Abstract
Background
The goal of this study was to identify genetic determinants of plasma NT-proatrial natriuretic peptide (NT-proANP) in the general community by performing a large-scale genetic association study and to assess its functional significance in in-vitro cell studies and on disease susceptibility.
Methods and Results
Genotyping was performed across 16,000 genes in 893 randomly selected individuals, with replication in 891 subjects from the community. Plasma NT-proANP1–98 concentrations were determined using a radioimmunoassay. Thirty-three genome-wide significant single nucleotide polymorphisms (SNPs) were identified in the MTHFR-CLCN6-NPPA-NPPB locus and were all replicated. To assess significance, in-vitro functional genomic studies and clinical outcomes for carriers of a SNP rs5063 (V32M) located in NPPA that represented the most significant variation in this genetic locus, were assessed. The rs5063 variant allozyme in transfected HEK293 cells was decreased to 55±8% of wild-type protein (p=0.01) as assessed by quantitative Western blots. Carriers of rs5063 had lower NT-proANP levels (1427 vs. 2291 pmol/L, p<0.001), higher diastolic blood pressures (75 vs. 73 mmHg, p=0.009) and were at an increased risk for stroke as compared to wild-type subjects independent of age, sex, diabetes, hypertension, atrial fibrillation, and cholesterol levels (hazard ratio 1.6, p=0.004).
Conclusions
This is the first large-scale genetic association study of circulating NT-proANP levels performed with replication and functional assessment that identified genetic variants in the MTHFR-CLCN6-NPPA-NPPB cluster to be significantly associated with NT-proANP levels. The clinical significance of this variation relates to lower NT-proANP levels, higher blood pressures and an increased risk for stroke in the general community.
Keywords: natriuretic peptide, heart failure, hypertension, genetics, association studies
Introduction
Atrial natriuretic peptide (ANP) has vasodilator and natriuretic effects and plays an important role in the pathophysiology of heart failure and hypertension.1 The NPPA gene encodes the 151 amino acid prepropeptide that is cleaved resulting in proANP26–151 that, in turn, undergoes proteolysis to form NT-proANP26–123 and ANP124–151. NT proANP levels correlate well with ANP levels, however the advantage of measuring NT-proANP level is that it is technically easier, more stable, reproducible and has a longer half-life than ANP.2, 3 We have previously shown that the propensity to develop hypertension is due to a relative deficiency in ANP.4 We have also demonstrated in subjects free of heart failure in the Olmsted County Community Cohort that NT-proANP level is independently predictive of death, development of heart failure and myocardial infarction (MI).5
Variation in ANP levels may be due in part to genetic variation. Disruption of the NPPA gene in mice leads to alteration of circulating ANP levels and hypertension and heterozygous mutants develop salt sensitive hypertension.6 Conversely, gain of function genetic variants in the NPPA gene have been shown to be associated with higher NT-proANP levels, lower systolic and diastolic blood pressures and a decreased risk for hypertension.7 There has been no large scale genetic association studies performed for circulating NT-proANP concentrations. Identifying the genetic determinants of NT-proANP levels may help us understand the pathophysiology of hypertension and sequelae such as stroke, MI and heart failure. Therefore, we undertook a genetic association study to identify possible genetic determinants of circulating NT-proANP and assess its significance by performing functional genomic studies in vitro and analyzing clinical outcomes in 1784 randomly selected subjects from Olmsted County, Minnesota.
Methods
The Mayo Clinic Institutional Review Board approved this study and written informed consent was obtained from all subjects.
Study Population
The cohort studied consisted of a random sample of residents from Olmsted County, Minnesota, age 45 years or older who were first characterized as part of the National Institutes of Health funded “Prevalence of Left Ventricular Dysfunction Study” (PAVD) and has been previously described.8 There were 2027 subjects in this cohort who had adequate quality and quantity of deoxyribonucleic acid (DNA) samples for genotyping. After quality control (see below) there were 1,784 subjects in whom plasma NT-proANP levels had been measured who were included in the final analysis. The subjects recruited were randomly separated into 2 cohorts. The Discovery Cohort that comprised of 893 randomly selected subjects from the PAVD study and the Replication Cohort consisting of the remaining 891 samples were used for the replication study.
Genotyping
Genotyping was performed using the “Metabochip”, a custom Illumina iSelect genotyping array. A total of 2,112 samples comprising both the Discovery and Replication Cohorts were genotyped, including duplicates and CEPH DNA controls. Samples were dropped if the call rate was <98% or if there were gender errors or duplicates. A high rate of concordance (99%) was observed for intentionally duplicated samples. PLINK software was used to estimate relatedness between samples and those with a PI HAT value >0.2, reflecting twins, parent-offspring or full sibling, or other close relatedness were identified and one sample from each of those pairs was excluded. Multidimensional scaling was performed to identify non-Caucasian subjects who were then dropped from the analysis (N=2). There were 196,725 SNPs that were genotyped across approximately 16,000 genes. Using a per SNP call rate >98%, 5.7% of the samples were dropped. There were 879 SNPs that failed the Hardy Weinberg equilibrium model and were flagged. SNPs with a minor allele frequency (MAF) < 0.01 were also dropped. After quality control, 124,590 SNPs and 1,931 samples remained for further analysis. NT-proANP data was available for 1,784 of these samples. SNPs in the autosomal chromosomes were phased using SHAPEIT v1 and imputed using IMPUTE v2.2.2 with the 1000 genomes PHASE 1 data as reference. Genetic variation in the natriuretic peptide system including the NPR1 (45 SNPs), NPR2 (44 SNPs), NPR3 (85 SNPs) and MME (747 SNPs) genes was covered by genotyping and imputation.
Natriuretic Peptide Assay
Plasma NT-proANP1–98 levels were measured in our laboratory using a radioimmunoassay (Phoenix Pharmaceuticals, Belmont, California).9 Inter-assay and intra-assay coefficients of variation for NT-proANP were measured and were 9% and 6% respectively.
Two-dimensional and Doppler Echocardiography
Two-dimensional and Doppler imaging echocardiography was performed as per a standardized protocol as previously described.8 Left ventricular (LV) dimension and mass and left atrial (LA) volume were calculated from M-mode and 2-dimensional measurements, respectively, and were indexed to body surface area.10 Presence of LV hypertrophy was defined based on LV mass index >130 g/m2 for men and >100 g/m2 for women.11
In-vitro ANP Protein Expression Analysis
The human NPPA cDNA and mutant rs5063 constructs were obtained from OriGene (Rockville, MD). HEK293 cells were cultured in DMEM (ATCC, 30-2002) with 10% fetal bovine serum. Transfections were performed with empty vector, WT and variant allozyme for NPPA using Lipofectamine 2000 (Invitrogen, Grand Island, NY). β-galactosidase DNA (Promega, Madison, WI) was co-transfected to correct for variation in transfection efficiency. The cells were harvested and whole cell lysate was prepared after 48 hours. Experiments were performed in triplicate.
Protein expression was determined by quantitative Western blot analysis. Whole cell lysates were subjected to electrophoresis on TGX gels, then transferred to PVDF membranes (BioRad, Hercules, CA), which were blocked with purified rabbit anti-NPPA antibody (Pheonix Pharmaceuticals, Burlingame, CA), followed by incubation with a secondary antibody. Immunoreactive proteins were detected with the Immun-Star WesternC kit (BioRad, Hercules, CA) and Gel Doc XR system (BioRad, Hercules, CA) were used to quantify proteins; data was normalized to β-galactosidase concentrations.
Clinical Outcome Measures
Participants were followed for a median follow up time of 12.2 years (IQR: 10.6–13.0, max=14.5). In addition to all-cause mortality, participants were monitored with respect to heart failure, MI, and cerebrovascular accident (CVA). Heart failure was defined as International Classification of Diseases-Ninth Revision (ICD-9) code 402 or 428. Stroke and transient ischemic attack were grouped together under the term CVA and included ICD-9 codes 430 to 438. MI was defined as ICD-9 code 410 or 412.
Statistical Analysis
Association analyses were performed using PLINK and R, separately for each cohort. The relationship between NT-proANP and genetic markers was modeled using ordinary multiple linear regression. NT-proANP was transformed on the log scale to remove skewness; SNP genotype effects were assumed to be additive; and age and gender were included in models to control for their effects on the phenotype.
Group characteristics, based on cohort and genotype subgroups, are presented with frequency and percentage and compared between groups using Pearson chi-square test for categorical variables. Continuous variables are presented as mean and standard deviation or median and quartiles, and compared between groups using two-sample t-test or non-parametric Wilcoxon rank sum test, depending on distribution. Due to the low minor allele frequency of rs5063, analyses presented in this report utilize the dominant genotype model. Time to event variables were defined based on date of event or last known follow-up for those without event. Kaplan-Meier curves were created to show the relationship between genotype and outcomes including death, MI, stroke, HF, and atrial fibrillation. The association of genotype and other baseline characteristics were analyzed using Cox proportional hazards regression methods. The proportional hazards assumption was tested by plotting and visual examination of scaled Schoenfeld residuals vs. time and significance was tested via correlation of residuals with rank time. No violations of the proportional hazards assumption were noted. All tests were conducted using two sided p-values. Mean protein values were compared using the standard two-sample t-test.
Results
Study Population
There were 1784 subjects who were randomly divided into Discovery (N=893) and Replication (N=891) cohorts. Baseline demographics for these subjects are listed in Supplementary Table 1. There were no significant differences between the two groups in age, gender or body mass index (BMI) or co-morbidities including hypertension, diabetes mellitus and coronary artery disease. Renal function measured by creatinine and calculated glomerular filtration rate was within normal limits and similar between the two groups. LVEF was preserved, LV mass and diastolic function parameters such as E/e’ ratio, were also similar in both groups. Plasma NT-proANP measurements including the variability in these measurements were similar in the Discovery and Replication cohorts, with a median NT-proANP level in the Discovery cohort of 2215 pmol/L (IQR: 1372-3337) and 2174 pmol/L (IQR: 1436- 3190) in the Replication cohort.
Genotyping Results in the Discovery and Replication Cohorts
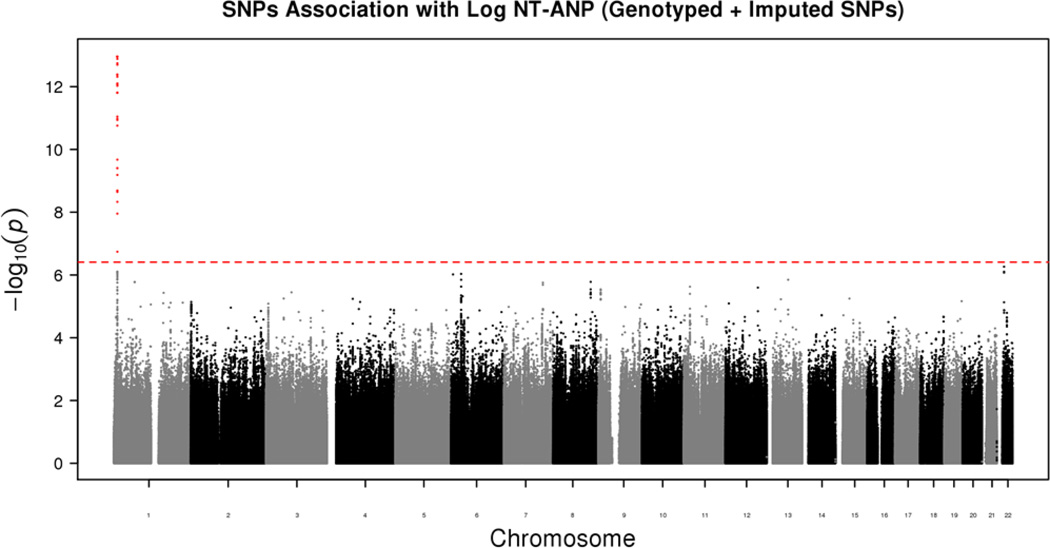
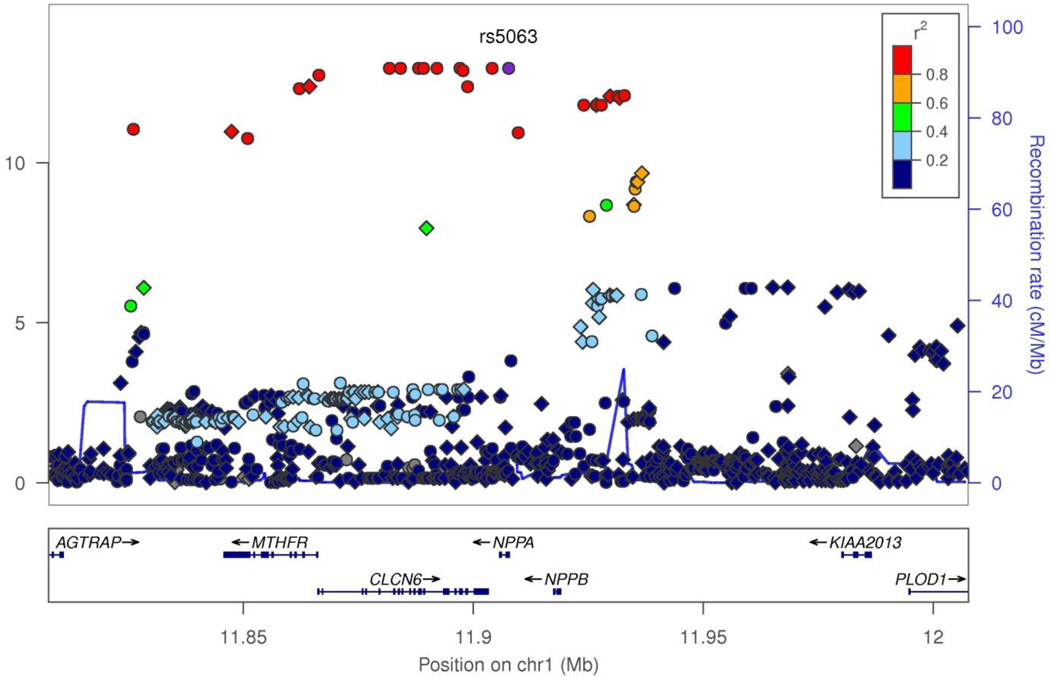
The Manhattan plot of data for both genotyped and imputed SNPs for the Discovery cohort is presented in Figure 1. The QQ plot that displays the potential for p-value inflation is shown in Supplementary Figure 1 with a genomic inflation factor of 0.994, demonstrating adequate adjustment for population stratification with no apparent inflation of Type 1 error. The significance threshold after correcting for multiple testing was 3.9×10−7. This threshold was also used for the replication study since the entire analysis that was performed in the Discovery cohort was repeated in the Replication cohort. In both cohorts, as shown in the Manhattan plot in Figure 1 for the Discovery cohort and in Supplemental Table 1 for both cohorts, the genome wide highly significant SNPs mapped to chromosome 1. Table 1 shows the results from combined analyses of all SNPs with association p≤9.6×10−7 in the Discovery cohort. There were 38 SNPs on chromosome 1 that were significant in the Discovery cohort, 33 of which were validated in the Replication cohort with similar p values. Amongst these 33 SNPs, 24 SNPs were genotyped and 9 were imputed using 1000 Genomes Project data as a reference. The top 10 SNPs were all genotyped SNPs and had association p values in the range of 10−13 and below. The SNP with the lowest p value, rs75747410 in the discovery cohort had a minor allele frequency of 5.3% and was located in an intron of CLCN6, a chloride ion channel. The effect of all of the significant SNPs on NT-proANP levels was in the range of 0.63 to 0.76-fold change per minor allele in the Discovery cohort and 0.62 to 0.81-fold change per minor allele in the Replication cohort. The directionality of the effect of these SNPs on NT-proANP levels was identical in both the Discovery and Replication cohorts. The location of these SNPs and information on their linkage disequilibrium (LD) is shown graphically in the Locus Zoom plot in Figure 2. Most of the significant SNPs mapped to the MTHFR-CLCN6-NPPA-NPPB locus were in LD (r2≥0.6) with each other, as seen in Supplementary Figure 2. The 33 significant SNPs in both the Discovery and Replication cohorts that were not within this gene cluster were present in LOC100506273, LOC100506273 and a small nuclear RNA gene, RNU5E-1. Amongst the significant variants, 15 were present in the 5’-upstream or promoter regions, 12 in introns and 6 were present in exons of various genes within the cluster. There were 2 nonsynonymous (ns) SNPs, rs2274976 (R594Q) in MTHFR, a gene involved in the homocysteine pathway and rs5063 (V32M) in the NPPA gene encoding the precursor peptide of NT-proANP. The SNP rs5063 was the most significant SNP (p=1.14×10−26) in the combined analysis (Table 1).
Figure 1.
Manhattan plot of the results of the large-scale genetic association study of circulating ANP levels in the general community. The red line represents a p value = 3.9 × 10−7, the level required for statistical significance.
Table 1.
Significant Genetic Associations with NT-proANP Concentrations in the Combined Cohort
| SNP | chromosome | position (bp) |
gene | gene type | variant location | genotyped or imputed |
R^2 imputation quality |
minor allele |
common allele |
MAF | fold change |
p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs5063 | 1 | 11907648 | NPPA/NPPA-AS1 | protein-coding/miscRNA | missense/rna_exon | genotyped | NA | T | C | 0.052 | 0.629 | 1.14E-26 |
| rs141308438 | 1 | 11906469 | NPPA/NPPA-AS1 | protein-coding/miscRNA | intron/intron | imputed | 0.9983 | T | C | 0.052 | 0.630 | 2.03E-26 |
| rs3737965 | 1 | 11866451 | CLCN6 | protein-coding | intron | genotyped | NA | A | G | 0.052 | 0.632 | 3.67E-26 |
| rs75747410 | 1 | 11881727 | CLCN6 | protein-coding | intron | genotyped | NA | A | G | 0.053 | 0.633 | 4.21E-26 |
| rs77072136 | 1 | 11888061 | CLCN6 | protein-coding | intron | genotyped | NA | T | C | 0.053 | 0.633 | 4.21E-26 |
| rs41275484 | 1 | 11889225 | CLCN6 | protein-coding | intron | genotyped | NA | A | G | 0.053 | 0.633 | 4.21E-26 |
| rs79811212 | 1 | 11892094 | CLCN6 | protein-coding | intron | genotyped | NA | C | T | 0.053 | 0.633 | 4.21E-26 |
| rs41275500 | 1 | 11897082 | CLCN6 | protein-coding | synonymous | genotyped | NA | T | C | 0.053 | 0.633 | 4.21E-26 |
| rs7552330 | 1 | 11904092 | NPPA-AS1 | miscRNA | rna_exon | genotyped | NA | A | G | 0.053 | 0.633 | 4.21E-26 |
| rs3753586 | 1 | 11864262 | MTHFR | protein-coding | intron | imputed | 0.9994 | C | T | 0.052 | 0.633 | 8.85E-26 |
| rs3753585 | 1 | 11864378 | MTHFR | protein-coding | intron | imputed | 0.9994 | A | G | 0.052 | 0.633 | 8.86E-26 |
| rs17037397 | 1 | 11862163 | MTHFR | protein-coding | intron | genotyped | NA | A | C | 0.052 | 0.633 | 9.84E-26 |
| rs2272803 | 1 | 11898789 | CLCN6 | protein-coding | intron | genotyped | NA | T | G | 0.052 | 0.636 | 1.68E-25 |
| rs2076003 | 1 | 11884147 | CLCN6 | protein-coding | intron | genotyped | NA | C | T | 0.052 | 0.636 | 1.69E-25 |
| rs2075539 | 1 | 11897758 | CLCN6 | protein-coding | intron | genotyped | NA | A | G | 0.053 | 0.637 | 1.91E-25 |
| rs3737967 | 1 | 11847449 | MTHFR/LOC100506310 | protein-coding/protein-coding | 3'UTR/missense | imputed | 0.9984 | A | G | 0.051 | 0.639 | 3.48E-24 |
| rs2274976 | 1 | 11850927 | MTHFR | protein-coding | missense | genotyped | NA | T | C | 0.051 | 0.643 | 1.26E-23 |
| rs77012921 | 1 | 11929761 | LOC390997 | pseudo | 5'upstream | imputed | 1 | C | T | 0.043 | 0.626 | 2.22E-23 |
| rs75030554 | 1 | 11931445 | LOC390997 | pseudo | 5'upstream | genotyped | NA | T | C | 0.043 | 0.626 | 2.22E-23 |
| rs116525098 | 1 | 11931772 | LOC390997 | pseudo | 5'upstream | imputed | 0.9972 | T | C | 0.043 | 0.626 | 3.01E-23 |
| rs79712000 | 1 | 11924030 | NPPB | protein-coding | 5'upstream | genotyped | NA | C | T | 0.043 | 0.629 | 3.07E-23 |
| rs77003042 | 1 | 11926747 | NPPB | protein-coding | 5'upstream | imputed | 1 | G | T | 0.043 | 0.629 | 3.07E-23 |
| rs74714707 | 1 | 11926758 | NPPB | protein-coding | 5'upstream | genotyped | NA | T | C | 0.043 | 0.629 | 3.07E-23 |
| rs75540316 | 1 | 11926898 | NPPB | protein-coding | 5'upstream | genotyped | NA | T | G | 0.043 | 0.629 | 3.07E-23 |
| rs79653311 | 1 | 11927878 | NPPB | protein-coding | 5'upstream | genotyped | NA | C | T | 0.043 | 0.629 | 3.07E-23 |
| rs61757273 | 1 | 11909736 | NPPA | protein-coding | 5'upstream | genotyped | NA | T | G | 0.045 | 0.640 | 3.69E-22 |
| rs114941496 | 1 | 11826085 | LOC100506273 | unknown | rna_exon | genotyped | NA | A | G | 0.048 | 0.649 | 8.77E-22 |
| rs79837245 | 1 | 11932847 | LOC390997 | pseudo | 5'upstream | genotyped | NA | A | T | 0.047 | 0.648 | 2.35E-21 |
| rs113823536 | 1 | 11935715 | LOC390997 | pseudo | 5'upstream | imputed | 1 | G | T | 0.056 | 0.703 | 3.86E-17 |
| rs79593079 | 1 | 11936644 | LOC390997 | pseudo | 5'upstream | imputed | 0.9953 | T | C | 0.056 | 0.703 | 4.64E-17 |
| rs60600557 | 1 | 11935166 | LOC390997 | pseudo | 5'upstream | genotyped | NA | A | G | 0.056 | 0.705 | 6.24E-17 |
| rs78097221 | 1 | 11935443 | LOC390997 | pseudo | 5'upstream | imputed | 1 | A | C | 0.056 | 0.706 | 1.16E-16 |
| rs58120150 | 1 | 11925244 | NPPB | protein-coding | 5'upstream | genotyped | NA | C | T | 0.057 | 0.711 | 2.57E-16 |
| rs80177860 | 1 | 11934890 | LOC390997 | pseudo | 5'upstream | imputed | 0.9996 | C | G | 0.060 | 0.726 | 3.75E-15 |
| rs7545290 | 1 | 11934928 | LOC390997 | pseudo | 5'upstream | genotyped | NA | C | T | 0.061 | 0.728 | 4.08E-15 |
| rs11803049 | 1 | 11928895 | LOC390997 | pseudo | 5'upstream | genotyped | NA | A | G | 0.070 | 0.744 | 4.62E-15 |
| rs112521149 | 1 | 11889815 | CLCN6 | protein-coding | intron | imputed | 0.8924 | A | G | 0.108 | 0.783 | 1.60E-13 |
| rs148216773 | 1 | 11969569 | RNU5E-1 | snRNA | 3'downstream | imputed | 0.9433 | G | C | 0.061 | 0.766 | 3.17E-10 |
Figure 2.
Genetic associations with ANP concentrations across the chromosome 1 region of interest: Manhattan plot of −log10 (P values) from conditional logistic regression for observed (blue circle) and imputed (blue diamond) SNP genotypes according to their physical location. The recombination rate in centimorgan per megabase (blue line) and the linkage disequilibrium (r2) of each SNP with the rs5063 SNP are shown.
In-vitro ANP Protein Expression Levels
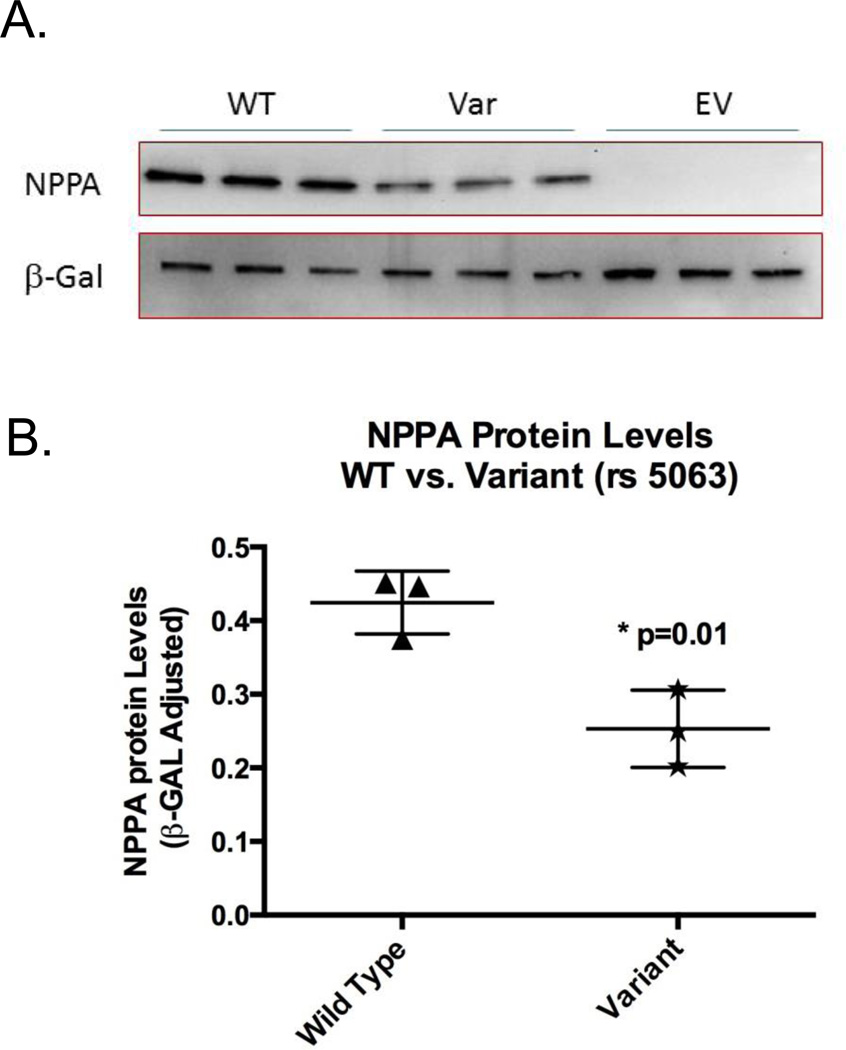
The ns SNP rs5063 located in the NPPA gene was in high linkage disequilibrium (r2>0.8) with 21 other genotyped SNPs that were significant in both cohorts and hence represented most of the significant genetic variation in the MTHFR-CLCN6-NPPA-NPPB locus. To determine the functional effect of this SNP on NPPA expression, WT cDNA and rs5063 variant construct were transfected into HEK293 cells and protein levels were measured by quantitative Western blots. The rs5063 allozyme was decreased to 55±8% of wild-type protein (p=0.01) (Figure 3).
Figure 3.
Atrial natriuretic peptide (NPPA) functional genomics: A. Western blot analysis showing NPPA wild-type (WT) protein expression as compared with rs5063 variant NPPA protein expression for constructs with nonsynonymous single-nucleotide polymorphisms expressed in HEK293 cells. EV denotes transfection with an empty vector. B. A dot plot with NPPA immunoreactive protein levels of rs5063 allozyme as compared to WT protein. Each dot represents independent transfection experiments. All values are corrected for transfection efficiency.
Genetic Association with Clinical Outcomes
To assess its functional significance on clinical outcomes and disease susceptibility, subjects homozygous for the minor allele (T/T) for rs5063 were pooled with heterozygous subjects (T/C) and compared with individuals homozygous for the major allele (C/C). The genotype frequencies for rs5063 in these groups were as follows: (C/C 90%, C/T or T/T 10%). The clinical characteristics of these subjects by genotype are presented in Table 2. Median NT-proANP levels were significantly lower and mean diastolic blood pressures were significantly higher in the carriers of the rs5063 minor allele (1427 pmol/L, 75±10.87 mmHg) as compared to subjects homozygous for the WT allele (2291 pmol/L, 73.4±10.18 mmHg, age and sex adjusted p<0.001 and p=0.009, respectively). From a cardiac structural and functional perspective, there was a weak trend for a greater proportion of subjects with left ventricular hypertrophy among the carriers (15%) than WT (11%) subjects (p=0.09), with the former group also demonstrating longer deceleration times (235±43 versus 229±39 msec, p=0.03), suggestive of early diastolic relaxation abnormalities.
Table 2.
Clinical Characteristics Including Cardiovascular Outcomes of the Study Population Based on the NPPA rs5063 Genotype
| rs5063 | P Values | |||
|---|---|---|---|---|
| Variable | C/C (N=1736) | C/T or T/T (N=195) | Unadj. | Age/Sex Adj. |
| Age at exam | 62.36 ± 10.30 | 61.93 ± 10.51 | 0.58 | |
| Gender, n (%) | 906 (52%) | 107 (55%) | 0.48 | |
| BMI of patient | 28.38 ± 5.26 | 28.55 ± 5.26 | 0.68 | 0.66 |
| Diabetes, n (%) | 118 (7%) | 13 (7%) | 0.95 | 0.96 |
| CAD, n (%) | 179 (10%) | 26 (13%) | 0.19 | 0.08 |
| Verified hypertension, n (%) | 480 (28%) | 44 (23%) | 0.13 | 0.14 |
| Former/Current smoker, n (%) | 852 (49%) | 102 (52%) | 0.39 | 0.31 |
| Afib/Flutter, n (%) | 66 (4%) | 6 (3%) | 0.61 | 0.73 |
| Total cholesterol | 203.64 ± 35.52 | 202.31 ± 36.25 | 0.62 | 0.51 |
| HDL cholesterol | 46.15 ± 14.47 | 44.69 ± 13.33 | 0.18 | 0.07 |
| Systolic blood pressure | 132.31 ± 20.95 | 134.11 ± 23.28 | 0.26 | 0.15 |
| Diastolic blood pressure | 73.40 ± 10.18 | 75.14 ± 10.87 | 0.024 | 0.009 |
| Heart rate | 66.19 ± 11.42 | 66.08 ± 10.50 | 0.89 | 0.84 |
| Creatinine, median (Q1, Q3) | 0.8 (0.7, 1.0) | 0.8 (0.7, 0.9) | 0.13 | 0.31 |
| Calculated GFR (MDRD) | 81.20 ± 17.78 | 82.63 ± 16.69 | 0.29 | 0.36 |
| NT-ProANP, median (Q1, Q3) | 2291.0 (1475.0, 3388.0) | 1427.0 (884.0, 2316.0) | <.001 | <.001 |
| Diastolic dysfunction, n (%) | 445 (28%) | 51 (29%) | 0.93 | 0.98 |
| Ejection fraction | 63.18 ± 6.48 | 63.17 ± 6.16 | 0.99 | 0.82 |
| Deceleration time | 229.22 ± 38.75 | 234.47 ± 42.63 | 0.08 | 0.03 |
| PA systolic pressure | 22.49 ± 4.81 | 22.73 ± 5.16 | 0.59 | 0.61 |
| LA volume | 46.87 ± 20.26 | 46.25 ± 16.29 | 0.69 | 0.95 |
| E/A ratio | 1.10 ± 0.39 | 1.10 ± 0.37 | 0.95 | 0.85 |
| E/e prime | 8.54 ± 3.02 | 8.86 ± 2.92 | 0.21 | 0.21 |
| LV hypertrophy, n (%) | 146 (11%) | 23 (15%) | 0.11 | 0.09 |
| Average LV mass index | 95.99 ± 21.34 | 97.28 ± 21.75 | 0.48 | 0.23 |
| LVESD | 2.94 ± 0.49 | 2.98 ± 0.44 | 0.41 | 0.33 |
| LVEDD | 4.89 ± 0.49 | 4.96 ± 0.47 | 0.08 | 0.04 |
| Death, K-M est. (cum. events) | 0.97 | 0.98 | ||
| 1 years | 1.00 (6) | 1.00 (0) | ||
| 5 years | 0.96 (75) | 0.96 (8) | ||
| 10 years | 0.89 (185) | 0.88 (21) | ||
| CHF, K-M est. (cum. # events) | 0.19 | 0.18 | ||
| 1 years | 0.99 (21) | 1.00 (0) | ||
| 5 years | 0.95 (79) | 0.96 (6) | ||
| 10 years | 0.90 (152) | 0.86 (21) | ||
| Stroke, K-M est. (cum. # events) | 0.012 | 0.009 | ||
| 1 years | 0.97 (46) | 0.95 (9) | ||
| 5 years | 0.91 (139) | 0.83 (30) | ||
| 10 years | 0.83 (256) | 0.74 (43) | ||
| Atrial fibrillation, K-M est. (cum. # events) | 0.82 | 0.78 | ||
| 1 years | 0.98 (29) | 0.98 (3) | ||
| 5 years | 0.94 (100) | 0.93 (12) | ||
| 10 years | 0.87 (188) | 0.86 (22) | ||
| MI, K-M est. (cum. # events) | 0.86 | 0.81 | ||
| 1 years | 0.98 (32) | 0.99 (2) | ||
| 5 years | 0.95 (80) | 0.95 (8) | ||
| 10 years | 0.90 (144) | 0.90 (16) | ||
| Combined Stroke, MI, CHF, Death, K-M est. (cum. # events) | 0.032 | 0.025 | ||
| 1 years | 0.94 (93) | 0.94 (11) | ||
| 5 years | 0.83 (280) | 0.77 (41) | ||
| 10 years | 0.69 (492) | 0.60 (67) | ||
Outcomes are summarized by 1, 5, and 10 year survival estimates from Kaplan-Meier analyses with cumulative numbers of events observed within each time period. Unadjusted and adjusted p-values for outcomes from Cox regression analyses
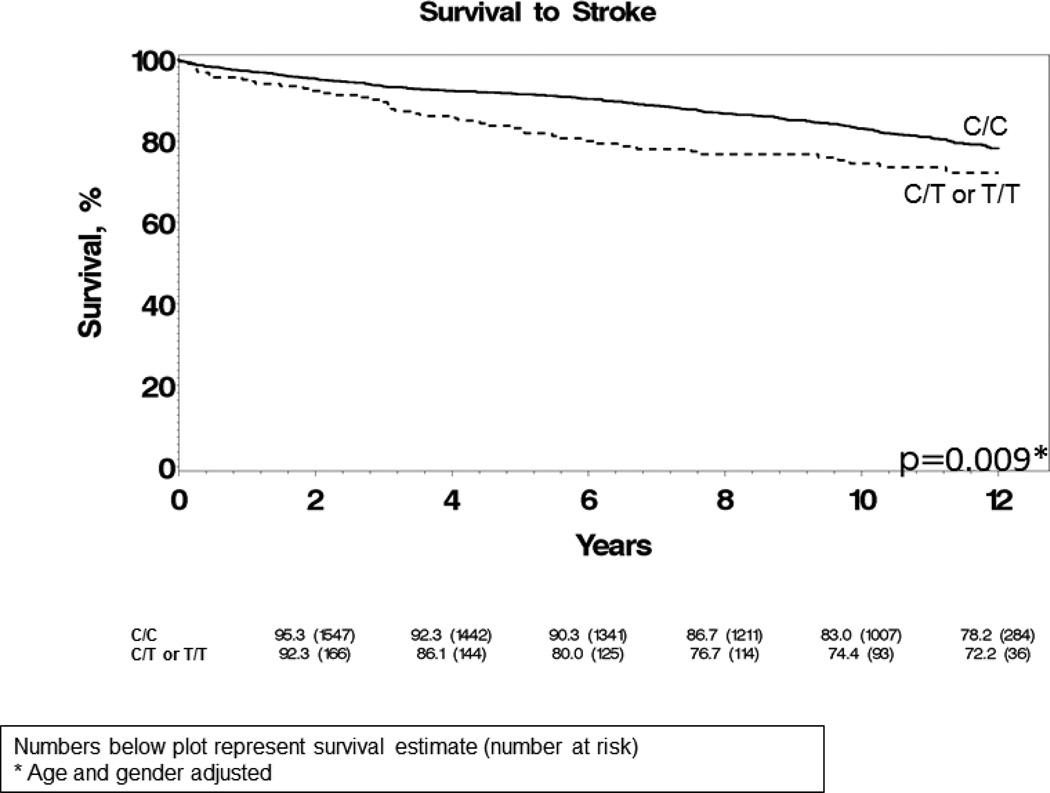
There was a significant difference in the occurrence of CVA between the 2 groups. At 10 years, 26% of subjects who were either homozygous or heterozygous for the rs5063 minor allele had a CVA, compared to 17% of WT subjects (age and sex adjusted p=0.009) (Figure 4). As depicted in Table 3, in addition to age and sex, after adjusting for other clinical covariates such as diabetes, hypertension, atrial arrhythmias and serum cholesterol, the carrier status of the rs5063 genotype remained strongly associated with stroke, with a hazard ratio of 1.6 (p=0.004) comparable to hypertension (HR 1.8) and atrial arrhythmias (HR1.6). The variables included in Table 3 are known risk factors for stroke.12, 13 Interestingly, the effect of the genotype on stroke was independent of NT-proANP plasma concentrations, as seen in Supplementary Table 2. In addition to clinical covariates, after adjusting for NT-proANP levels in Supplementary Table 2, the rs5063 genotype remains significantly associated with stroke (HR 1.6, p=0.006). Carriers of rs5063 had a higher combined composite endpoint of stroke, MI, heart failure and death as compared to WT subjects (age and sex adjusted p=0.025). However, this endpoint was primarily driven by an increased incidence of stroke.
Figure 4.
Kaplan-Meier curve demonstrating stroke free survival according to carrier status of the NPPA rs5063 genotype. Numbers below the figure represent the survival estimate (number at risk). P-value from Cox regression analysis including age and gender as covariates.
Table 3.
Multivariable Logistic Regression Model for Stroke
| Variable | P Value | Hazard Ratio | 95% LCL | 95% UCL |
|---|---|---|---|---|
| Age at Exam | <0.0001 | 1.08 | 1.07 | 1.09 |
| Female Gender | 0.07 | 0.81 | 0.65 | 1.02 |
| Diabetes | 0.35 | 1.18 | 0.83 | 1.68 |
| Verified Hypertension | <0.0001 | 1.80 | 1.44 | 2.25 |
| Afib/Flutter | 0.02 | 1.58 | 1.07 | 2.34 |
| Cholesterol | 0.76 | 1.00 | 1.00 | 1.00 |
| BMI | 0.69 | 1.00 | 0.98 | 1.03 |
| Former/Current Smoker | 0.01 | 1.34 | 1.07 | 1.67 |
| rs5063- C/T or T/T | 0.003 | 1.61 | 1.18 | 2.21 |
Discussion
This is the first large-scale genetic association study of circulating NT-proANP levels performed that identified and replicated genetic markers in the MTHFR-CLCN6-NPPA-NPPB genetic locus to be associated with variation in NT-proANP levels. The genetic regulation of ANP has important implications in the pathophysiology and treatment of cardiovascular disease. We performed this genetic association study using DNA from 893 randomly selected subjects and replicated these results in an additional 891 subjects from Olmsted County, Minnesota. We have demonstrated the functional significance of this genetic variation by relating it to reduced NT-proANP levels, higher blood pressures and an increased risk for stroke in the general community. This is also the first study to demonstrate that the association of this genotype with stroke was independent of other important clinical predictors of stroke such as age, sex, hypertension, diabetes, atrial arrhythmias and serum cholesterol levels. The hazard ratio of the association of this allele with stroke, 1.6, was comparable to that of hypertension and atrial arrhythmias. The strength of this study is that the association of this genetic variant with stroke was evaluated in a large community-based cohort and, hence, these results may be applicable to a general and not just a disease-specific population of Caucasian ancestry. An additional strength was measurement of NT-proANP levels and detailed phenotypic description could provide a pathophysiological explanation of the association of the rs5063 genotype with the clinical outcome of stroke. A limitation of this study is that subjects from Olmsted County, MN where the study is based at are primarily Caucasians and, therefore, results of this cannot be generalized to subjects of other ancestries.
Genetic variants in the 5’-upstream region of NPPA or promoter variants (e.g. C-664G, A-2843G) have been associated with decreased circulating ANP levels.14, 15 Both the 5’-upstream and 3’-downstream regions, −3.2 kbp and +3.7 kbp relative to the transcription start site of NPPA, have multiple transcription factor binding sites that could serve as activator or repressor sequences and could regulate NPPA gene expression.16 We have identified a genetic variant 1,334 base pairs (bp) upstream from the 5’ region of NPPA, rs61757273 that was significantly associated with NT-proANP levels (p=1.15×10−14 in the Discovery and p=7.64×10−12 Replication cohort). A biologically plausible nsSNP rs5063, that substitutes valine with methionine at residue 32 in NPPA was also significantly associated with NT-proANP levels in our study.
A study by Newton-Cheh et al genotyped for only 13 SNPs in the NPPA-NPPB locus in 29,717 subjects and resulted in the identification of rs5068 (p=8×10−70) in NPPA and rs198358 (p=8×10−30) in the NPPA-antisense RNA 1 (NPPA-AS1) gene as being associated with higher NT-proANP levels, lower systolic blood pressure and a reduced risk of hypertension.7 In our study, when using a large-scale genotyping array covering 16,000 genes, neither of these SNPs (rs5068, p=0.002 and rs198358, p=0.01) met our study’s significance threshold (3.9×10−7). Newton-Cheh et al also identified those SNPs to be significant genetic determinants of BNP levels, however a more recent genome wide association study of circulating NT-proBNP levels did not validate either rs5068 or rs198358 to be significantly associated with BNP concentrations.17 Our large-scale genetic association study with NT-proANP levels and the GWAS of NT-proBNP levels highlights the importance of an agnostic approach in determining genetic variants and the potential bias of candidate gene studies.
Although the NPPA-AS1 rs198358 SNP was not validated in our study in our cohort, rs7552330, a SNP in that same gene was significantly associated with NT-proANP levels (p=1.11×1013 Discovery and 1.25×10−14 Replication cohort). The transcripts for NPPA-AS1 and NPPA can form RNA duplexes and result in post–transcriptional down regulation of NPPA expression and, therefore, could affect NT-proANP levels.18 Therefore, the NPPA-AS1 gene could play a role in the regulation of NT-proANP levels.
The importance of the gene encoding chloride channel 6 (CLCN6) in the regulation of natriuretic peptides was demonstrated in a GWAS performed in 3,071 subjects to identify genetic determinants of circulating BNP levels.17 An intronic SNP in CLCN6, rs1023252 (meta-analysis p=3.5×10−14) was identified and replicated as a GWA significant determinant of BNP levels. In our study, 27% of the SNPs that met significance in both the Discovery and Replication cohorts were all in the intronic region of CLCN6. It is being increasingly recognized that introns can serve as binding sites for transcription factors and intronic transcripts can be a type of long noncoding RNA that can regulate gene expression.19 Therefore, it is conceivable that CLCN6 could play an important role in not only regulating the expression of NPPB but also NPPA. Finally, 6 SNPs in our study that were in the 5’ flanking region of NPPB were significantly associated with NT-proANP levels. It is possible that these regions share transcription factor binding motifs that regulate the expression of both natriuretic peptides.
Identifying significant genetic variants by genetic association studies alone does not support their causal role and, hence, functional characterization is also an important component of such association studies. To assess functional significance of these variants, in-vitro cell based studies and clinical outcomes and disease susceptibility of carriers of rs5063 that represented the most significant variation in this genetic locus were analyzed. ANP protein expression levels were reduced by 45% in rs5063 transfected HEK293 cells as compared to WT, indicative of a direct functional effect of the SNP on ANP protein levels. The most common mechanism responsible for decreased levels of protein in association with nsSNPs is accelerated degradation involving the ubiquitin-proteasome system and autophagy as we have previously demonstrated.20, 21
Variation in circulating NT-proANP levels has important clinical implications and therefore identifying genetic modifiers of NT-proANP levels has clinical relevance. Circulating NT-proANP is significantly reduced in patients with metabolic syndrome, in hypertensive patients and is associated with increased LV mass.22 We have also previously shown that the propensity to develop hypertension is due to a relative deficiency in natriuretic peptides including NT-proANP.2 Higher NT-proANP levels were predictive of a higher cardiovascular risk score and of cardiovascular disease independent of risk assessment profiles such as the Progetto Cuore or Framingham risk scores.23 We previously confirmed these findings in subjects free of heart failure in the Olmsted County Community Cohort by showing that NT-proANP is predictive of subsequent death, development of heart failure and myocardial infarction, even after adjustment for age, sex, body mass index and other CV risk factors.3 NT-proANP plasma concentration is not only an independent predictor of prognosis in patients with heart failure with preserved ejection fraction but it is also a marker for asymptomatic left ventricular dysfunction.24 After myocardial infarction, baseline NT-proANP is associated with adverse cardiac remodeling and is predictive of subsequent CV events, independent of cardiac remodeling despite normal plasma NT-proBNP levels.25, 26
Our study is the first to show a correlation between genetic variation, in-vitro NT-proANP protein and in-vivo circulating plasma levels and occurrence of subsequent adverse cardiovascular events in the general community. Our study demonstrates that genetic variation in the MTHFR-CLCN6-NPPA-NPPB genetic locus is associated with lower NT-proANP levels, higher diastolic blood pressure and stroke, suggesting that these variants may be loss-of-function alleles. In our cohort, there also appears to be a trend in these subjects to have atherosclerotic disease with lower HDL levels and a trend towards a higher prevalence of coronary artery disease. We have previously shown that genetic variation in NPPA can have an effect on the cardio-metabolic profile of carriers and that natriuretic peptides not only play an important role in fluid and blood pressure homeostasis but could also affect the atherosclerotic process.27, 28 Atrial natriuretic peptides therefore likely play a “protective” role in the prevention of hypertension and its sequelae such as stroke. Identifying high-risk patients by genotype may allow earlier intervention to prevent the occurrence of such adverse outcomes in the general community. Furthermore, in our study, the rs5063 genotype was independent of NT-proANP levels in predicting adverse outcomes. This finding is not surprising because, although BNP has been a useful marker for predicting adverse cardiovascular events in community-based cohorts, the clinical utility of ANP levels as a biomarker has been controversial.29 Although the finding of stroke risk can be explained by lower ANP levels such as higher blood pressures, unfavorable cardio-metabolic profile, and adverse cardiac remodeling, one could speculate that a cross-sectional single value obtained by measurement of a biomarker (NT-proANP) at a given time in the general community may not reflect a true “deficiency’ state of natriuretic peptides due to the genotype and its effect over time. Furthermore, the relationship of ANP levels and hypertension is complex and may be manifested as a lack of compensatory increase as hypertension stage progresses rather than an actual decrease in circulating levels.4 Finally, the genetic variation that occurs in this locus may result in the alteration of not only ANP but also other natriuretic peptides such as BNP or other neurohormonal triggers, which could lead to adverse cardiovascular outcomes and would not be reflected by measurement of a single biomarker.
We have identified genetic determinants of circulating NT-proANP levels in the MTHFR-CLCN6-NPPA-NPPB locus by performing the first large-scale genetic association study. This genetic variation was associated with lower ANP protein expression and circulating plasma levels, higher diastolic blood pressures and stroke. Our study confirms the important role of the natriuretic peptide system in the pathophysiology of hypertension and its sequelae such as stroke and lays the foundation for the study of the potential therapeutic benefit of manipulating the natriuretic peptide system in treating these conditions.
Supplementary Material
Acknowledgments
We thank Luanne Wussow for her assistance with the preparation of this manuscript.
Funding Sources: This work was supported in part from CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), Heart Failure Clinical Research Network grant U01 HL 084907 and grant U10 HL11026, NIH grant U19 GM61388 (The Pharmacogenomics Research Network), and NIH grants R01 HL36634 and P01 HL76611.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Munagala VK, Burnett JC, Jr, Redfield MM. The natriuretic peptides in cardiovascular medicine. Curr Probl Cardiol. 2004;29:707–769. doi: 10.1016/j.cpcardiol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Boomsma F, Bhaggoe UM, Man in't Veld AJ, Schalekamp MADH. Comparison of N-terminal pro-atrial natriuretic peptide and atrial natriuretic peptide in human plasma as measured with commercially available radioimmunoassay kits. Clin Chim Acta. 1996;252:41–49. doi: 10.1016/0009-8981(96)06311-5. [DOI] [PubMed] [Google Scholar]
- 3.McDowell G, Patterson C, Maguire S, Shaw C, Nicholls DP, Hall C. Variability of Nt-proANP and C-ANP. Eur J Clin Invest. 2002;32:545–548. doi: 10.1046/j.1365-2362.2002.01031.x. [DOI] [PubMed] [Google Scholar]
- 4.Macheret F, Heublein D, Costello-Boerrigter LC, Boerrigter G, McKie P, Bellavia D, et al. Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J Am Coll Cardiol. 2012;60:1558–1565. doi: 10.1016/j.jacc.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKie PM, Cataliotti A, Sangaralingham SJ, Ichiki T, Cannone V, Bailey KR, et al. Predictive utility of atrial, N-Terminal pro-atrial, and N-terminal pro-B-type natriuretic peptides for mortality and cardiovascular events in the general community: a 9-year follow-up study. Mayo Clinic Proc. 2011;86:1154–1160. doi: 10.4065/mcp.2011.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John SWKJ, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. Erratum in: Science 1995;1267:1753. [DOI] [PubMed] [Google Scholar]
- 7.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–353. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, et al. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 9.Burnett JCKP, Jr, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 10.Shub CKA, Zachariah PK, Bailey KR, Tajik AJ. Determination of left ventricular mass by echocardiography in a normal population: effect of age and sex in addition to body size. Mayo Clinic Proc. 1994;69:205–211. doi: 10.1016/s0025-6196(12)61058-1. [DOI] [PubMed] [Google Scholar]
- 11.Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: The Framingham heart study. Am J Cardiol. 1987;59:956–960. doi: 10.1016/0002-9149(87)91133-7. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 14.Rubattu S, Bigatti G, Evangelista A, Lanzani C, Stanzione R, Zagato L, et al. Association of atrial natriuretic peptide and type A natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J Am Coll Cardiol. 2006;48:499–505. doi: 10.1016/j.jacc.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 15.Xue H, Wang S, Wang H, Sun K, Song X, Zhang W, et al. Atrial natriuretic peptide gene promoter polymorphism is associated with left ventricular hypertrophy in hypertension. Clin Sci. 2008;114:131–137. doi: 10.1042/CS20070109. [DOI] [PubMed] [Google Scholar]
- 16.Houweling AC, van Borren MM, Moorman AFM, Christoffels VM. Expression and regulation of the atrial natriuretic factor encoding gene Nppa during development and disease. Cardiovasc Res. 2005;67:583–593. doi: 10.1016/j.cardiores.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Del Greco MF, Pattaro C, Luchner A, Pichler I, Winkler T, Hicks AA, et al. Genome-wide association analysis and fine mapping of NT-proBNP level provide novel insight into the role of the MTHFR-CLCN6-NPPA-NPPB gene cluster. Hum Mol Genet. 2011;20:1660–1671. doi: 10.1093/hmg/ddr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annilo T, Kepp K, Laan M. Natural antisense transcript of natriuretic peptide precursor A (NPPA): structural organization and modulation of NPPA expression. BMC Mol Biol. 2009;10:81. doi: 10.1186/1471-2199-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 20.Pereira NL, Lin D, Pelleymounter L, Moon I, Stilling G, Eckloff BW, et al. Natriuretic peptide receptor-3 gene (NPR3): nonsynonymous polymorphism results in significant reduction in protein expression because of accelerated degradation. Circ: Cardiovasc Genet. 2013;6:201–210. doi: 10.1161/CIRCGENETICS.112.964742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira NL, Aksoy P, Moon I, Peng Y, Redfield MM, Burnett JC, Jr, et al. Natriuretic peptide pharmacogenetics: membrane metallo-endopeptidase (MME): common gene sequence variation, functional characterization and degradation. J Mol Cell Cardiol. 2010;49:864–874. doi: 10.1016/j.yjmcc.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubattu S, Sciarretta S, Ciavarella GM, Venturelli V, De Paolis P, De Tocci G, et al. Reduced levels of N-terminal-proatrial natriuretic peptide in hypertensive patients with metabolic syndrome and their relationship with left ventricular mass. J Hypertens. 2007;25:833–839. doi: 10.1097/HJH.0b013e32803cae3c. [DOI] [PubMed] [Google Scholar]
- 23.Rubattu SBA, Marchitti S, Iacone R, Di Castro S, Evangelista A, Stanzione RIR, Sciarretta S, Palmieri L, Volpe M, Strazzullo P. Olivetti Heart Study Research Group. Determinants of N-terminal proatrialnatriuretic peptide plasma levels in a survey of adult male population from Southern Italy. J Hypertens. 2010;28:1638–1645. doi: 10.1097/HJH.0b013e32833a39aa. [DOI] [PubMed] [Google Scholar]
- 24.Andersson B, Hall C. N-terminal proatrial natriuretic peptide and prognosis in patients with heart failure and preserved systolic function. J Card Fail. 2000;6:208–213. doi: 10.1054/jcaf.2000.8836. [DOI] [PubMed] [Google Scholar]
- 25.Hole T, Hall C, Skjærpe T. N-terminal proatrial natriuretic peptide predicts two-year remodelling in patients with acute transmural myocardial infarction. Eur Heart J. 2004;25:416–423. doi: 10.1016/j.ehj.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 26.Jarai R, Iordanova N, Jarai R, Raffetseder A, Woloszczuk W, Gyöngyösi M, et al. Risk assessment in patients with unstable angina/non-ST-elevation myocardial infarction and normal N-terminal pro-brain natriuretic peptide levels by N-terminal pro-atrial natriuretic peptide. Eur Heart J. 2005;26:250–256. doi: 10.1093/eurheartj/ehi038. [DOI] [PubMed] [Google Scholar]
- 27.Cannone V, Boerrigter G, Cataliotti A, Costello-Boerrigter LC, Olson TM, McKie PM, et al. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll Cardiol. 2011;58:629–636. doi: 10.1016/j.jacc.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casco VH, Veinot JP, de Bold MLK, Masters RG, Stevenson MM, de Bold AJ. Natriuretic peptide system gene expression in human coronary arteries. J Histochem Cytochem. 2002;50:799–809. doi: 10.1177/002215540205000606. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. New Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.