decrease of neutrophils, basophils and monocytes thus observed may be related to leucocyte infiltrations in the liver and lung revealed by histopathological analysis of these organs.
A significant decrease of hepatic proteins levels was noted, moreover, liver relative weight also increased. These parameters are indicators of hepatic toxicity. Furthermore, a significant increase of AST and ALT in serum was also observed. It is well known that many toxic compounds accumulate in the liver where they are detoxified [23]. Liver damage and its recovery are usually assessed by measuring the level of serum transaminases, particularly ALT. Indeed, changes in their serum level are biological markers of liver dysfunctioning and/or damage [24]. Thus, S. barteri extract may be associated with hepatotoxicity. These findings were further confirmed by the histopathological studies on the liver which revealed marked necrosis, vascular congestions, peri- portal inflammations and cell vacuolizations.
Urine creatinine decreased while serum creatinine was not affected. Creatinine is a marker of kidney toxicity, its levels increased in the serum when the cortex and/ or the glomerula are damaged [25]. Glomerula damage is also indicated by the increase of the urine protein levels [26]. No variation of serum creatinine and urine protein levels indicates that the kidneys are normal as shown by histopathological study.
A significant increase in HDL-cholesterol levels in the treated females and reduction in LDL-cholesterol and triglycerides levels in some treated animals were observed. This showed that the extract had some beneficial effects by reducing cardiovascular risk factors, which contribute to death of diabetic patient [27].
Histopathological examination revealed many abnormalities. Vascular congestions on the liver section could be due to the inflammation, blockage or vasoconstriction action of the S. barteri extract on the walls of blood vessels. This extract could contain some substances capable of acting like non steroidal anti-inflammatory drugs that provoke hypersensibility reaction which led the lung and liver inflammations observed [28]. The presence of the empty vacuole-like spaces in the hepatocytes could be due to abnormal infiltration of extracellular substances into the hepatocytes or to malfunctioning of the latter [20]. The joined basal membrane of the seminar tube could be due to cellular retraction with reduction of cytoplasmic compounds or cells loss caused by apoptosis [29]. It may also be due to the osmotic gradient modification through the cytoplasmic membrane [30]. The congestion of glomeruli and widening of the urinary space was observed. Drug concentration in the blood is affected by capillary constriction, leading to a decrease in glomerular filtration of that drug which minimizes its effect and protects the tubular cells [31]. This may affect the shrinkage and atrophy of the glomeruli. At the same time, the mesangial cell processes may be retracted due to the contraction of their filaments, which may be stimulated by angiotensin II present in these cells.
Conclusion
S. barteri extract is relatively harmless by acute oral administration. Although sub-acute administration is associated with side effects on the central nervous system, immune system, liver and testis. Therefore, for a S. barteri extract based treatment, the dose, frequency and duration of the treatment should be carefully defined to avoid adverse effect of the plant extract.
Acknowledgements
This works was supported by AIRES-Sud (Appuis Intégrés pour le Renforcement des Equipes Scientifiques du Sud), a program of the French Ministry of Foreign and European Affair implemented by the “Institut de Recherche pour le Developpement” (IRD-DSF). We are grateful to Dr Désiré Dzeufiet for histopathological analysis, Pr. Antoine Mvondo Ze, and Pr. Telesphore Nguelefack for their technical assistance.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SSA is the field investigator. RANN and JRK designated the study and supervised the work. RSM is co-field investigator and conceived the manuscript. ATT prepared the plant extract. JDT contributed to the phytochemical studies. HNM and MLKM contributed in manuscript writing and editing. RMEE and LB revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Serge Secco Atsafack, Email: atsafackserge@yahoo.fr.
Jules-Roger Kuiate, Email: jrkuiate@yahoo.com.
Raymond Simplice Mouokeu, Email: moraysi@yahoo.fr.
Martin Luther Koanga Mogtomo, Email: koanga@yahoo.com.
Alembert Tiabou Tchinda, Email: alembertt2002@yahoo.fr.
Tamokou Jean De Dieu, Email: jtamokou@yahoo.fr.
Huguette Magnifouet Nana, Email: nanahug@yahoo.fr.
Rébecca Madeleine Ebelle Etame, Email: Ebelleetame@yahoo.fr.
Lucie Biyiti, Email: Biyitilu@yahoo.fr.
Rosalie Annie Ngono Ngane, Email: angono@yahoo.com.
References
- 1.Mouokeu RS, Ngono Ngane RA, Lunga PK, Koanga MM, Tiabou AT, Njateng GSS, et al. Antibacterial and dermal toxicological profiles of ethyl acetate extract from Crassocephalum bauchiense (Hutch.) Milne-Redh (Asteraceae) BMC Complement Altern Med. 2011;11:43. doi: 10.1186/1472-6882-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Njateng GSS, Kuiate JR, Gatsing D, Tamokou JD, Mouokeu RS, Kuete V. Antidermatophytic activity and dermal toxicity of essential oil from the leaves of Ageratum houstonianum (Asteraceae) J Biol Sci. 2010;10(5):448–54. doi: 10.3923/jbs.2010.448.454. [DOI] [Google Scholar]
- 3.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 2010;76(14):91–1479. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 4.Aguinaga YJ, Claudiano SG, Marcusso PF, Ikefuti C, Ortega GG, Eto FS, et al. Acute Toxicity and Determination of the Active Constituents of Aqueous Extract of Uncaria tomentosa Bark in Hyphessobrycon eques. Hindawi Publishing Corporation Journal of Toxicology 2014, Article ID 412437, 5p. [DOI] [PMC free article] [PubMed]
- 5.Wright HE, Mc Cullough J, Alonso LE, Diallo MS. Une Évaluation Biologique Rapide de Trois Forêt Classées du Sud-est de la Guinée. Washington D.C: Bulletin RAP d’Evaluation Rapide 40. Conservation International; 2006. p. 249 p. [Google Scholar]
- 6.Neba NE. Traditional health care system and challenges in developing ethnopharmacology in Africa: Example of Oku, Cameroon. Ethno Med. 2011;5(2):133–9. [Google Scholar]
- 7.Nguemo DD, Foko J, Pinta JY, Ngouo LV, Tchoumboue J, Zango P. Inventaire et identification des plantes mellifères de la zone soudano-guinéene d’altitude de l’Ouest Cameroun. Tropicultura. 2004;22(3):139–45. [Google Scholar]
- 8.Cunningham AB. Cunningham AB. Peuples, parc et plantes. Recommandations pour les zones à usages multiples et les alternatives de développement autour du parc national de Bwindi Impénétrable, Ouganda. UNESCO Paris: Document de travail Peuples et Plantes no 4; 1996. p. 68p. [Google Scholar]
- 9.Harbone JB. Phytochemical methods: a guide to modern techniques of plant analysis. London: Chapman and Hall Ltd; 1973. p. 116 p. [Google Scholar]
- 10.Adedapo AA, Omoloye OA, Ohore OG. Studies on the toxicity of an aqueous extract of the leaves of Abrus precatorius in rats. Onderstepoort J Vet Res. 2007;74:31–6. doi: 10.4102/ojvr.v74i1.137. [DOI] [PubMed] [Google Scholar]
- 11.WHO . Guidelines for Methodologies of Research and Evaluation of Traditional Medicine. Geneva: WHO; 2000. p. 80 p. [Google Scholar]
- 12.Manjarekar PN, Jolly CI, Narayan S. Comparative studies of immunomodulatory activity of Tinospora cordifolia and Tinospora sinesis. Fitoterapia. 2000;71:254–7. doi: 10.1016/S0367-326X(99)00167-7. [DOI] [PubMed] [Google Scholar]
- 13.Gornall AG, Barwill GS, David MM. Determination of serum protein by means of the buiret reaction. J Biol Chem. 1949;177:751–66. [PubMed] [Google Scholar]
- 14.Venkataranganna MV, Gopumadhavan S, Sundaram R, Ghaise P, Mitra SR. Pharmacodynamics and toxicological profile of PartySmart, a herbal preparation for alcohol hangover in Wistar rat. Indian J Med Res. 2008;127:460–6. [PubMed] [Google Scholar]
- 15.Fielden MR, Kolaja KL. The role of early in vivo toxicity testing in drug discovery toxicology. Expert Opin Drug Saf. 2008;7:107–10. doi: 10.1517/14740338.7.2.107. [DOI] [PubMed] [Google Scholar]
- 16.Jeffrey AK, Emily O, Megan EP, Debra B, Traci S, Melinda MA, et al. Early toxicology signal generation in the mouse. Toxicol Pathol. 2010;38:452–71. doi: 10.1177/0192623310364025. [DOI] [PubMed] [Google Scholar]
- 17.Hodge HC, Sterner JH. Determination of substance acute toxicity by LD50. Am Ind Hyg Assoc. 1943;10:93.
- 18.Kengni F, Tala DS, Djimeli MN, Fodouop SP, Kodjio N, Magnifouet NH, et al. In vitro antimicrobial activity of Harungana madagascriensis and Euphorbiaprostrata extracts against some pathogenic Salmonella sp. Int J Biol Chem Sci. 2013;7(3):1106–18. doi: 10.4314/ijbcs.v7i3.17. [DOI] [Google Scholar]
- 19.Gatsing D, Tchakoute V, Ngamga D, Kuiate J-R, Tamokou JDD, Nji-Nkah BF, et al. In Vitro Antibacterial activity of Crinum Purpurascens herb leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran J Med Sci. 2009;34(2):126–36. [Google Scholar]
- 20.Magnifouet MH, Ngono Ngono RA, Kuiate J-R, Koanga MLM, Tamokou JD, Ndifor F, et al. Acute and sub-acute toxicity of the methanolic extract of the Pteleopsis hylodendron stem bark. J Ethnopharmacol. 2011;137:70–6. doi: 10.1016/j.jep.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Froment D. L’insuffisance rénale chronique. Symposium francophone de médecine. 2008;4:31–4. [Google Scholar]
- 22.Rosidah Mun FY, Amirin S, Mariam A, Gabriel AA, Mohd ZA. Toxicology evaluation of standardized methanol extract of Gynura procumbens. J Ethnopharmacol. 2009;123:244–9. doi: 10.1016/j.jep.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Clarke EGC, Clarke ML. Veterinary toxicology. London: Cassel and Collier Macmillan Publishers; 1977. pp. 268–77. [Google Scholar]
- 24.Al-boushi S, Safer A, Afzal M, Moussa AS. Green tea modulates reserpine toxicity in animal models. J Toxicol Sci. 2009;34(1):77–87. doi: 10.2131/jts.34.77. [DOI] [PubMed] [Google Scholar]
- 25.Tiez NW. Fundamentals of clinical chemistry. 2. Philippines: Martila; 1982. p. 38. [Google Scholar]
- 26.Bakoush O, Grubb A, Rippe B, Tencer J. Urine excretion of protein HC in proteinuric glomerular diseases correlates to urine IgG but not to albuminuria. Kidney Int. 2001;60:1904–09. [DOI] [PubMed]
- 27.Barnett HA, O’Gara G. Diabetes and the heart. Clinical practice series. Edinburgh UK: Churchill Livingstone; 2003. pp. 7–30. [Google Scholar]
- 28.Lionel F, Baker B. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606–15. doi: 10.1016/j.clindermatol.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G. Cell death assays for drug discovery. Natural Revue Drug Discov. 2011;10(3):221–37. doi: 10.1038/nrd3373. [DOI] [PubMed] [Google Scholar]
- 30.Langworthy MM. The role of nfatc1 in proximal tubule injury and repair. PhD Thesis. Vanderbilt University, Nashville, Tennessee; 2008. p. 161.
- 31.Stevens A, Lowe J. Histology. London, New York: Gower Medical Publishing; 1997. p. 322-47.


