Abstract
Background
Cortical stimulation mapping (CSM) commonly uses visual naming to determine resection margins in the dominant hemisphere of epilepsy patients. Visual naming alone may not identify all language sites in resection-prone areas, prompting additional tasks for comprehensive language mapping.
Objective
To demonstrate word-finding distinctions between visual, auditory, and reading modalities during CSM and the percentage of modality-specific language sites within dominant hemisphere subregions.
Methods
Twenty-eight epilepsy patients underwent CSM using visual, auditory, and sentence completion tasks. Hierarchical logistic regression analyzed errors to identify language sites and provide modality-specific percentages within subregions.
Results
The percentage of sites classified as language sites based on auditory naming was twice as high in anterior temporal regions compared to visual naming, marginally higher in posterior temporal areas, and comparable in parietal regions. Sentence completion was comparable to visual and auditory naming in parietal regions, and lower in most temporal areas. Of 470 sites tested with both visual and auditory naming, 95 sites were distinctly auditory while 48 sites were distinctly visual. The remaining sites overlapped.
Conclusion
Distinct cortical areas were found for distinct input modalities, with language sites in anterior tip regions found most often using auditory naming. The vulnerability of anterior temporal tip regions to resection in this population and distinct sites for each modality suggest a multimodality approach may be needed to spare crucial language sites, if sparing those sites can be shown to significantly reduce the rate of post-operative language deficits without sacrificing seizure control.
Keywords: auditory naming, cortical stimulation, language, reading, visual naming
Introduction
Epilepsy patients often undergo pre-resection cortical stimulation mapping (CSM) to remove maximum pathological tissue while simultaneously minimizing post-operative functional deficits. To minimize deficits, it is necessary to identify the location of functional cortex that is dedicated to speech, language, sensory, and motor processing so they may be spared, particularly near the boundaries of pathological tissue.
The removal of an epileptic focus or lesion is particularly challenging within the dominant hemisphere due to a lack of specific correspondence between anatomical landmarks and higher cognitive functions that parallels pre- or post-central gyri corresponding to motor and sensory areas.1 For temporal lobectomies used to treat intractable epilepsy or for lesion resections in temporal or parietal areas, language is mapped for each patient by electrically stimulating discrete areas of cortex to create a temporary and reversible lesion while a specific language task is performed. If stimulation repeatedly interrupts the patient's ability to perform the task, that site is inferred to be essential for generalized language function.2, 3
Confrontation naming has been the language task of choice since early accounts of language mapping in the 1950's (Penfield, 1958). An external visual stimulus, usually a line drawing, remains the current “gold standard” in many epilepsy surgery centers for creating language maps that influence resection margin decisions.2, 4, 5 Recently, however, this gold standard has been called into question. While several studies report that visual confrontation naming is successful insofar as patients appear to perform well on post-operative visual naming tests or present with deficits that resolve within 6 months,6-8 it is recognized that declines on visual confrontation naming develop or are made more severe in individuals following anterior temporal lobectomies under various circumstances, e.g. in the presence of hippocampal pathology, late seizure onset, or later age of acquisition of object names, possibly regardless of surgical approach. 9-14 Using broader outcome measures, other studies show that post-operative word-finding deficits are more generalized than visual naming alone following temporal resections. 15, 16 It is reported that many left temporal lobe epilepsy (LTE) patients who experience daily speech-based word finding difficulties show significant post-operative deficits in auditory relative to visual naming when auditory naming is not specifically mapped or preserved.15-19 Although reading sites have been found in temporal zones anteriorly to visual naming sites,20 and sentence completion tasks have been found in middle temporal gyrus or inferior parietal areas,21, 22 this function is rarely tested in intraoperative settings. When comparisons between preoperative and postoperative testing in reading are reported following resection of discrete areas within the left inferotemporal cortex, deficits emerge that appear to be related to the processing of visual word forms.23 In short, deficits that are not specific to visual confrontation naming may remain under-documented since they are rarely tested intraoperatively or compared pre- and post-operatively.24 Before exploring pre- and post-operative functional abilities related to the sparing or resection of additional language sites, however, it should be determined how often a distinct language site is found within various subregions of the dominant hemisphere based on multiple word finding input modalities such as visual objects, auditory definitions, and sentence-stem reading.
In previous studies, the distribution of visual and auditory naming sites has typically relied on qualitative descriptions showing a general localization of auditory naming sites in anterior temporal regions,15-18 visual naming sites in posterior temporal or temporoparietal regions,17, 25 and reading sites throughout temporal and parietal areas.21, 22 Schwartz et al., provide function-specific areas in posterior areas of the temporal lobe; however, resections for standard temporal lobectomies to treat intractable epilepsy routinely involve anterior portions of the superior and middle temporal gyri, while lesional resections may involve language areas in the parietal lobe. Given the potential for post-operative deficits and distinct functional areas in visual, auditory, and reading modalities, the objective of the current study was to determine what percentage of sites could be classified as a language site in dominant temporal and parietal subregions based on these three word-finding tasks, with the first hypothesis that the percentage of language sites within specific temporal and parietal subregions would significantly vary between modalities. Additionally, our second hypothesis was that the word-finding paradigms in each of these input modalities would involve distinct cortical sites. This study is a preliminary step towards answering a larger question regarding sparing versus resection of distinct naming sites, whether sparing of such sites leads to a difference in seizure control, and how postoperative function correlates with sparing or resection of such sites.
Methods
Subjects
A consecutive series of 28 epilepsy patients [thirteen females, fifteen males; mean age (SD) 37.0 (12.7) years, range 16.3-58.7 years] who underwent CSM for language (27 intraoperative, 1 extraoperative) were included in this study. Written informed consent was obtained in accordance with the guidelines of the Internal Review Board of Duke University Medical Center (DUMC). Eligibility criteria were: 1) a clinically-mandated exposure area of frontal, temporal, and parietal lobes in the left (dominant) hemisphere, 2) a diagnosis of epilepsy via prolonged video EEG monitoring, 3) English as a native language, and 4) left hemisphere language dominance determined by intracarotid amobarbital (Wada) testing, or left hemisphere intraoperative identification of language sites. Exclusionary criteria were patients less than 16 years of age. Word-finding tasks used three input modalities to locate a language site, specifically: 1) visual object naming (V), 2) auditory naming (A), and 3) sentence completion (S). Institutional standard of care procedures dictated the visual modality be presented first. Visual naming only was done in one patient, visual + auditory naming was done in an additional 12 patients, and visual + auditory + sentence-completion was done in an additional 15 patients. The sentence-completion paradigm was developed after the first 8 patients were completed, and was not administered to an additional 4 subjects due to fatigue (3) or lack of cooperation (1). One patient had sentence completion omitted from the statistical analysis due to a paucity of non-stimulated control trials. Line drawings are as described previously,1 auditory definitions and sentence-completion items were designed by the authors (see Figures and Text, Supplemental Content 1 for lists of visual, auditory, and sentence-completion stimuli). Sentence completion is used to expressly include a word-finding component, as previous evidence indicates that resections near word-finding sites best predict postoperative generalized deficits.26 Errors for reading, word finding, or both were documented specifically in the current analysis. Presence of hippocampal sclerosis (HS) was diagnosed by MRI (HS=17, no HS=11) and included as a regressor in the model as a possible confounder. It was not found to influence the percentage of language sites in any of the subregions, so was omitted from further analyses, though it is possible that a higher number of subjects would show an effect.
Demographic and sample characteristics are presented in Table 1. Using a prospective significance level of P < .05 to indicate significance and independent sample t-tests (SPSS, version 19), no significant differences were present with respect to gender in age, education, age of seizure onset, seizure duration before surgery, or Weschler's Abbreviated Intelligence Scale (WASI) Verbal (VIQ), Performance (PIQ), or Full IQ (FIQ) scores (all p > .2). The difference in the number of subjects who participated in the sentence-completion modality versus those who did not raised the possibility of group differences in demographic and/or neuropsychological profiles that could potentially influence the percentage of language sites across subregions. Prior to proceeding with logistic regression analysis of the complete cohort, independent sample t-tests showed no significant differences between sentence and no-sentence groups in demographic variables (all p > .1), although seizure duration trended towards the sentence group having a longer seizure duration than the no-sentence group (p = .06). Neuropsychological data for sentence-completion participants (7/15 to 11/15, varies by test) and no sentence-completion participants (3/13 to 6/13, varies by test) showed no significant differences in VIQ, PIQ, FIQ, or Reading Recognition (independent sample t–tests, all p > .1) or in Lexical Fluency (California Oral Word Association Test (COWAT)), Semantic Fluency (COWAT), or Visual Naming (Multilingual Aphasia Examination (MAE)) (independent samples Mann-Whitney U test, all p > .2). In light of these non-significant differences between the sentence-completion and non-sentence-completion participant groups, we proceeded to include the complete cohort in the hierarchical logistic regression model.
Table 1.
Patient demographics.
| Subject | Gender | Age | Yrs Ed | Hnd | Seizure Type | Age Seizure Onset | Seizure Duration Yrs | Hippocampal Sclerosis | Febrile Seizures | Engel Classification |
|---|---|---|---|---|---|---|---|---|---|---|
| 0404 | M | 26.2 | 18 | L | CP, TC | 19.0 | 7.2 | N | N | I |
| 0714 | F | 36.0 | 12 | R | CP | 1.0 | 35.0 | Y | Y | I |
| 0808 | F | 51.3 | 16 | R | CP | 5.4 | 45.8 | Y | N | I |
| 0822 | F | 23.9 | 16 | L | CP | 9.0 | 8.9 | N | N | I |
| 0908 | M | 58.7 | 20 | R | CP | 18.0 | 40.7 | Y | N | I |
| 0914 | M | 42.0 | 12 | R | SP | 6.4 | 35.5 | Y | Y | I* |
| 1116 | F | 50.9 | 12 | R | CP | 3.1 | 4.8 | Y | N | I |
| 1305 | F | 45.1 | 12 | R | CP | 40.8 | 4.4 | N | N | I |
| 1504 | F | 47.7 | 10 | R | CP | 10.1 | 37.6 | Y | N | I |
| 1515 | F | 52.1 | 16 | R | CP | 1.5 | 50.7 | Y | Y | I |
| 1519 | F | 22.2 | 16 | R | CP | 12.1 | 10.1 | N | N | I |
| 1622 | M | 30.5 | 12 | R | CP | 0.7 | 25.8 | Y | Y | I |
| 1711 | M | 18.9 | 13 | R | CP | 17.4 | 1.5 | Y | Y | I |
| 1717 | F | 32.0 | 18 | R | CP | 17.0 | 3.0 | N | N | II |
| 1723 | F | 43.1 | 17 | R | SP | 2.1 | 41.0 | N | Y | I |
| 1725 | M | 16.3 | 10 | R | CP | 2.1 | 14.2 | Y | N | I |
| 2011 | M | 31.9 | 12 | R | TC | 2.0 | 29.8 | Y | Y | I |
| 2108 | M | 37.8 | 18 | R | CP | 30.1 | 7.7 | N | N | I |
| 2211 | M | 55.9 | 22 | L | CP | 51.0 | 5.0 | Y | N | I |
| 2216 | M | 34.3 | 15 | R | CP | 10.0 | 24.3 | N | N | II |
| 2308 | M | 36.1 | 12 | R | CP, G | 26.1 | 10.0 | N | N | I |
| 2319 | F | 55.6 | 12 | R | TC, G | 6.0 | 49.6 | Y | N | I |
| 2323 | M | 18.4 | 12 | R | CP | 1.5 | 16.9 | Y | Y | IV |
| 2324 | F | 25.7 | 12 | R | CP, TC | 1.1 | 2.7 | N | Y | I |
| 2508 | M | 27.8 | 14 | R | CP | 6.4 | 11.3 | Y | N | I |
| 2521 | M | 22.6 | 16 | L | CP | 20.0 | 2.6 | N | N | I |
| 2622 | M | 49.5 | 14 | R | CP | 0.5 | 49.0 | Y | N | I |
| 2604 | F | 43.6 | 12 | R | CP | 14.1 | 29.5 | Y | N | I |
Abbreviations: Yrs Ed = Years of Education, Hnd = Handedness, Seizure Type (CP = Complex Partial, SP = Simple Partial, TC = Tonic-Clonic, G = Generalized), presence (Y) or absence (N) of hippocampal sclerosis and febrile seizures
Engel classification at 3 months post-surgery.
Intraoperative mapping methods
Intraoperative mapping used a bipolar stimulator with interelectrode distance of 5mm (Ojemann Cortical Stimulator; Radionics, Burlington, MA, USA). Digital photography and schematic diagrams documented functional sites on the cortical surface with a sterile 5-mm2 tag. Current amplitude began at 4mA and progressively increased by 1mA to a maximum of 14mA to determine after-discharge threshold. We used a standard stimulation procedure7 with biphasic square-wave pulses of 1msec at 60Hz and a maximum train duration of four seconds until after-discharge activity was evoked or current amplitude reached 14mA. Extraoperative mapping was performed on one patient (1717) using a 6x8cm (48 contact) grid array over the frontoparietotemporal region, with 5mm-diameter electrodes embedded in Silastic with center-to-center interelectrode distances of 1cm (Ad-Tech, Racine, WI, USA).
Language paradigm administration
All CSM stimuli used for language mapping with each patient were correctly named and/or read at a minimum of two separate pre-operative occasions, with removal of items not named correctly. Items were chosen pseudo-randomly from the lists shown in Supplemental Content 1 to allow a balance of items from “natural” and “constructed” semantic categories. Stimulation was administered during two of every three trials on average, immediately before the appearance of the stimulus. Before each overt response, the patient spoke the carrier phrase “This is a ...” (visual/auditory naming) or “This says ...” (sentence completion) to ensure attentiveness to the task and a non-general speech arrest. Stimulation was initiated after the aural cue with a minimal delay (< 500ms), allowing stimulation to occur immediately after the item was presented but before an oral response of the carrier phrase began.
Error analysis and Trial Coding
Standard examples27 of error types in word-finding were noted: 1) semantic paraphasias, 2) phonological paraphasias, 3) semantic/phonological blends, 4) off-target responses, i.e., unrelated to the correct response, 5) no-target responses (carrier phrase given but object not named), 6) perseverations within 5 trials, 7) apraxic errors, 8) phonological reductions, 9) neologisms, and 10) temporal delays. Additional error types in reading are as follows: 1) slow/effortful reading (apraxic), 2) syntactic errors, 3) sentence stem additions, 4) sentence stem omissions, and 5) mixed sentence stem errors (additions + omissions). See text in Supplemental Content 2 for examples of each error type.
Each trial was coded as follows: 1) stimulation (st) versus no stimulation (nst), 2) patient response – error versus no error, 3) input modality (visual, auditory, or sentence completion), 4) subregion localization (described below), 5) an indicator where a stimulation error carried over to a subsequent non-stimulated trial, and 6) presence versus absence of afterdischarges, where neurons continued to fire intermittently following the cessation of stimulation. The total number of trials coded was 3402 for visual naming (1625nst, 1777st), 1711 for auditory naming (455nst, 1256st), and 756 for sentence completion (257nst, 499st). Per patient, mean (SD) stimulation trials numbered 63.5 (16.2) for visual, 46.5 (11.8) for auditory, and 29.4 (10.9) for sentence-completion modalities, reflecting relative time requirements to present each trial within each modality.
Brain regions and localization
A cortical parcellation system (CPS) using the terminology of the NeuroNames hierarchy28 in the Foundational Model of Neuroanatomy29 was used as the basis for stimulation site localization. Further described in Corina et al. (2005),30 this system divides the surface of the cortex (by using landmarks and projections) into 37 distinct regions. For the current study, a schematic representation of the lateral left hemisphere used 11 of these distinct subregions within temporal and parietal lobes, with an extension of the central sulcus dividing the temporal lobe into four anterior temporal (PolSTG, ASTG, PolMTG, AMTG) and four posterior temporal subregions (MSTG, PSTG, MMTG, PMTG). Three parietal subregions (ASMG, PSMG, AnG) are defined, with inferior aspects of the SMG defined relative to a posterior extension of the Sylvian fissure (Figure 1).
Figure 1.

Shaded grey areas show subregions of temporal and parietal lobes for language site analyses. Temporal lobe: Superior Temporal Gyrus (STG), Middle Temporal Gyrus (MTG); Parietal lobe: Angular Gyrus (AnG), Supramarginal Gyrus (SMG). Prefixes used are Pol, Anterior (A), Medial (M), and Posterior (P). An extension of the Central Sulcus divides anterior from posterior areas of the temporal lobe (red line); parietal areas are bordered by the Sylvian fissure and inferior side of the SMG/AnG structures (yellow line).
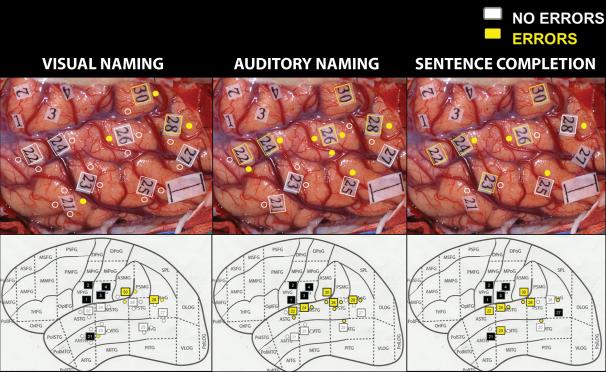
For each patient, digital photographs with sterile tags provided landmarks to stimulated cortical areas for generalized speech arrest (Broca's area, tag 10), pre- and post-central gyri to show face/hand motor and sensory areas respectively (tags 1-5), and language mapping (tags 20-31). Tag locations were transposed to the schematic brain drawing (SS). Discrete sites producing an error on a stimulation trial were noted for subregion localization. Photos were de-identified and tagged landmarks/stimulation sites were independently confirmed by a separate observer for subregion localization (see example in Figure 2). Concordance between observers exceeded 95%, with the second observer resolving any differences (MMH).
Figure 2.
Multimodality intraoperative stimulation sites transferred from digital photo to schematic subregions for visual naming (column 1), auditory naming (column 2), and sentence completion (column 3) (patient 1504). Error-free stimulated sites (white), stimulated sites with error trials (yellow), sites not stimulated for language (black). VPrG=Ventral Precentral Gyrus (motor strip), VPoG=Ventral Postcentral Gyrus (sensory strip), 1cm indicator shown in posterior/inferior corner of digital photos.
Statistical modeling
Fisher's exact test was used intraoperatively to compare the trial error rates for the non-stimulated trials and the sequence of stimulated trials at a given site for each patient; rejection of the null hypothesis at a 60% error rate suggested that the location was a language site. Fisher's test has limitations, however, for comparing modalities across all patients, as tests are not independent across subjects, as each site utilizes the same non-stimulated set of trials to provide a baseline, and the test does not allow one to incorporate other variables that may affect error rates. To determine whether there were subregional differences in the localization of language sites across modalities, hierarchical logistic regression models were fit to the trial error indicators. The hierarchical logistic model incorporated individual differences in the baseline error rate among subjects through subject-specific random-effects, and allowed the probability of an error on a given trial to depend on the semantic category of the presented item, the presence of an afterdischarge from the previous stimulated trial, and a (latent) indicator that the site was a language site. For each modality, the latent indicator that the site was a language site was modeled using a second stage logistic regression model, where the probability that the site was a language site was allowed to depend on the subregion of the brain and subject-specific characteristics (presence of febrile seizures and hippocampal sclerosis). This model incorporates the varying number of stimulation and non-stimulation trials across modalities and patients, i.e., it is not necessary for stimulation sites to be repeated across modalities within patients nor is it necessary to have an equal number of stimulation and non-stimulation trials across modalities or across patients. Markov chain Monte Carlo (MCMC) was used to provide the posterior distribution that each site was a language site, to construct Bayesian confidence intervals for the probability of a site being a language site in each subregion under the three modalities, and to determine differences among the probabilities under each modality. Each site was classified as a language site if the posterior probability that the site was a language site exceeded 0.60. See Supplemental Content 3, which describes details of the statistical model and model fitting.
Results
As is common across cohorts of ATL patients, a variable number of subjects were stimulated within each subregion for a given modality, and a variable number of stimulations were administered for each modality and within each subregion. The presence of an error at a stimulated site is not sufficient to classify it as a language site. An error rate of approximately 60% (corresponding approximately to p < .05 on the Fisher's exact test) at a given site is needed before classifying it as a language site for clinical purposes. Using this criteria, a combination of distinct cortical sites (i.e., 1 modality only) as well as overlapping cortical sites (i.e., > 1 modality) with various combinations of tasks was found across patients; the number and location of distinct and overlapping language sites was also variable across patients. Table 2 shows the ratio of subjects stimulated as well as the error rate for each subregion within each modality across all stimulated sites. Subject ratios and errors rates that are limited to only those sites with trials across modalities (V+A, V+S, A+S, or V+A+S) are shown in Table 3. With these raw error rates, auditory naming shows the highest rates in anterior and posterior temporal areas compared to visual and sentence-completion, while sentence-completion shows the highest rates in parietal areas. See Figures A-C, Supplemental Content 4 for the number (as percentage of the total) of distinct sites (A), the average number of sites per patient (B), and the number of overlapping sites for each modality in each subregion (C) across all stimulated sites. See Figures A-C, Supplemental Content 5 for those sites limited to stimulations across modalities.
Table 2.
Number and % of subjects stimulated and error rate (ratio of errors to stimulation trials) by modality and subregion for all trials.
| Subregion | # Subjects stimulated | # Errors/Stimulations | ||||
|---|---|---|---|---|---|---|
| Modality | V (%) | A (%) | S (%) | V (%) | A (%) | S (%) |
| PolSTG | 23/28 (82) | 22/27 (81) | 10/15 (67) | 21/141 (15) | 28/83 (34) | 12/35 (34) |
| ASTG | 28/28 (100) | 27/27 (100) | 15/15 (100) | 37/266 (14) | 87/237 (37) | 31/90 (34) |
| PolMTG | 18/28 (64) | 14/27 (52) | 3/15 (20) | 8/87 (9) | 5/27 (19) | 2/11 (18) |
| AMTG | 27/28 (96) | 24/27 (89) | 12/15 (80) | 31/271 (11) | 38/135 (28) | 6/38 (16) |
| Anterior Temporal Region Combined | -- | -- | -- | 97/765 (13) | 158/482 (33) | 51/174 (29) |
| MSTG | 26/28 (93) | 25/27 (93) | 14/15 (93) | 39/248 (16) | 87/214 (41) | 28/78 (36) |
| PSTG | 23/28 (82) | 23/27 (85) | 13/15 (87) | 43/151 (28) | 55/128 (43) | 25/55 (45) |
| MMTG | 25/28 (89) | 23/27 (85) | 11/15 (73) | 33/193 (17) | 39/145 (27) | 6/36 (17) |
| PMTG | 24/28 (86) | 23/27 (85) | 12/15 (80) | 32/127 (23) | 44/106 (42) | 16/47 (34) |
| Posterior Temporal Region Combined | -- | -- | -- | 147/729 (20) | 225/593 (38) | 75/216 (35) |
| AnG | 21/28 (75) | 17/27 (63) | 8/15 (53) | 31/118 (26) | 25/64 (39) | 18/48 (38) |
| ASMG | 17/28 (61) | 16/27 (59) | 7/15 (47) | 30/78 (38) | 25/61 (41) | 15/29 (52) |
| PSMG | 21/28 (75) | 19/27 (70) | 8/15 (53) | 20/86 (23) | 13/56 (23) | 18/32 (56) |
| Parietal Region Combined | -- | -- | -- | 81/282 (29) | 63/181 (35) | 51/109 (47) |
| 28/28 | 27/27 | 15/15 | -- | -- | -- | |
| Baseline | M(SD) | M(SD) | M(SD) | |||
| Error Rate % (no stimulation) | 5.3 (6.8) | 10.2 (10.4) | 2.5 (4.5) | |||
Intraoperative baseline error % rates across the cohort (Mean (Standard Deviation)) are listed for each modality. V=Visual, A=Auditory, S=Sentence Completion.
Table 3.
Number and % of subjects stimulated and error rate (ratio of errors to stimulation trials) by modality and subregion for sites stimulated across modalities.
| Subregion | # Subjects stimulated | # Errors/Stimulations | ||||
|---|---|---|---|---|---|---|
| Modality | VA (%) | VS or AS (%) | VAS (%) | V (%) | A (%) | S (%) |
| PolSTG | 11/12 (92) | 1/15 (7) | 10/15 (67) | 20/119 (17) | 28/81 (35) | 12/35 (34) |
| ASTG | 12/12 (100) | 0/15 (0) | 15/15 (100) | 34/223 (15) | 83/227 (37) | 30/88 (34) |
| PolMTG | 6/12 (50) | 4/15 (27) | 3/15 (20) | 4/46 (9) | 2/21 (10) | 2/11 (18) |
| AMTG | 10/12 (83) | 3/15 (20) | 11/15 (73) | 22/177 (12) | 35/127 (28) | 6/38 (16) |
| Anterior Temporal Region Combined | -- | -- | -- | 80/565 (14) | 148/456 (32) | 50/172 (29) |
| MSTG | 10/12 (83) | 1/15 (7) | 14/15 (93) | 38/216 (18) | 81/194 (42) | 28/76 (37) |
| PSTG | 7/12 (58) | 0/15 (0) | 13/15 (87) | 39/120 (33) | 53/118 (45) | 25/55 (45) |
| MMTG | 11/12 (92) | 1/15 (7) | 10/15 (67) | 24/135 (18) | 35/125 (28) | 6/35 (17) |
| PMTG | 8/12 (67) | 3/15 (20) | 10/15 (67) | 29/102 (28) | 40/91 (44) | 14/42 (33) |
| Posterior Temporal Region Combined | -- | -- | -- | 130/573 (23) | 209/528 (40) | 73/208 (35) |
| AnG | 6/12 (50) | 3/15 (20) | 8/15 (53) | 30/92 (33) | 24/61 (39) | 17/47 (36) |
| ASMG | 9/12 (75) | 0/15 (0) | 7/15 (47) | 25/62 (40) | 25/61 (41) | 15/29 (52) |
| PSMG | 7/12 (58) | 2/15 (13) | 8/15 (53) | 16/72 (22) | 11/53 (21) | 18/31 (58) |
| Parietal Region Combined | -- | -- | -- | 71/226 (31) | 60/175 (34) | 50/107 (47) |
| 28/28 | 27/27 | 15/15 | -- | -- | ||
| Baseline | M(SD) | M(SD) | M(SD) | |||
| Error Rate % (No Stimulation) | 5.3 (6.8) | 10.2 (10.4) | 2.5 (4.5) | |||
Intraoperative baseline error % rates across the cohort (Mean (Standard Deviation)) are listed for each modality. V=Visual, A=Auditory, S=Sentence Completion. One patient who only received visual naming was excluded.
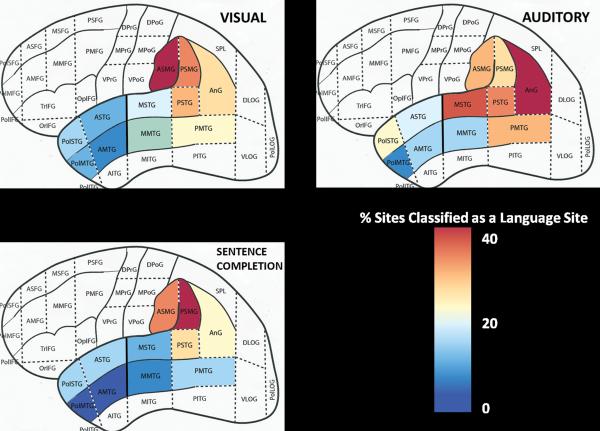
Conventional statistical quantification is problematic with such multi-level clustered data because sites do not necessarily receive the same number of stimulations across modalities. To adjust for this issue, a hierarchical logistic regression model was used instead to show the percentage of sites classified as language sites for each modality. Aggregated over all stimulated sites and patients for each input modality, the percentages of sites classified as language sites within each subregion were calculated (Table 4) and rescaled to an 11-class color gradient map (Figure 3). Figure 3 shows that visual (picture-based) language sites returned relatively low percentages in anterior temporal subregions, progressing higher posteriorly in the temporal lobe and superiorly into the parietal lobe. Combined posterior subregion percentages (middle and posterior STG/MTG) are 2.7 times higher than those in combined anterior subregions (pole and anterior STG/MTG). In the parietal lobe, the anterior SMG shows the highest percentage (39%) of language sites using visual naming, and is consistently higher than values in the middle/posterior areas of superior or middle temporal gyri (Table 4). Statistically, marginal 95% confidence intervals for the aggregate probability show that visual naming had significantly more sites only in the middle MTG and only compared to sentence-completion (Table 4).
Table 4.
Percentage of sites classified as language sites based on task and region using the hierarchical model.
| Subregions | % Visual | % Auditory | % Sentence-Completion | V | CIs A | SC |
|---|---|---|---|---|---|---|
| PolSTG | 11 | 19 | 11 | (0.21, 0.32) | (0.24, 0.4) | (0.22, 0.41) |
| ASTG | 8 | 16 | 14 | (0.2, 0.3) | (0.28, 0.38) | (0.25, 0.39) |
| PolMTG | 8 | 6 | 0 | (0.18, 0.29) | (0.16, 0.34) | (0.09, 0.23) |
| AMTG | 4 | 11 | 0 | (0.2, 0.27) | (0.2, 0.31) | (0.18, 0.27) |
| Anterior Temporal Region Combined | 7 | 14 | 9 | -- | -- | -- |
| MSTG | 18 | 34 | 11 | (0.32, 0.41) | (0.36, 0.49) | (0.27, 0.4) |
| PSTG | 27 | 30 | 25 | (0.38, 0.51) | (0.33, 0.49) | (0.33, 0.5) |
| MMTG | 13 | 12 | 4 | (0.33, 0.41) | (0.28, 0.38) | (0.2, 0.31) |
| PMTG | 19 | 28 | 11 | (0.37, 0.47) | (0.32, 0.47) | (0.28, 0.43) |
| Posterior Temporal Region Combined | 19 | 26 | 13 | -- | -- | -- |
| AnG | 22 | 38 | 22 | (0.35, 0.49) | (0.34, 0.54) | (0.26, 0.48) |
| ASMG | 39 | 29 | 31 | (0.43, 0.6) | (0.29, 0.49) | (0.3, 0.56) |
| PSMG | 30 | 24 | 40 | (0.36, 0.51) | (0.27, 0.43) | (0.29, 0.63) |
| Partietal Region Combined | 29 | 30 | 30 | -- | -- | -- |
Marginal 95% confidence intervals (CI) for the probability of a site being a language site by modality. Intervals in bold indicate regions where that difference in probabilities for the two modalities are significantly different at the 0.05 level (95% confidence interval excludes zero). Modality abbreviations Visual = V (n=28), Auditory = A (n=27), Sentence Completion = SC (n=15). Area abbreviations Pol=Pole, A=Anterior, M=Middle, P=Posterior. Gyri abbreviations STG=superior temporal gyrus, MTG=middle temporal gyrus, AnG=angular gyus, SMG=supramarginal gyrus.
Figure 3.
Heat maps displaying the % of sites classified as a language site by modality using the hierarchical logistic regression model. Legend displays an 11-class color gradient map representing low (blue) to high (red) percentages. See Table 4 for subregion and combined region values and significance testing.
In contrast, the percentage of sites classified as language sites using auditory naming were higher compared to visual naming-based sites within most of the anterior temporal lobe (pole and anterior STG/anterior MTG), most of the posterior temporal lobe (middle and posterior STG, posterior MTG), and the AnG. Auditory-based site percentages were twice as high in the pole, middle, and posterior regions of the STG and twice as high in the compilation of anterior regions compared to visual-based sites (14% versus 7%). Statistically, auditory naming had significantly more sites than visual naming in the anterior STG subregion only. Overall, the percentage of auditory-based language sites are slightly higher in combined posterior areas compared to visual-based percentages (27% versus 22%). Only anterior and posterior SMG and the middle MTG subregions had percentages of visual-based language sites higher than auditory-based ones, although differences were not statistically significant.
The percentage of language sites identified using sentence completion was higher in the anterior STG compared to visual naming (14% vs. 8%). In posterior temporal areas, percentages of reading-based language sites were comparable to visual-based language sites in the middle/posterior portions of the STG and posterior MTG. In the middle MTG, reading-based language sites were much weaker than both visual- and auditory-based language sites (4% vs. 13% and 12%, respectively). Comparisons in the parietal lobe are mixed, with the highest probabilities for reading-based language sites in the posterior SMG (40%), and although it is robust in the anterior SMG and AnG, visual- and auditory-based language sites have higher percentages in these areas respectively. Despite these seemingly large differences, no subregion showed sentence-completion percentages to be statistically higher than visual or naming modalities at the 0.05 level.
To summarize, auditory naming identifies significantly more language sites than visual naming in the anterior STG subregion. Visual naming identifies significantly more language sites than sentence completion in the middle MTG subregion. Within the supramarginal gyrus, although auditory naming is strong, a combination of visual naming (anterior SMG) and sentence completion (posterior SMG) yields the highest numbers of sites, but statistically no modality identifies a significantly higher number of sites than another.
Although the differences in percentage of sites within each modality are significantly higher only in the MMTG for visual naming and the ASTG for auditory naming, it does not imply that language sites overlap across modalities. Using the previously described 60% error rate to identify a language site, Figure 4 shows that out of the 473 sites that were stimulated using both Visual and Auditory naming modalities, 93 sites were identified with auditory naming only and not visual naming (sites in the upper left quadrant). These 93 sites were distributed about evenly in temporal and parietal areas, with 33 in combined anterior temporal areas (PolSTG/MTG and Anterior STG/MTG), 26 in combined posterior temporal areas (M + P STG/MTG), and 34 in combined parietal areas (AnG/A + P SMG). Another 52 sites were identified with visual naming alone and not auditory naming (sites in the lower right quadrant), with 9 in anterior temporal areas, 19 in posterior temporal areas, and 24 in parietal areas. Sixteen sites were identified with both auditory and visual naming (sites in the upper right quadrant), with 6 in anterior temporal areas, 3 in posterior temporal areas, and 7 in parietal areas. The remaining 312 sites stimulated in both visual and auditory modalities were not classified as language sites at the 60% error rate (sites in the lower left quadrant). Of 47 sites tested in both visual naming and sentence completion, 4 sites showed distinct visual naming and 3 sites showed distinct sentence completion sites (over all areas). Of 37 sites tested with both auditory naming and sentence completion, one distinct site was found with sentence completion while auditory naming identified an additional 7 sites over all areas.
Figure 4.
Estimates of the probability that a site for an individual is a language site under each Visual and Auditory modalities, with associated standard deviations of the estimates indicated by the grey error bars. Each point represents a site where both visual and auditory tests were conducted. Black represents anterior temporal sites (Pol + Ant STG/MTG), green represents posterior temporal sites (M + P STG/MTG), and red represents parietal sites (A+P SMG/AnG). The horizontal and vertical lines indicate the threshold of 60% used to classify a site as a language site.
Discussion
Prior work in intraoperative cortical stimulation mapping has demonstrated a variety of topographic regions for multiple language tasks. Each modality was found in areas consistent with previous reports, with visual naming sites being found in middle to posterior areas of the temporal cortex,1, 8, 17, 21 including the superior temporal gyrus 31 as well as the supramarginal gyrus. Although the latter area has been associated with sentence reading errors, 8, 17 anomia from visually presented objects in the supramarginal area has been found in paradigms involving subcortical stimulation of the postero-superior loop of the arcuate fasciculus, where it coincides with the inferior area of the supramarginal gyrus just superior to the Sylvian fissure.32 Auditory naming was found in anterior temporal areas, consistent with previous cortical stimulation reports,15-17, 25 and also in posterior temporal areas which have been shown to be involved in auditory processing, auditory imagery, and word retrieval in the imaging literature.33 Language sites using sentence completion were found in the supramarginal and angular gyri, consistent with previous reports insofar as those reports refer broadly to the inferior parietal lobe as well as the angular gyrus when semantic decisions are required from written words, 21, 22, 34 for reading function using sentence-completion,26 and to a certain extent using single words,8 which together may be interpreted as being part of a neural system that maps orthography to phonology.35
Percentage of sites within subregions and across modalities
In the present study, our first hypothesis was that the percentage of language sites within specific temporal and parietal subregions would vary significantly between modalities.
Consistent with this hypothesis to a limited extent, we found that the percentage of language sites identified using visual naming was significantly higher in the middle portion of the middle temporal gyus compared to sentence completion. The middle temporal gyrus is identified by a variety of imaging studies during word retrieval tasks that require semantics-to-phonology processes (see Indefrey and Levelt, 2004 for a review),40 processing of visual word forms or semantic reading (see Price, 2012 for a review),33 or reading aloud,35 but these appear to be concentrated in the posterior areas of this gyrus, where previous cortical stimulation studies show qualitatively comparable rates of visual naming and reading, 21, 37 and the present study found comparable site percentages between these two modalities. Higher instances of visual naming specifically in middle areas of the middle temporal gyrus compared to reading has been found previously,21 which also corresponds to lesion literature showing acute anomias following insult to this area.41 While it is not clear that this area is modality-specific to visual naming, this area may be modality-primed for visual inputs.
The percentage of language sites identified using auditory naming was significantly higher in the anterior superior temporal gyrus compared to visual naming. Imaging studies consistently identify anterior portions of the superior temporal gyrus in auditory processing of speech sounds and semantic associations during speech comprehension.33 Compared to previous cortical stimulation studies that have demonstrated generalized anterior representations of auditory naming15-17, 42, 43 or greater representation of auditory sites in both anterior and posterior regions of the temporal lobe,25 the current study provides quantitative evidence that auditory naming elicits a larger percentage of sites versus visual naming in this resection-prone area. Such quantitative analysis has important clinical relevance for neurosurgeons and patients, as the area within 2.5 cm of the superior temporal pole is at very high risk for resection in anterior temporal lobectomies, with previous reports indicating that such resections lead to irreversible language deficits when visual word-finding is used exclusively to map language.1, 2, 4, 6, 16, 26, 27 Despite auditory naming having a higher raw percentage of sites identified compared to either visual naming or sentence completion throughout most of the temporal lobe, fewer subregions than anticipated had significantly higher percentages of language sites in one modality over another, particularly in the auditory naming modality. This result raised the question that despite comparable percentages of sites across modalities in temporal and parietal subregions, did cortical stimulation mapping identify language sites that were multi-modality or modality-specific?
Distinct vs Overlapping Sites
Our second hypothesis was that word-finding for visual, auditory, and sentence-completion input modalities elicits distinct cortical sites within patients and across temporoparietal subregions. Confining our analysis to combined anterior temporal, posterior temporal, and parietal subregions for those sites stimulated across modalities, we found distinct sites for both visual and auditory naming with far greater frequency (93 Auditory (A) sites, 52 Visual (V) sites) than overlapping sites (16 V + A sites). Although auditory naming may be considered a more sensitive or statistically equivalent paradigm to identify language sites than visual naming (the current protocol for standard of care) or sentence completion, if auditory naming were used as the sole paradigm to map language it is possible that essential language sites would be overlooked during resection planning since these sites may be distinct from each other. The data shown in Figure 4 for visual versus auditory naming illustrates that the different paradigms used together may be more effective at identifying language sites for a comprehensive map than using any naming paradigm alone. Further studies are needed, however, to determine whether sparing these additional distinct sites will preserve function.
The question of resection extent and neuropsychological outcome is ongoing, with various extents of lateral and mesial resections associated with declines in visual naming.13 Most neuropsychological advantages associated with selective amygdalohippocampectomy over lobectomy concern mainly memory (see review in Schramm 2008),44 so whether sparing additional lateral sites to preserve language function in multiple modalities leads to better naming outcomes or poorer seizure control are potential issues that need further exploration. Furthermore, how significant naming declines are to the patients is unclear – although an average rate of visual naming decline of 34% was found from four studies, few patients themselves note significant declines in language despite objective measurements.45 This discrepancy is also found with memory, where subjective ratings from patients appear to correlate more with seizure outcome (good seizure outcome associated with higher subjective scores), medication side-effects, and mood (fewer side effects and better mood associated with higher subjective scores) than with objective memory ratings that show significant declines.46 Auditory naming has, to date, been assessed infrequently14 so it is currently unclear how resecting these sites will impact post-operative function and whether objective declines will correspond to subjective ratings; however, this question is the topic of ongoing research at our institution. Although the advantages of using visual naming paradigms are efficiency, relative ease of performance, and common usage across many surgical epilepsy centers, standardized auditory naming paradigms are available18, 47 and preoperative patient rehearsal practices can minimize task difficulty and time restrictions to provide a comparable mapping experience for both the patient and the surgical team.
Limitations of the study
The limitations of this study are similar to other studies that employ intraoperative or extraoperative cortical stimulation to map cognitive function. The stimulation is conducted on only one hemisphere, making it unknown whether or how the contralateral hemisphere can contribute or participate in these language tasks. Furthermore, the identification of language sites is limited to the operative field of view, and additional sites in the inferior temporal lobe were not accounted for. Adding proper noun naming to stimuli sets could supplement this paradigm for an ATL population, as proper noun naming has been associated with left temporal pole areas in imaging and lesion-deficit studies,48 and is impaired in left ATL patients with late seizure-onset age.49 Since unique sites have been identified using proper nouns,50 further studies are needed to identify distinct proper noun naming sites and whether resecting any such additional sites lead to subsequent clinically significant post-operative deficits. A relatively high level of patient cooperation during testing is needed to successfully perform the language tasks described above, and stimulation is applied only to the surface of the cortex, ostensibly omitting any stimulation and resulting language sites within sulcal areas. Factors such as a low after-discharge threshold must be taken into account for sites that may not receive an adequate level of stimulation, possibly resulting in false negatives. Since it is a clinical procedure for epilepsy or brain tumors, it is by nature performed on populations with brain pathology, rendering conclusions that may not extend to the general population in terms of normal language processing. Nevertheless, it is a tool that is valuable for epilepsy and brain lesion patient populations, as it has the potential to spare essential language functions and preserve a superior quality of life.
Conclusion
The purpose of this study was to show varying percentages for dominant hemisphere temporo-parietal subregions and to demonstrate word-finding distinctions between visual, auditory, and reading modalities during CSM. While the percentage of auditory naming sites are significantly higher in the anterior portion of the STG compared to visual naming, and are comparable to visual naming in both anterior and posterior portions of the superior/middle temporal and angular gyri, using a combination of word-finding modalities may provide a more comprehensive language map since these language sites are distinct from one another in anterior portions of the temporal lobe that are vulnerable to resection in standard temporal lobectomies.
Supplementary Material
Acknowledgements
We would like to acknowledge Lisa Fornnarino and Lisa Hughes for contributions to editing and recording the auditory and sentence-completion stimuli. We are also grateful to our patients and their families for their support.
Funding:
This work was supported by grants from the National Institute of Neurological Disorders and Stroke [NRSA-5F32NS43031 and R01NS055142] to SS.
Footnotes
Disclosure:
The authors have no personal or institutional financial interest in any materials described in this submission.
References
- 1.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989 Sep;71(3):316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 2.Ojemann GA. Functional mapping of cortical language areas in adults: intraoperative approaches. Advances in Neurology. 1993;63:155–163. [PubMed] [Google Scholar]
- 3.Lesser R, Gordon B, Uematsu S. Electrical stimulation and language. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 1994 Mar;11(2):191–204. doi: 10.1097/00004691-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Ojemann GA. Cortical organization of language. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1991 Aug;11(8):2281–2287. doi: 10.1523/JNEUROSCI.11-08-02281.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald DB, Cosgrove GR, Ronner S, et al. Location of language in the cortex: a comparison between function MR imaging and electrocortical stimulation. American Journal of Neuroradiology. 1997;18(8):1529–1539. [PMC free article] [PubMed] [Google Scholar]
- 6.Hermann B, Wyler AR. Comparative Results of Dominant Temporal Lobectomy under General or Local-Anesthesia - Language Outcome. Journal of Epilepsy. 1988 Sep-Oct;1(3):127–134. [Google Scholar]
- 7.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994 Apr;34(4):567–576. doi: 10.1227/00006123-199404000-00001. discussion 576. [DOI] [PubMed] [Google Scholar]
- 8.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. The New England journal of medicine. 2008 Jan 3;358(1):18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 9.Davies KGBB, Bush AJ, Hermann BP, Dohan FC, Jaap AS. Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocamps. Epilepsia. 1998;39:407–419. doi: 10.1111/j.1528-1157.1998.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 10.Stafiniak PSA, Sperling MR, Kester DB, Robinson LJ, O'Connor MJ, Gur RC. Acute naming deficits following dominant temporal lobectomy: prediction by age and 1st risk for seizure. Neurology. 1990;40:1509–1512. doi: 10.1212/wnl.40.10.1509. [DOI] [PubMed] [Google Scholar]
- 11.Langfitt JTRR. Word-finding deficits persist after left anterotemporal lobectomy. Archives Neurology. 1996;53:72–76. doi: 10.1001/archneur.1996.00550010090021. [DOI] [PubMed] [Google Scholar]
- 12.Bell BDDK, Hermann P, Walters G. Confrontation naming after anterior temporal lobectomy is related to age of acquisition of the object names. Neuropsychologia. 2000;38:83–92. doi: 10.1016/s0028-3932(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 13.Hermann BP, Perrine K, Chelune GJ, et al. Visual confrontation naming following left anterior temporal lobectomy: a comparison of surgical approaches. Neuropsychology. 1999 Jan;13(1):3–9. doi: 10.1037//0894-4105.13.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Ives-Deliperi VL, BJ. Naming outcomes of anterior temporal lobectomy in epilepsy patients: A systematic review of the literature. Epilepsy and Behavior. 2012;24:194–198. doi: 10.1016/j.yebeh.2012.04.115. [DOI] [PubMed] [Google Scholar]
- 15.Hamberger MJ, Tamny TR. Auditory naming and temporal lobe epilepsy. Epilepsy Res. 1999 Jul;35(3):229–243. doi: 10.1016/s0920-1211(99)00023-6. [DOI] [PubMed] [Google Scholar]
- 16.Hamberger MJ, Seidel WT, McKhann GM, 2nd, Perrine K, Goodman RR. Brain stimulation reveals critical auditory naming cortex. Brain. 2005 Nov;128(Pt 11):2742–2749. doi: 10.1093/brain/awh621. [DOI] [PubMed] [Google Scholar]
- 17.Hamberger MJ, Goodman R, Perrine K, Tamny T. Anatomical dissociation of auditory and visual naming in the lateral temporal cortex. Neurology. 2001;56:56–61. doi: 10.1212/wnl.56.1.56. [DOI] [PubMed] [Google Scholar]
- 18.Hamberger MJ, Seidel WT. Auditory and visual naming tests: normative and patient data for accuracy, response time, and tip-of-the-tongue. Journal of the International Neuropsychological Society : JINS. 2003 Mar;9(3):479–489. doi: 10.1017/s135561770393013x. [DOI] [PubMed] [Google Scholar]
- 19.Bell BD, Seidenberg M, Hermann BP, Douville K. Visual and auditory naming in patients with left or bilateral temporal lobe epilepsy. Epilepsy Res. 2003 Jun-Jul;55(1-2):29–37. doi: 10.1016/s0920-1211(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 20.Ojemann G, Cawthon D, Lettich E. Localization and physiological correlates of language and verbal memory in human lateral temporoparietal cortex. Neurobiology of Higher Cognitive Function. 1990:185–202. [Google Scholar]
- 21.Schwartz TH, Devinsky O, Doyle W, Perrine K. Function-specific high-probability “nodes” identified in posterior language cortex. Epilepsia. 1999 May;40(5):575–583. doi: 10.1111/j.1528-1157.1999.tb05559.x. [DOI] [PubMed] [Google Scholar]
- 22.Roux FE, Lubrano V, Lauwers-Cances V, Tremoulet M, Mascott CR, Demonet JF. Intra-operative mapping of cortical areas involved in reading in mono- and bilingual patients. Brain. 2004 Aug;127(Pt 8):1796–1810. doi: 10.1093/brain/awh204. [DOI] [PubMed] [Google Scholar]
- 23.Gaillard R, Naccache L, Pinel P, et al. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006 Apr 20;50(2):191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychology review. 2007 Dec;17(4):477–489. doi: 10.1007/s11065-007-9046-6. [DOI] [PubMed] [Google Scholar]
- 25.Malow BA, Blaxton TA, Susumu S. Cortical stimulation elicits regional distinctions in auditory and visual naming. Epilepsia. 1996;37(3):245–252. doi: 10.1111/j.1528-1157.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 26.Ojemann GA, Dodrill CB. Verbal memory deficits after left temporal lobectomy for epilepsy. Mechanism and intraoperative prediction. J Neurosurg. 1985 Jan;62(1):101–107. doi: 10.3171/jns.1985.62.1.0101. [DOI] [PubMed] [Google Scholar]
- 27.Corina D. Coding for Ojemann Speech Errors. In: University of Washington, Seattle, editor. Cognitive Neuropsychology Laboratory Internal Circular. University of Washington; 2001. pp. 1–2. [Google Scholar]
- 28.BowdenDM MR. NeuroNames Brain Hierarchy. Neuroimage. 1995;2:63–83. doi: 10.1006/nimg.1995.1009. [DOI] [PubMed] [Google Scholar]
- 29.Martin RFMJ, Bowden DM, Brinkley J, Rosse C. Foundational Model of Neuroanatomy: Implications for the Human Brain Project. Paper presented at: American Medical Informatics Association. 2001 [PMC free article] [PubMed] [Google Scholar]
- 30.Corina DP, Gibson EK, Martin R, Poliakov A, Brinkley J, Ojemann GA. Dissociation of action and object naming: evidence from cortical stimulation mapping. Hum Brain Mapp. 2005 Jan;24(1):1–10. doi: 10.1002/hbm.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson JM, Gilmore R, Roper S, et al. Conduction aphasia and the arcuate fasciculus: A reexamination of the Wernicke-Geschwind model. Brain Lang. 1999 Oct 15;70(1):1–12. doi: 10.1006/brln.1999.2135. [DOI] [PubMed] [Google Scholar]
- 32.Duffau H, Capelle L, Sichez N, et al. Intraoperative mapping of the subcortical language pathways using direct stimulations - An anatomo-functional study. Brain. 2002 Jan;125:199–214. doi: 10.1093/brain/awf016. [DOI] [PubMed] [Google Scholar]
- 33.CJ P. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62:816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price CJ, Mechelli A. Reading and reading disturbance. Current opinion in neurobiology. 2005 Apr;15(2):231–238. doi: 10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR. Neural systems for reading aloud: a multiparametric approach. Cereb Cortex. 2010 Aug;20(8):1799–1815. doi: 10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devinsky O, Perrine K, Llinas R, Luciano DJ, Dogali M. Anterior temporal language areas in patients with early onset of temporal lobe epilepsy. Ann Neurol. 1993 Nov;34(5):727–732. doi: 10.1002/ana.410340517. [DOI] [PubMed] [Google Scholar]
- 37.Devinsky O, Perrine K, Hirsch J, McMullen W, Pacia S, Doyle W. Relation of cortical language distribution and cognitive function in surgical epilepsy patients. Epilepsia. 2000 Apr;41(4):400–404. doi: 10.1111/j.1528-1157.2000.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz TH, Devinsky O, Doyle W, Perrine K. Preoperative predictors of anterior temporal language areas. J Neurosurg. 1998 Dec;89(6):962–970. doi: 10.3171/jns.1998.89.6.0962. [DOI] [PubMed] [Google Scholar]
- 39.Fuerst D, Shah J, Kupsky WJ, et al. Volumetric MRI, pathological, and neuropsychological progression in hippocampal sclerosis. Neurology. 2001 Jul 24;57(2):184–188. doi: 10.1212/wnl.57.2.184. [DOI] [PubMed] [Google Scholar]
- 40.Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004 May-Jun;92(1-2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Raymer AM, Foundas AL, Maher LM, et al. Cognitive neuropsychological analysis and neuroanatomic correlates in a case of acute anomia. Brain Lang. 1997 Jun 1;58(1):137–156. doi: 10.1006/brln.1997.1786. [DOI] [PubMed] [Google Scholar]
- 42.Hamberger MJ, McClelland S, McKhann GM, Williams AC, Goodman RR. Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying tmeporal lobe lesions. Epilepsia. 2007;48(3):531–558. doi: 10.1111/j.1528-1167.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- 43.Hamberger MJ, Seidel WT, Goodman RR, et al. Evidence for cortical reorganization of language in patients with hippocampal sclerosis. Brain. 2007 Nov;130(Pt 11):2942–2950. doi: 10.1093/brain/awm187. [DOI] [PubMed] [Google Scholar]
- 44.J S. Temporal lobe epilepsy surgery and the quest for optimal extent of resection: A review. Epilepsia. 2008;49(8):1296–1307. doi: 10.1111/j.1528-1167.2008.01604.x. [DOI] [PubMed] [Google Scholar]
- 45.Sherman EM, Wiebe S, Fay-McClymont TB, et al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia. 2011 May;52(5):857–869. doi: 10.1111/j.1528-1167.2011.03022.x. [DOI] [PubMed] [Google Scholar]
- 46.Sawrie SM, Martin RC, Kuzniecky R, et al. Subjective versus objective memory change after temporal lobe epilepsy surgery. Neurology. 1999 Oct 22;53(7):1511–1517. doi: 10.1212/wnl.53.7.1511. [DOI] [PubMed] [Google Scholar]
- 47.Hammeke TA, Kortenkamp SJ, Binder JR. Normative data on 372 stimuli for descriptive naming. Epilepsy Res. 2005 Aug-Sep;66(1-3):45–57. doi: 10.1016/j.eplepsyres.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 48.D T. The left temporal pole is important for retrieving words for unique concrete entities. Aphasiology. 2009;23(7-8):867–884. doi: 10.1080/02687030802586498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yucus CJTD. Preserved proper naming following left anterior temporal lobectomy is associated with early age of seizure onset. Epilepsia. 2007;48(12):2241–2252. doi: 10.1111/j.1528-1167.2007.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winstanley SFSS, Sabsevitz D, Hammeke T, Mueller W, Mushtaq R, Raghavan M. Category specific naming protocol during electrical stimulation mapping identifies wider cortical language representation. American Epilepsy Society; Seattle: p. WA2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.