Abstract
Background:
Persistent somatoform pain disorder (SPD) is a condition in which the patient suffers from persistent, severe and distressing pain; and from associated physical and psychological distress. While presence of restless leg syndrome (RLS) in SPD is understudied, their association might have an impact on general well-being and quality of life (QoL) in SPD.
Aims and Objectives:
Present study aimed at evaluating the prevalence of RLS in SPD patients attending outpatient department services at a tertiary care institute in eastern India.
Materials and Methods:
Two hundred and forty consecutive patients with SPD were screened initially and after applying appropriate inclusion and exclusion criteria, 192 subjects (male = 85, female = 107) were included in the study. Severity of RLS was assessed using a questionnaire of the International Restless Legs Syndrome Study Group and QoL was measured on QoL Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF).
Results:
Revealed a 28% prevalence of RLS is in patients with SPD, which is much higher than its estimated population prevalence. A larger proportion of those with RLS had continuous course of SPD, longer duration of SPD, and higher daytime sleepiness. They also had poorer scores on Q-LES-Q-SF, indicating a poorer QoL overall.
Discussion and Conclusion:
This is the first report, to the best of our knowledge, on this aspect from India. While this association between RLS and SPD may have biological explanation based on abnormal monoaminergic neurotransmission system, the findings call for more vigilant approach to SPD patients in order to improve their QoL and add to their well-being.
Keywords: Dopamine, restless leg syndrome, somatoform pain disorder
Introduction
Somatoform disorder is defined by presence of multiple physical symptoms that suggest a general medical condition but is not fully explained by the latter. Persistent somatoform pain disorder (SPD) is a subtype in which predominant complaint is of persistent, severe, and distressing pain; often causing significant physical and psychological distress.
The prevalence of restless leg syndrome (RLS) in the general population is estimated between 8% and 18%.[1,2] Criteria for the diagnosis of RLS were first proposed by the International Restless Leg Syndrome Study Group (IRLSSG) in 2003.[3,4] According to these, the urge to move the legs, worsening of symptoms with rest or inactivity, relief with movement and deterioration during evening or night hours compared to daytime are the diagnostic of RLS. The diagnosis is further supported by a positive family history, periodic limb movement and symptomatic improvement with levodopa therapy.[4] The symptoms of RLS can either be primary (idiopathic) or secondary to causes like iron-deficiency, end stage renal disease, diabetes mellitus, Parkinson's disease, venous insufficiency and even pregnancy.
Literature shows that a considerable proportion of patients with SPD has sleep disturbances,[5] and patients with sleep disorders have an increased risk of developing somatoform symptoms.[1] It has been proposed that in spite of its noninclusion in diagnostic criteria, sleep disturbances may constitute one of the most important dimensions of somatoform disorder besides depression, somatic distress and phobic anxiety.[6] Put in this context, as RLS occurs predominantly at night and profoundly affects sleep, it would be interesting to note its contribution to sleep disturbances in SPD. Though there are studies substantiating the relationship between SPD and sleep, and sleep disturbances and RLS; very few studies have been conducted so far to explore the relationship between the SPD and RLS.[7] Present study aims to fulfill this void by measuring the prevalence of RLS in SPD patients and its effect on sleep and quality of life (QoL) in this group.[8]
Materials and Methods
Sample
The present study was a cross-sectional study conducted in a Tertiary Care Teaching Hospital in Eastern India. It was approved by the institutional ethics committee. Two hundred and forty consecutive patients diagnosed for the 1st time with SPD, diagnosed as per IRLSSG criteria,[4] were selected using purposive sampling, and those aged 18–60 years and with adequate cognitive functions to perform the interview were recruited. Subjects with any psychiatric, substance-related (except for tobacco) or medical comorbidities like end stage renal disease, diabetes mellitus, Parkinson's disease, venous insufficiency etc., were excluded from the study. To exclude iron-deficiency state, we followed a three-step procedure: All patients were screened clinically for pallor, and those with pallor were instructed to have their hemogram done. A laboratory diagnosis of iron-deficiency anemia led to their exclusion.
Thirty-five patients did not meet the inclusion criteria or met exclusion criteria. Twenty-three patients did not give valid consent. The study was thus completed with 192 patients giving valid informed consent. Sociodemographic data were collected, and patients were examined clinically using RLS diagnostic criteria[4] (IRLSSG) for the occurrence of RLS.
Instruments
The severity of RLS was assessed using the questionnaire of the IRLSSG. This scale is a self-rated instrument which rates RLS in the areas of discomfort, need to move and relief after moving, sleep disturbance, tiredness during the day, severity, frequency, duration, overall effects on daily life and resultant mood disturbance on a scale of 1–5. QoL in our patients was measured using QoL Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF).[9] Higher scores on the QLES-Q are indicative of greater enjoyment or satisfaction. Daytime sleepiness was measured on Epworth sleepiness scale (ESS).[10] The ESS consists of 8 items, with a 4-point scale (0–3). A total score of 10 or higher suggests excessive daytime sleepiness and indicates a sleep disorder. The Cronbach's alpha of this scale was 0.737 indicating good reliability. Finally, all these instruments were applied by trained psychiatrists.
Statistical analysis
SPSS-version 20 (SPSS Inc., IBM Corporation, New York, US) was used for statistical analysis. For comparisons between the groups, t-test was used for continuous variables and Chi-square tests were used for categorical variables. The relationship between QOL, Duration of SPD, RLS score, and other clinical characteristics was assessed using Pearson's correlation analysis. Significance was determined at P < 0.05.
Results
Prevalence data
Among 192 study participants, 56 patients (male = 24, female = 32) met the IRLSSG diagnostic criteria for RLS, whereas 136 patients (male = 61, female = 75) did not. Hence the prevalence rate was estimated at 29.16%.
Sociodemographic data
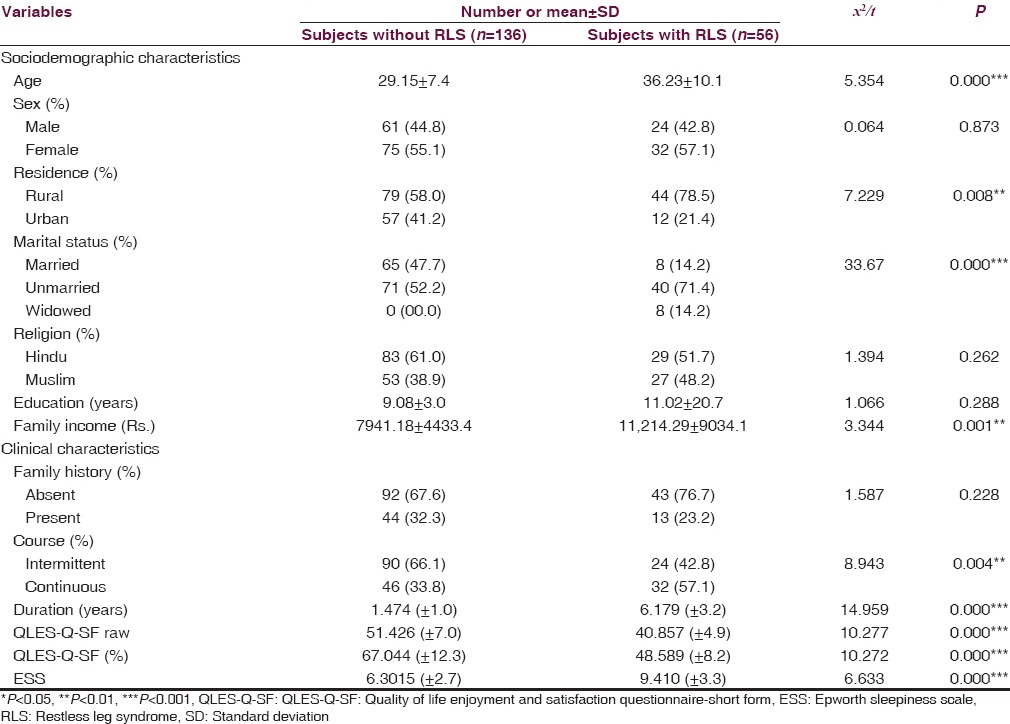
Table 1 describes sociodemographic and clinical characteristics of the study subjects. The total sample (n = 192) had a mean age of 31.21 ± 9.65 years. Majority of the subjects were female (55%), were from the rural background (64.1%), married (57.8%) and having 9.65 ± 11.44 years of mean education. On comparing the non-RLS and RLS group statistically, significant difference was observed for the variables of age, residence, marital status and family income. Patients with RLS group to were older in age, were predominantly from the rural background, were more likely to be unmarried, and had higher family income.
Table 1.
Comparison of sociodemographic and clinical characteristics between those with and without RLS (n=192)
Clinical variables
Since we had recruited SPD at first presentation, most of our samples were not taking psychotropic medications when they were assessed. Out of a total of 192 subjects, 118 had not taken any medicines for their symptoms in last 1-month, 53 were taking nonsteroidal antiinflammatory drugs on an “as-required” basis, 5 were on regular nonsteroidal antiinflammatory medication; and 16 were taking antidepressants prescribed by a physician (9 tri-cyclic antidepressants, 5 Selective serotonin reuptake inhibitors, 2 Serotonin norepinephrine reuptake inhibitors). None of the subjects had taken neuroleptic medications in the past. Among the RLS group, 18 patients had mild (1–10) and 48 patient had moderate (score 11–20) degree of RLS. Table 1 shows the clinical variables and their comparison between RLS and non-RLS group. Compared to the non-RLS group, a larger proportion of RLS group had a continuous course of SPD (χ2 = 8.943, P < 0.01), longer duration of SPD (χ2 = 14.959, P < 0.001), poorer QoL as assessed by Q-LES-Q-SF (χ2 = 10.272, P < 0.001), and higher score on ESS suggestive of higher daytime sleepiness (χ2 = 6.633, P < 0.001).
Quality of life
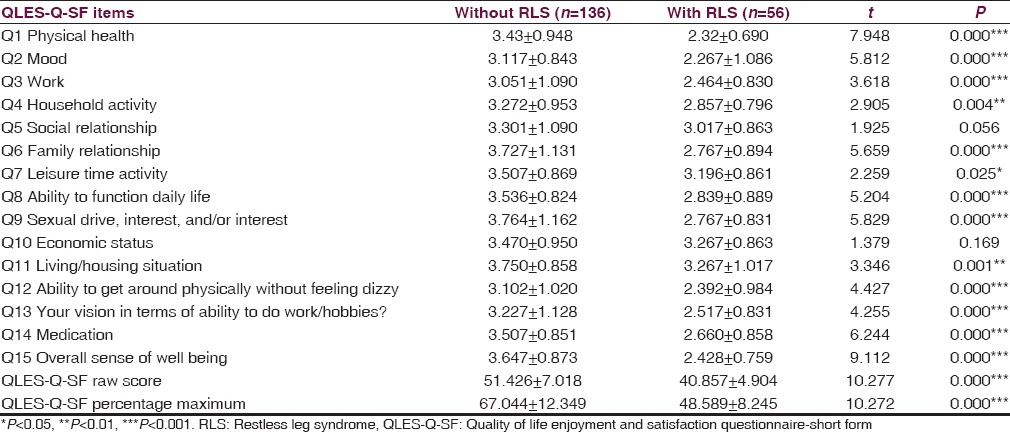
To study the QoL raw scores on Q-LES-Q-SF were converted to percentage maximum scores (QLESPER). The evaluation of Q-LES-Q-SF life quality scale showed that all items and total score of the scale were significantly lower in the RLS group [Table 2].
Table 2.
Comparison of QLES-Q-SF item scores between RLS and non-RLS groups (independent sample t-test)
Correlation
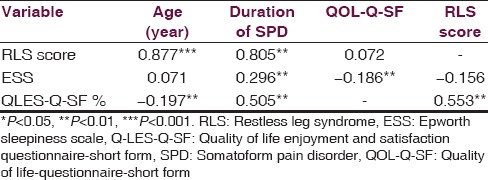
The relationship between Q-LES-Q-SF percentage maximum scores, clinical features, ESS score and IRLSSG scale scores were investigated. Though there was a significant difference between the groups in terms of QLESPER, it failed to correlate significantly with RLS scale score. Both, however, correlated significantly with age of presentation and duration of SPD [Table 3].
Table 3.
Pearson's correlation matrix between variables in patients of SPD with RLS (n=56)
Discussion
Our results reveal a 29.16% prevalence of RLS is in patients with SPD. Earlier studies addressing the prevalence of RLS in community samples reported rates between 0.9% and 8.3% in Asians and 17.7% in Americans.[11,12,13] A population study of RLS that applied standard diagnostic criteria in 15,391 subjects (≥18-year-old) from the United States and five European countries (France, Germany, Italy, Spain, and the United Kingdom) reported prevalence of RLS to be 7.2%.[14] Though Asian and particularly Indian population studies are sparse and few, the study by Rangarajan et al. reported 2.1% prevalence of RLS in 1266 urban population sample in India.[15] Hence, our finding reports a much higher prevalence of RLS in SPD, when compared to the general population at large.
In our study, sex-distribution, education in years, monthly income and family history of somatoform disorder were not found to differ significantly between those patients of SPD with and without RLS. However, those with RLS had an older age, longer duration of illness, continuous course and had remained unmarried at study recruitment; findings that are in agreement with available literature in this regard.[16] Interestingly, nearly 50% with patients with RLS had reported a positive family history in past studies, which was not replicated in the present study, and we obtained a much lower rate of 23%. Though a high heritability of idiopathic RLS has been proposed by authors in past,[2,3] our finding may be explained on the basis of nondiagnosis of cases in families of most of our subjects who came from a rural background with poor medical facilities. Rural Indian society also has greater tolerance for and acceptance of somatic symptoms that might have resulted in lesser impairments, further excluding the cases from our diagnostic net.
Thus, while we report a much-higher prevalence of RLS in SPD, what might be the neurobiological basis of this common association? We suggest that this might be due to an underlying disturbance in monoaminergic neurotransmission system. During chronic pain, glucocorticoid induced monoamine depletion may lead to loss of the monoaminergic tone, causing a reduction in descending inhibitory impulses to the spinal cord causing an intensification of pain sensation.[17] Schwartz and others[18] found that low levels of 5-HIAA and tryptophan were related to higher pain scores in fibromyalgia patients. There was also a tendency of higher pain scores to be related to higher serum concentrations of the neuropeptide substance P, pointing to the antagonistic interactions between substance P and serotonergic system in nociception. Interestingly, monoaminergic dysfunction, especially involving the dopaminergic system, is also thought to play a role in the pathophysiology of RLS.[19] As the dopamine is a prerequisite for the synthesis of noradrenaline, dopaminergic dysfunction in RLS affects noradrenergic neurotransmission as well. A normal level of norepinephrine may be necessary for opioid responsiveness as shown in a recent study.[20] This may explain the co-occurrence of SPD and RLS, and is consistent with the fact that opioids have less-than-usual efficacy in the treatment of chronic somatoform pain.[21]
Another possibility relates to sleep disturbances secondary to RLS. Our study found a significantly higher ESS score in subjects with RLS, compared to those without (P < 0.001). It has been shown in past studies that there is a high prevalence of sleep disturbance in SPD,[5] with frequent arousals. Nonrestorative sleep might lead to metabolic dysfunction of the brain with sleep-related alterations in immunological and neurotransmitter functions (serotonin, substance P, endorphins),[22,23] and converge on previously mentioned neurotransmitter systems. Thus, sleep disturbances might alter physiology of pain and fatigue, aggravating SPD and RLS.
Somatoform disorder itself is an important reason for having lower QoL.[16] The presence of RLS seems to further worsen the QoL, according to the findings of our study. We found on QoL scale that almost in all areas, SPD with RLS had a significantly poorer QoL compared to those without. While diagnosing RLS is relatively simple, this finding calls for a more vigilant approach to SPD so as to not miss a diagnosis of coexistent RLS. With this caution, we might be able to serve our patients better.
This study had a good sample size of 192 subjects. We had also used relatively robust exclusion criteria to rule out causes of secondary RLS. As the study center was a tertiary care referral facility, subjects were representative of the population attending psychiatry outpatient department in eastern parts of India. Therefore, findings are more or less generalizable to at least this population group. Present study is the first to report prevalence of RLS in SPD from India to the best of our knowledge. This study sheds light on this prevalent but often neglected comorbidity that can reduce chances of remission and compromise QoL in SPD.
Limitations and future direction
Our study had several limitations. Since we had used recall method for data collection, recall biases cannot be ruled out. In order to exclude secondary causes of RLS like iron-deficiency states, we relied on clinical examination and hemoglobin estimation due to logistical constraints at our end. However, a direct measure of serum ferritin would have been more robust. Our study also lacked a control group, which limits the possibility of discussing the findings in relation to a comparable healthy population.
While our results are pioneering, large-sample prospective studies with an age-sex matched healthy control group and exclusion criteria based on serum ferritin levels are suggested to reach a definitive conclusion on this issue.
Acknowledgment
We acknowledge our gratitude toward all the colleagues and seniors of our department for their help and support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Saletu B, Brandstätter N, Frey R, Saletu-Zyhlarz GM, Dantendorfer K, Berger P, et al. Clinical aspects of sleep disorders – Experiences with 817 patients of an ambulatory sleep clinic; comment. Wien Klin Wochenschr. 1997;109:390–9. [PubMed] [Google Scholar]
- 2.Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 3.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 4.Aurora RN, Kristo DA, Bista SR, Rowley JA, Zak RS, Casey KR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults – An update for 2012: Practice parameters with an evidence-based systematic review and meta-analyses: An American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35:1039–62. doi: 10.5665/sleep.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aigner M, Graf A, Freidl M, Prause W, Weiss M, Kaup-Eder B, et al. Sleep disturbances in somatoform pain disorder. Psychopathology. 2003;36:324–8. doi: 10.1159/000075833. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie N, Kirk KM, Heath AC, Martin NG, Hickie I. Somatic distress as a distinct psychological dimension. Soc Psychiatry Psychiatr Epidemiol. 1999;34:451–8. doi: 10.1007/s001270050219. [DOI] [PubMed] [Google Scholar]
- 7.Aigner M, Prause W, Freidl M, Weiss M, Izadi S, Bach M, et al. High prevalence of restless legs syndrome in somatoform pain disorder. Eur Arch Psychiatry Clin Neurosci. 2007;257:54–7. doi: 10.1007/s00406-006-0684-0. [DOI] [PubMed] [Google Scholar]
- 8.Winter AC, Berger K, Glynn RJ, Buring JE, Gaziano JM, Schürks M, et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in men. Am J Med. 2013;126:228–35. doi: 10.1016/j.amjmed.2012.06.039. 235.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: A new measure. Psychopharmacol Bull. 1993;29:321–6. [PubMed] [Google Scholar]
- 10.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 11.Cho SJ, Hong JP, Hahm BJ, Jeon HJ, Chang SM, Cho MJ, et al. Restless legs syndrome in a community sample of Korean adults: Prevalence, impact on quality of life, and association with DSM-IV psychiatric disorders. Sleep. 2009;32:1069–76. [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KW, Yoon IY, Chung S, Shin YK, Lee SB, Choi EA, et al. Prevalence, comorbidities and risk factors of restless legs syndrome in the Korean elderly population – Results from the Korean Longitudinal Study on Health and Aging. J Sleep Res. 2010;19:87–92. doi: 10.1111/j.1365-2869.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- 13.Froese CL, Butt A, Mulgrew A, Cheema R, Speirs MA, Gosnell C, et al. Depression and sleep-related symptoms in an adult, indigenous, North American population. J Clin Sleep Med. 2008;4:356–61. [PMC free article] [PubMed] [Google Scholar]
- 14.Allen RP, Auerbach S, Bahrain H, Auerbach M, Earley CJ. The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol. 2013;88:261–4. doi: 10.1002/ajh.23397. [DOI] [PubMed] [Google Scholar]
- 15.Rangarajan S, Rangarajan S, D’Souza GA. Restless legs syndrome in an Indian urban population. Sleep Med. 2007;9:88–93. doi: 10.1016/j.sleep.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Chandrashekhar CR, Reddy V, Isaac MK. Life events and somatoform disorders. Indian J Psychiatry. 1997;39:166–72. [PMC free article] [PubMed] [Google Scholar]
- 17.Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: Coincidence or consequence? J Neuroendocrinol. 2001;13:1009–23. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz MJ, Späth M, Müller-Bardorff H, Pongratz DE, Bondy B, Ackenheil M. Relationship of substance P, 5-hydroxyindole acetic acid and tryptophan in serum of fibromyalgia patients. Neurosci Lett. 1999;259:196–8. doi: 10.1016/s0304-3940(98)00937-9. [DOI] [PubMed] [Google Scholar]
- 19.Trenkwalder C, Paulus W. Why do restless legs occur at rest? – Pathophysiology of neuronal structures in RLS. Neurophysiology of RLS (part 2) Clin Neurophysiol. 2004;115:1975–88. doi: 10.1016/j.clinph.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Jasmin L, Boudah A, Ohara PT. Long-term effects of decreased noradrenergic central nervous system innervation on pain behavior and opioid antinociception. J Comp Neurol. 2003;460:38–55. doi: 10.1002/cne.10633. [DOI] [PubMed] [Google Scholar]
- 21.Rief W, Barsky AJ. Psychobiological perspectives on somatoform disorders. Psychoneuroendocrinology. 2005;30:996–1002. doi: 10.1016/j.psyneuen.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Moldofsky H. Sleep and fibrositis syndrome. Rheum Dis Clin North Am. 1989;15:91–103. [PubMed] [Google Scholar]
- 23.Ghosh A, Basu D. Restless legs syndrome in opioid dependent patients. Indian J Psychol Med. 2014;36:85–7. doi: 10.4103/0253-7176.127262. [DOI] [PMC free article] [PubMed] [Google Scholar]


