Abstract
Background:
Depression is one of the most frequent neuropsychiatric disturbances after a cerebrovascular stroke. The frequency of depression in stroke patients has varied widely in different populations. Post stroke depression is an important factor limiting recovery and rehabilitation in acute stroke patients.
Settings and Design:
A cross-sectional hospital-based study was performed in acute stroke patients admitted in the department of Medicine of a rural teaching tertiary care hospital in central India.
Materials and Methods:
In all consecutive acute stroke inpatients, the intensity of depression was assessed by a trained person through a questionnaire, Montgomery-Asberg Depression Rating Scale (MADRS), who is blind of the diagnosis and investigations of the patient. Another study person collected the data including demographics, co-morbid diseases or risk factors. Radiological imaging data was noted from the CT/MRI head reports of stroke patients.
Results:
Of the total 107 stroke patients, 60 (56%) were males and 47 (44%) were females. Sixty-one (57%) of the 107 stroke patients had depression. Of the 107 stroke patients, 35 (33%) had mild depression, 22 (20%) had moderate depression and 4 (4%) had severe depression. The age, gender, education status and co-morbidities of the stroke patient were not associated with depression. The association of socio-economic status and left-sided lesions with depression was found to be statistically significant (P < 0.05). Type and location of the lesion were not associated with depression.
Conclusion:
Post-stroke depression was present in more than half of the stroke patients and was related to socio-economic status and left-sided hemisphere lesions.
Keywords: Assessment of depression, depression, determinants, prevalence, stroke
Background
Cerebrovascular (CV) stroke is a major global health problem. In 2010, an estimated 11.5 million incident ischaemic strokes (63% in low-income and middle-income countries) and 5.3 million incident hemorrhagic strokes (80% in low-income and middle-income countries) took place.[1] An estimated 5.8 million individuals died from stroke in a year.[1,2] CV stroke is the leading cause of disability in adults and each year millions of stroke survivors have to adapt to a life with restrictions in activities of daily living as a consequence of cerebrovascular disease. Although CV stroke has an impact on the physical, social, cognitive, emotional or psychological functioning of individuals, research in this area has focused primarily on physical functioning, and psychiatric co-morbidity has been relatively neglected and under-recognized. In addition to neurological issues such as location of the stroke and the nature of the motor impairment, psychological factors are also important in determining quality of life (QoL) and functional outcome in stroke survivors.[3] The prevalence of depression in stroke patients ranges from 6% to 66%.[4,5,6] Post-stroke depression impacts negatively on QoL, functional recovery, cognitive function and rehabilitation of acute stroke patients.[7,8,9,10,11] In addition, it increases mortality and is associated with a higher risk of recurrent stroke at 1 year.[12,13,14,15] Apart from having an impact on the stroke survivors themselves, post-stroke depression also affects family.[16] There is a paucity of literature from India about the prevalence of depression and its correlation with various factors in acute stroke patients.
As there is uncertainty regarding the frequency and determinants of depression, this study was planned to assess the magnitude of depression in acute stroke patients admitted in a rural tertiary care hospital and to analyze the association of demographic variables (age, gender, socio-economic status and educational status), co-morbid conditions or risk factors and radiological imaging variables with depression.
Materials and Methods
Setting
This study was conducted between April 1, 2013 and September 30, 2013 in the department of Medicine, Mahatma Gandhi Institute of Medical Sciences, Sevagram which is a 750-bedded teaching rural tertiary care hospital located in a town in central India. Most patients visiting the hospital come from rural areas. The patients with the diagnosis CV stroke are admitted in the intensive care unit or general indoor ward of department of Medicine.
Study design
A cross-sectional design was used to assess the magnitude of depression through a questionnaire and to collect data related to demographic variables, co-morbid conditions, clinical and imaging variables.
Ethics
The study was approved by the institutional ethics committee of Mahatma Gandhi Institute of Medical Sciences (IRB00003623). We obtained a written informed consent from all study patients before enrolling in the study.
Subject
Inclusion criteria
(1) All consecutive patients admitted with a diagnosis of acute stroke (as defined by the World Health Organization criteria—a rapidly developing clinical symptoms or signs of focal or global disturbance of cerebral function that last more than 24 hours, with no apparent non-vascular causes (primary and metastatic neoplasms, dural hematoma, head trauma, etc)) with a radiological (computed tomography (CT) scan or magnetic resonance imaging (MRI) head) evidence of CV stroke. Acute stroke included acute ischemic stroke, intracerebral/intraventricular hemorrhage, and subarachnoid hemorrhage; (2) age of patient 18 years or older.
Exclusion criteria
(1) Presence of communicative problems that preclude a psychiatric examination (e.g. due to reduced level of consciousness, severe hearing or visual impairment, aphasia or severe dysarthria, severe cognitive dysfunction); (2) history of depression/psychiatric illness or on anti-depressant drugs; (3) illicit drug dependence; (4) patients who scored less than 10 on the Glasgow Coma Scale (GCS); (5) history of chronic liver disease or liver enzymes (ALT/AST) more than three times the upper limit of normal, end-stage renal disease, malignancy or thyroid disease; (6) refusal to consent.
Data collection
A research assistant collected the data of acute stroke patients including demographics (age, gender, per capita income (PCI), education-status), and comorbid diseases or risk factors such as hypertension (history of hypertension or anti-hypertension drug), diabetes mellitus (DM) (history of diabetes or anti-hyperglycemic drug), history of previous CV stroke, history of ischemic heart disease (IHD), current smokers (persons who report smoking at least 100 cigarettes in their life and who currently smoke every day or on some days), and high alcohol consumption (≥5 standard drink/day). Socioeconomic status (SES) of the stroke patients was assessed by per capita monthly household income (PCI) and was categorized into PCI quartiles. Education status was categorized as illiterate, primary school, middle school and high school and above. Reports of laboratory investigations like serum creatinine, sodium, potassium, blood sugar, urea and liver function tests were collected from the patient's medical records. The radiological imaging data (type, location and size of the stroke lesion) was noted from the CT/MRI head reports.
The intensity of depression in patients with stroke was assessed by an another trained person who was blind of the diagnosis and investigations of the patient, through a face-to-face interview based questionnaire (see Text S1), Montgomery-Asberg Depression Rating Scale (MADRS).[17] It is a 10-item diagnostic questionnaire to measure the severity of depressive episodes. The reliability and validity of MADRS questionnaire for the assessment of depression have been established in the previous studies.[18,19] Higher MADRS score indicates more severe depression, and each item yields a score of 0 to 6. The overall score ranges from 0 to 60. The assessments of depression in stroke patients were carried out during hospitalization.
Cutoff points for categorization of mild, moderate and severe depression were:
0 to 6 – normal/no depression
7 to 19 – mild depression
20 to 34 – moderate depression
>34 – severe depression.
Data analysis
We used SPSS software (version 16.0) to analyze the characteristics of the study population. The demographic variables, comorbid diseases and radiological characteristics of the stroke patients with depression were compared with those without depression using the Chi-square test or the Fisher exact test as appropriate. Categorical variables were presented as proportions and compared using the Chi-square test. A P < 0.05 was regarded as being statistically significant.
Results
A total of 107 stroke patients were studied. The mean age was 59.13 ± 11.66 years, ranging from 28 to 80 years. There were 21 (20%) stroke patients below the age of 50 years and 86 (80%) patients above the age of 50 years. Of the total 107 stroke patients, 60 (56%) were males and 47 (44%) were females [Table 1]. Seventy (65%) patients had their PCI below Rs. 3000 per month [Table 2]. There were 17 (16%) illiterates, 36 (34%) educated up to primary school, 26 (24%) educated up to middle school and 28 (26%) educated high school and above of the total 107 patients [Table 3].
Table 1.
Gender distribution of stroke patients

Table 2.
Distribution of stroke patients by per capita income quartiles

Table 3.
Distribution of stroke patients by education status

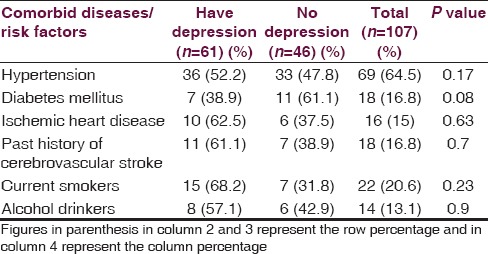
Sixty-one (57%) of the 107 stroke patients had depression. Of the 107 stroke patients, 35 (33%) had mild depression, 22 (20%) had moderate depression and 4 (4%) had severe depression [Table 4]. SES (PCI) was significantly associated with depression in our stroke patients (P < 0.05) [Table 5]. The association of age of the patient (<50 years vs > 50 years), gender, and education status with depression was not statistically significant (P > 0.05) [Table 5]. Of the 107 patients, 69 (64%) had hypertension, 18 (17%) had DM, 16 (15%) had IHD and 18 (17%) had previous history of CV stroke. The association of co-morbid illnesses, i.e. hypertension, DM, IHD, and previous history of stroke with depression was not statistically significant [Table 6]. There were 22 (20%) current smokers and 14 (13%) alcohol drinkers of the total 107 stroke patients. The association of depression with either current smoking or alcohol drinking was not statistically significant [Table 6].
Table 4.
Distribution of depression among stroke patients

Table 5.
Distribution of demographic variables among patients with or without depression
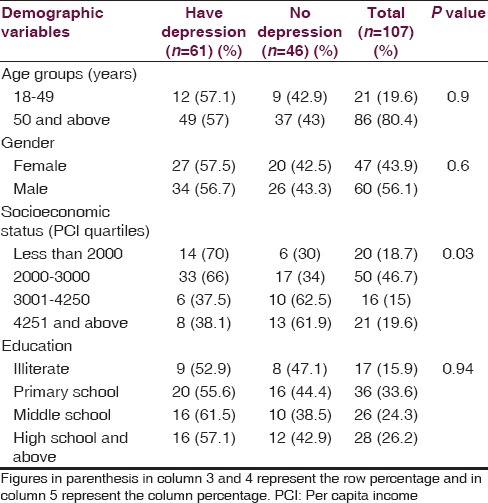
Table 6.
Distribution of comorbid diseases/risk factors among patients with or without depression
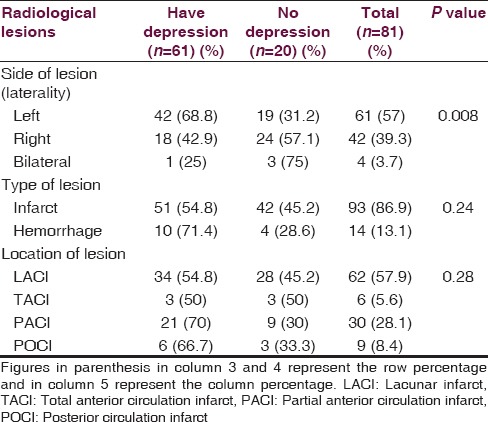
In the radiological imaging (CT/MRI head) of CV stroke patients, 61 (57%) patients had left-sided lesions, 42 (39%) patients had right-sided lesions and 4 (4%) had bilateral lesions. Ninety-three (87%) patients had cerebral infarcts and 14 (13%) had intracerebral hemorrhages (ICH). Sixty-two (58%) of the 107 stroke patients had lacunar (LAC) infarcts, 6 (6%) had total anterior circulation (TAC) infarcts, 30 (28%) had partial anterior circulation (PAC) infarcts and 9 (8%) had posterior circulation infarct (POC) infarcts. The association of left-sided lesions with depression was found to be statistically significant (P < 0.05). Type of lesion (infarct/ICH) and location of the lesion (LAC/TAC/PAC/POC) were not associated with depression (P > 0.05) [Table 7].
Table 7.
Distribution of imaging variables among patients with or without depression
Discussion
The overall magnitude of depression was 57% (61 of the 107 stroke patients had depression). Of the 107 stroke patients, 35 (33%) had mild depression, 22 (20%) had moderate depression and 4 (4%) had severe depression. In a study done by Hsieh et al., depressive symptoms and severe depression were found in 34.3% patients and 7.7% patients, respectively.[9] Forty-four percent patients were found to have post-stroke depression (PSD) in a study done by Camoes Barbosa et al.[20] In a study done by Raju et al. on 162 patients (interviewed after 1 month post stroke), 60 patients (37%) had depression.[3] Of the 80 stroke patients, 53 (66%) were depressed, 41 (51%) were mildly depressed and 12 (15%) were moderately to severely depressed in a study done by Glamcevski et al.[6] In our study, the stroke patients were assessed for depression within a week of occurrence of stroke, i.e. during hospitalization. In most of the previous studies, depression was assessed in stroke patients from 1 month to 2 years after the occurrence of stroke. Acute stress exposure due to more functional dependency and physical disability during the acute phase of stroke might be responsible for high magnitude of depression in our stroke patients as compared to that in previous studies. In addition, use of different tools for the assessment of depression in different studies might lead to varied overall frequency of depression. However, the magnitude of moderate to severe depression (24%) in our study is comparable with previous studies.
In our study, the association of gender with depression was not found to be statistically significant. This finding is in contrast to previous study done by Hsieh et al., in which depressive symptoms were more common in females.[9] The association of female gender with depression was also observed in a study done by Broomfield et al.[21]
Patient's SES was significantly associated with depression which is consistent with the previous studies.[21,22] Worsening effects of financial strain, poverty, or in deprivation of personal resources may link SES with depression. The association of patient's education status with depression was not statistically significant in our study.
In our study, co-morbid illnesses like hypertension, DM, IHD and previous history of stroke were not associated with depression. Ellis et al. observed that individuals with both stroke and depression were more likely to have history of hypertension, DM and heart disease compared with other groups.[15] The association of depression with either current smoking or alcohol drinking was not statistically significant in our study.
Stroke patients with left hemisphere lesions were significantly more depressed in comparison to patients with right hemisphere lesions. The similar finding was also observed in a meta-analysis done by Narushima et al.[23] Depression was significantly associated with left hemisphere lesions in previous studies done by Robinson et al. and Rajashekaran et al.[24,25] The reason hypothesized by the authors that the behavioral and catecholaminergic response to injury on the left side of brain is different from the right side of brain. However, Carson and colleagues in their systemic review did not find an association between depression and lesion side.[26] Berg et al. and Aben et al. in their respective studies also did not find any significant differences in depression prevalence between right- and left-hemispheric lesions.[27,28] Type of lesion (infarct/ICH) and location of the lesion (LAC/TAC/PAC/POC) were not associated with depression in our study. Robinson et al. reported an association of frontal lobe location with depression in stroke patients.[25]
Our study has several strengths. Our patients represent typical rural Indian patients from central India. By including every consecutive stroke inpatient we avoided the selection bias. We made a blind assessment (filling the MADRS questionnaire and data collection) of the patients. We used a validated tool which can be used easily bedside.
The limitation of our study is small sample size. If our sample size has been large with more number of patients with hypertension or DM or cardiovascular risk factors (smoking or alcohol drinking), we could get a significant association of these cardiovascular diseases or risk factors with depression. The patients with low GCS scores or aphasia which may be related to more severe strokes were excluded from the study. As our study is a hospital-based study, its results could not be generalized to the community or chronic CV stroke patients.
Conclusion
Post-stroke depression was present in more than half of the acute stroke patients and was related to left-sided lesions and socioeconomic status. Moderate depression and severe depression were present in 20% and 4% of all the stroke patients, respectively.
Suggestion
This study shows that depression is fairly common after acute stroke. Post-stroke depression is an under-recognized and under-treated entity. Early diagnosis and effective treatment of depression might improve functional recovery, cognitive performance, drug compliance, quality of life, and social and rehabilitation outcome of stroke patients. There is a need for further research to improve clinical practice in this area of stroke care.
Footnotes
Source of Support: This study was partly funded by the Indian Council of Medical Research, New Delhi, India. Ajitabh Suman, a medical student, was awarded the short term studentship (2013) to conduct this study (URL: http://www.icmr.nic.in/shortr.htm). The Indian Council of Medical Research had no role in study design, data collection, and analysis, or preparation of the manuscript. No additional external funding received for this study.
Conflict of Interest: None declared.
References
- 1.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global Burden of Diseases, I-njuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: Findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–81. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strong K, Mathers C, Bonita R. Preventing stroke: Saving lives around the world. Lancet Neurol. 2007;6:182–7. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- 3.Raju RS, Sarma PS, Pandian JD. Psychosocial problems, quality of life, and functional independence among Indian stroke survivors. Stroke. 2010;41:2932–7. doi: 10.1161/STROKEAHA.110.596817. [DOI] [PubMed] [Google Scholar]
- 4.Caeiro L, Ferro JM, Santos CO, Figueira ML. Depression in acute stroke. J Psychiatry Neurosci. 2006;31:377–83. [PMC free article] [PubMed] [Google Scholar]
- 5.Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: A systematic review of observational studies. Stroke. 2005;36:1330–40. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 6.Glamcevski MT, 2nd, Pierson J. Prevalence of and factors associated with poststroke depression: A Malaysian study. J Stroke Cerebrovasc Dis. 2005;14:157–61. doi: 10.1016/j.jstrokecerebrovasdis.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Whyte EM, Mulsant BH. Post stroke depression: Epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52:253–64. doi: 10.1016/s0006-3223(02)01424-5. [DOI] [PubMed] [Google Scholar]
- 8.Gillen R, Eberhardt TL, Tennen H, Affleck G, Groszmann Y. Screening for depression in stroke: Relationship to rehabilitation efficiency. J Stroke Cerebrovasc Dis. 1999;8:300–6. doi: 10.1016/s1052-3057(99)80004-4. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh LP, Kao HJ. Depressive symptoms following ischemic stroke: A study of 207 patients. Acta Neurol Taiwan. 2005;14:187–90. [PubMed] [Google Scholar]
- 10.Bilge C, Koçer E, Koçer A, Türk Börü U. Depression and functional outcome after stroke: The effect of antidepressant therapy on functional recovery. Eur J Phys Rehabil Med. 2008;44:13–8. [PubMed] [Google Scholar]
- 11.Hackett ML, Anderson CS. Predictors of depression after stroke: A systematic review of observational studies. Stroke. 2005;36:2296–301. doi: 10.1161/01.STR.0000183622.75135.a4. [DOI] [PubMed] [Google Scholar]
- 12.Bartoli F, Lillia N, Lax A, Crocamo C, Mantero V, Carrà G, et al. Depression after stroke and risk of mortality: A systematic review and meta-analysis. Stroke Res Treat 2013. 2013 doi: 10.1155/2013/862978. 862978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris PL, Robinson RG, Andrzejewski P, Samuels J, Price TR. Association of depression with 10-year poststroke mortality. Am J Psychiatry. 1993;150:124–9. doi: 10.1176/ajp.150.1.124. [DOI] [PubMed] [Google Scholar]
- 14.Yuan HW, Wang CX, Zhang N, Bai Y, Shi YZ, Zhou Y, et al. Poststroke depression and risk of recurrent stroke at 1 year in a Chinese cohort study. PLoS One. 2012;7:e46906. doi: 10.1371/journal.pone.0046906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis C, Zhao Y, Egede LE. Depression and increased risk of death in adults with stroke. J Psychosom Res. 2010;68:545–51. doi: 10.1016/j.jpsychores.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson CS, Linto J, Stewart-Wynne EG. A population-based assessment of the impact and burden of caregiving for long-term stroke survivors. Stroke. 1995;26:843–9. doi: 10.1161/01.str.26.5.843. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 18.Davidson J, Turnbull CD, Strickland R, Miller R, Graves K. The Montgomery-Asberg depression scale: Reliability and validity. Acta Psychiatr Scand. 1986;73:544–8. doi: 10.1111/j.1600-0447.1986.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 19.Kang HJ, Stewart R, Kim JM, Jang JE, Kim SY, Bae KY, et al. Comparative validity of depression assessment scales for screening poststroke depression. J Affect Disord. 2013;147:186–91. doi: 10.1016/j.jad.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Camões Barbosa A, Sequeira Medeiros L, Duarte N, Meneses C. Predictors of poststroke depression: A retrospective study in a rehabilitation unit. Acta Med Port. 2011;24(Suppl 2):175–80. [PubMed] [Google Scholar]
- 21.Broomfield NM, Quinn TJ, Abdul-Rahim AH, Walters MR, Evans JJ. Depression and anxiety symptoms post-stroke/TIA: Prevalence and associations in cross-sectional data from a regional stroke registry. BMC Neurol. 2014;14:198. doi: 10.1186/s12883-014-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorant V, Deliège D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: A meta-analysis. Am J Epidemiol. 2003;157:98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 23.Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. J Neuropsychiatry Clin Neurosci. 2003;15:422–30. doi: 10.1176/jnp.15.4.422. [DOI] [PubMed] [Google Scholar]
- 24.Rajashekaran P, Pai K, Thunga R, Unnikrishnan B. Post-stroke depression and lesion location: A hospital based cross-sectional study. Indian J Psychiatry. 2013;55:343–8. doi: 10.4103/0019-5545.120546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson RG, Price TR. Post-stroke depressive disorders: A follow-up study of 103 patients. Stroke. 1982;13:635–41. doi: 10.1161/01.str.13.5.635. [DOI] [PubMed] [Google Scholar]
- 26.Carson AJ, MacHale S, Allen K, Lawrie SM, Dennis M, House A, et al. Depression after stroke and lesion location: A systematic review. Lancet. 2000;356:122–6. doi: 10.1016/S0140-6736(00)02448-X. [DOI] [PubMed] [Google Scholar]
- 27.Berg A, Palomäki H, Lehtihalmes M, Lönnqvist J, Kaste M. Poststroke depression: An 18-month follow-up. Stroke. 2003;34:138–43. doi: 10.1161/01.str.0000048149.84268.07. [DOI] [PubMed] [Google Scholar]
- 28.Aben I, Lodder J, Honig A, Lousberg R, Boreas A, Verhey F. Focal or generalized vascular brain damage and vulnerability to depression after stroke: A 1-year prospective follow-up study. Int Psychogeriatr. 2006;18:19–35. doi: 10.1017/S104161020500270X. [DOI] [PubMed] [Google Scholar]


