Abstract
Polymersome vesicles and wormlike filomicelles self-assembled with amphiphilic, degradable block copolymers have recently shown promise in application to cancer therapy. In the case of filomicelles, dense, hydrophilic brushes of poly(ethylene glycol) on these nanoparticles combine with flexibility to nonspecifically delay clearance by phagocytes in vivo, which has motivated the development of “self” peptides that inhibit nanoparticle clearance through specific interactions. Delayed clearance, as well as robustness of polymer assemblies, opens the dosage window for delivery of increased drug loads in the polymer assemblies and increased tumor accumulation of drug(s). Antibody-targeting and combination therapies, such as with radiotherapy, are emerging in preclinical animal models of cancer. Such efforts are expected to combine with further advances in polymer composition, structure, and protein/peptide functionalization to further enhance transport through the circulation and permeation into disease sites.
Keywords: drug delivery, vesicle, wormlike micelle, stealthiness, degradable block copolymer
INTRODUCTION
Cancer is one of the leading causes of death in the world, and effective treatments remain a challenge. Despite the many serious side effects of chemotherapeutic agents, their administration in cancer therapies is almost inevitable. Drug formulations that maximize therapeutic efficacy while minimizing side effects have thus been an important aspect of pharmaceutical research for decades. Shortly after Bangham et al. (1) first described liposomes as bilayered phospholipid vesicles in 1965, Gregoriadis & Ryman (2) proposed the application of such assemblies as drug delivery systems. Subsequent studies of liposome-entrapped drugs demonstrated enhanced bioactivity (3) and altered biodistribution (4). Liposome formulations have been improved over the years, and after extensive development (e.g., scale-up, sterility, stability), some formulations have made their way into wide clinical use today (5, 6). However, despite many advantageous features, some properties of liposomes, such as membrane stability and encapsulant retention, can be limiting. Vesicle-forming, amphiphilic block copolymers have provided a means to overcome such limitations and broadened the possibilities through a wide range of accessible chemistries, molecular weights, and functionalities (7).
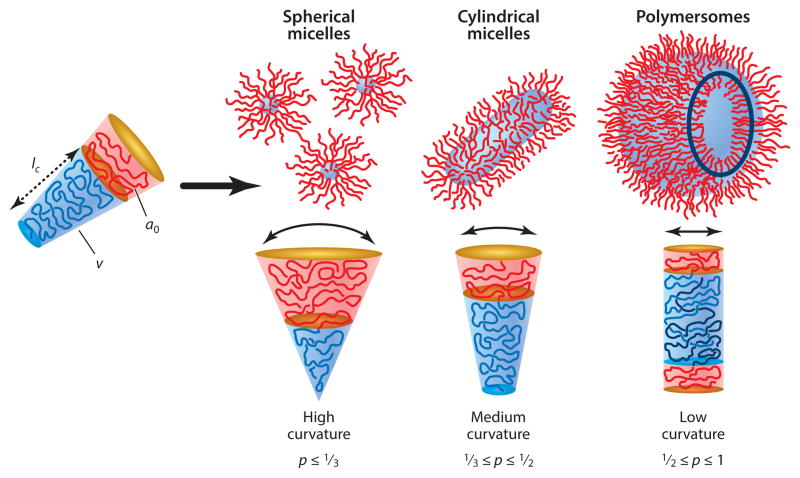
Amphiphilic block copolymers have been explored extensively in drug delivery (8–10). Their self-assembly in water can be tuned by modifying the molecular weight of the hydrophilic and/or hydrophobic blocks to obtain several stable morphologies beyond vesicles (Figure 1). The structure of the assemblies can be predicted to some extent from the packing parameter (p) that relates to the sizes of both blocks (9). Block copolymers with large hydrophilic chains will generally assemble into spherical micelles and successively, by increasing the size of the hydrophobic block, into wormlike micelles (or filomicelles) and vesicles (or polymersomes). In drug delivery, differences in morphology can greatly influence performance (11). Polymersomes possess a thick hydrophobic core capable of accommodating hydrophobic drugs or dyes and also possess an aqueous lumen that can encapsulate hydrophilic drugs or dyes. Spherical and wormlike micelles, however, can integrate hydrophobic molecules only in their cores. The structural diversity has fostered the development of various functional systems with different properties for various applications (8, 12–15).
Figure 1.
Self-assembled structures from amphiphilic block copolymers in a block-selective solvent depending on the size of the hydrophilic and hydrophobic blocks. p is the packing parameter defined as p = v/(a0 • lc), where v is the volume of the hydrophobic chains, a0 is the optimal area of the hydrophilic head group, and lc is the length of the hydrophobic tail. Reprinted with permission from Reference 9 © 2009 John Wiley & Sons.
Polymer composition also plays a very important role in function and application. Biocompatible and biodegradable block copolymers are particularly important in biomedical applications, with the ideal being degradation products that are nontoxic and easily eliminated by the organism. Many drug delivery systems make use of such materials (16–18). This review focuses on some of the latest work in drug delivery using self-assembled nanocarriers (i.e., polymersomes and filomicelles) from degradable block copolymers. The review covers a few key aspects of design, synthesis, and application of such systems, emphasizing applications to cancer therapy. Due to the large number of contributions to this topic, only a selection of the most relevant citations in the field is reviewed here.
SYNTHESIS OF DEGRADABLE BLOCK COPOLYMERS
Aliphatic polyesters are among the most commonly used polymers in drug delivery (19, 20) because their degradation products are nontoxic and can be easily eliminated from the body. They can be synthesized by polycondensation using diols and a diacid (or acid derivative), or from a hydroxy acid (Scheme 1). This method has been used traditionally for polyester synthesis, but it presents significant drawbacks that are difficult to circumvent. These involve the need for high temperatures, long reaction times, and formation of undesired by-products that limit the synthesis of high–molecular weight polymers.
Scheme 1.
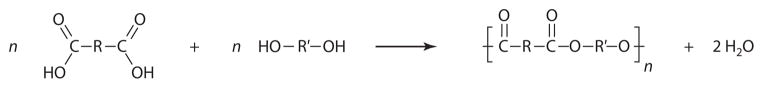
Synthesis of aliphatic polyesters by polycondensation of diols and diacids.
Two polyesters synthesized using this method include poly(tetramethylene adipate) (21) and poly(ethylene glycol) (PEG)/poly(ethylene succinate) (22). In the latter case, high molecular weights of the product were obtained by postpolycondensation chain extension reaction. An alternative and efficient way to obtain high–molecular weight polyesters is the ring opening polymerization of lactones, which involves the use of initiators and the presence of a catalyst. Poly(ε-caprolactone) (PCL), poly(lactic acid) (PLA), and poly(lactic-co-glycolic acid) can be obtained by reacting the initiator (usually an alcohol) with the respective lactones (i.e., caprolactone and lactide or lactide and glycolide) in the presence of stannous octoate (Scheme 2). Amphiphilic block copolymers that are suitable for biomedical application can be readily obtained by using hydrophilic polymers like PEG as macroinitiators. Furthermore, this synthetic strategy allows for the introduction of end-functional groups in the block copolymer that can be used for the incorporation of relevant molecules for application in drug delivery (23, 24). Alternatively, both polymers can be linked after being polymerized independently, provided that both possess functional end groups that can react with each other.
Scheme 2.

Synthesis of poly(ε-caprolactone) by ring-opening polymerization using isopropyl alcohol as the initiator and stannous octoate as the catalyst.
ASSEMBLY OF BLOCK COPOLYMERS INTO POLYMERSOMES AND FILOMICELLES
Even with weakly hydrophobic polymers, the weight fraction of the polymer blocks in a block copolymer as a hydrophilic/hydrophobic ratio generally dictates the preferred morphology of polymer assemblies in water (7, 9, 25, 26). Temperature and preparation procedures are also important factors (25) with, for example, high fluid shear stresses capable of fragmenting long wormlike micelles into kinetically stable spherical micelles (11); this is no doubt why there are many reports of block copolymers that make spherical micelles when one would predict a formation of wormlike micelles or vesicles. Different strategies have been developed for the preparation of assemblies from amphiphilic block copolymers, such as solvent evaporation (26), dialysis, thin-film hydration (27), emulsion-based methods, and solvent injection (27). Numerous variations of these and other techniques to modify the aggregates include sonication, extrusion, and freeze-thawing. The method and modification techniques used will influence the resulting morphology, size, and distribution of the aggregates, but a useful rule of thumb is the higher the input energy (e.g., stirring vigorously), the smaller the aggregate. Therefore, the method of choice will depend on the application of the resulting aggregates.
The most broadly used methods for the preparation of filomicelles and polymersomes are solvent evaporation and thin-film hydration. In the solvent evaporation method, a solution of the block copolymer in an organic solvent is carefully added to water. Slow stirring of the mixture prompts the evaporation of the organic solvent, leading to the aggregation of the block copolymers into the preferred morphology. When the organic solvent in which the block copolymer is dissolved is miscible with water, the dialysis method can be used. The gradual removal of this solvent by dialysis results in the formation of the assemblies. The thin-film hydration method involves the formation of a thin film of the block copolymer on the surface of a vial via slow evaporation of the organic solvent in which the compound is initially dissolved. Addition of water and slow stirring at moderate temperatures lead to aggregate formation. Polymersomes can also be prepared via a solvent-injection method in which a solution of the block copolymer in a miscible organic solvent is slowly added to water while shaking. The formation of aggregates is noticeable through turbidity, with further removal of organic solvent required, such as by dialysis. Aggregates formed by any of these methods can be visualized by fluorescence microscopy, especially when hydrophobic fluorophores, such as PKH26, are incorporated into hydrophobic compartments (Figure 2a) (26). Low doses of dye-functionalized polymers, which integrate into the assembled structures without altering their properties, have also been employed to aid in visualization by fluorescence microscopy (Figure 2b) (28). Other techniques used for visualizing the assemblies include (cryo)-transmission electron microscopy (TEM) (29) and atomic force microscopy (30), but fluorescence microscopy is faster.
Figure 2.
Fluorescence microscopy images of (a) assemblies from poly(ethylene glycol)-block-poly(ε-caprolactone) (PEO-b-PCL, or OCL) containing fluorophore PKH26. (b) OCL filomicelles blended with 5% of rhodamine-PCL. Reprinted with permission from Reference 26 © 2010 American Chemical Society.
NANOCARRIER DESIGN
Nanocarrier design is important to drug delivery, with factors such as morphology affecting circulation time in vivo or cellular uptake, whereas chemistry will affect integration of drugs as well as release of encapsulants in a controlled or uncontrolled manner.
Stealthiness
One of the major challenges when developing a new drug delivery system is avoiding rapid clearance in vivo. The longer the nanocarrier remains in circulation, the greater the chance it exits a blood vessel and permeates a tumor to accumulate. This enhanced permeability and retention (EPR) effect (31) is a key mechanism for the delivery of anticancer therapeutics into tumors using polymeric nanocarriers. Keeping nanocarriers in circulation is generally difficult. They are tagged by physisorption of abundant serum proteins (particularly immunoglobulin and complement) as foreign bodies and then cleared in minutes or hours (up to days) by phagocytic cells lining the vasculatures of the spleen and liver or residing in other tissues, including tumors. Various strategies have been developed to increase the circulation time of nanocarriers in the bloodstream. One widely explored approach for the past two decades involves attachment of hydrophilic polymers such as PEG. PEG has been conjugated to drugs (32), proteins (33), and antibodies (34), in addition to lipids in liposomes (35), providing some degree of stealthiness to all of the systems. PEG is often used as the hydrophilic block in block copolymers because a wide range of molecular weights are readily available and the resulting assemblies in water display PEG chains on the surface of the nanocarrier that delay the physisorption of serum proteins. Despite growing evidence of immunological responses to PEG (36, 37) that can in principle limit therapeutic efficacy, it remains a widely used hydrophilic polymer in drug formulations. Attractive alternatives include zwitterionic polymers (38) that are just emerging in polymersome applications to tumors (39).
Other approaches for achieving long circulation times in vivo are just beginning to emerge based on the use of molecules found on host cells that help passivate or avoid phagocytes. A marker of self protein CD47 on host red blood cells (RBCs) as well as all other cells of the body specifically engages a phagocyte receptor to inhibit uptake by phagocytes. We have recently designed and synthesized “self ” polypeptides from human CD47 and attached them to antibody-targeted polystyrene nanoparticles, and we found that “self ” increased circulation times compared with nanoparticles with either PEG or a scrambled version of the peptide (Figure 3a) (40). Although such commercially available nanoparticles are far from optimal for imaging or delivery, the “self ” peptide has thus far shown significant benefits in tumor imaging and tumor delivery of an anticancer drug, regardless of whether or not the dye/drug-loaded nanoparticles were antibody targeted to the tumor cells.
Figure 3.
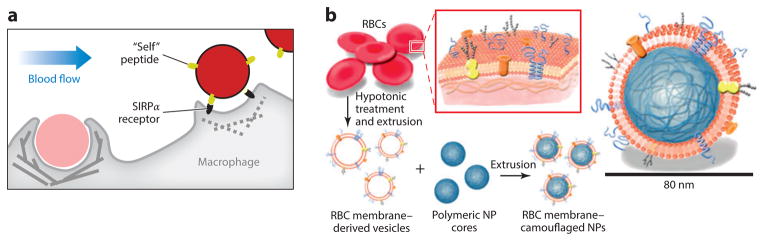
Different strategies to avoid the clearance of nanoparticles from the circulation. (a) “Self ” peptide–functionalized nanobeads are recognized by SIRPα receptor on the macrophage surface to signal “self ” and inhibit phagocytic engulfment. Reprinted with permission from Reference 40 © 2013 Am. Assoc. Adv. Sci. (b) The red blood cell (RBC) membrane is used to wrap polymeric nanoparticles and elude the immune system. Reprinted with permission from Reference 41 © 2011 Natl. Acad. Sci. USA. Abbreviation: NP, nanoparticle.
Perhaps related to the “self ” approach, Zhang and coworkers (41) used the natural RBC membrane to coat synthetic nanoparticles (RBC-NPs) and showed reduced susceptibility to macrophage uptake (Figure 3b). Membrane asymmetry may need more attention and control because lipids such as phosphatidylserine are normally found only on the inner leaflet of cells and flip to the outer layer as a mark for clearance by phagocytosis. A polysialic acid capsule on Escherichia coli K1 helps to evade the host immune system (42), which motivated Deonarain and coworkers (43) to study the influence of polysialylation on antibody pharmacokinetics, concluding that it too increased blood residency and tumor accumulation.
In addition to composition, nanocarrier shape and flexibility can also lead to more persistent circulation (11, 44). In vivo studies comparing flexible filomicelles and PEGylated stealth polymersomes showed that filomicelles were able to circulate for up to a week, which is severalfold longer than polymersomes can. To elucidate the type of interaction of filomicelles with phagocytic cells, fluorescently labeled micelles were incubated in vitro with human-derived macrophages. Microscope images revealed that only filomicelles shorter than 2.5 μm were internalized by cells (Figure 4a), with no fluorescence detected for longer filomicelles. Further experiments in steady flow of spherical micelles and filomicelles past phagocytes showed facile internalization of spherical micelles after contact with phagocytes (Figure 4b). In contrast, filomicelles aligned with the flow so that hydrodynamic shear could pull them off of the phagocyte, thereby escaping internalization.
Figure 4.
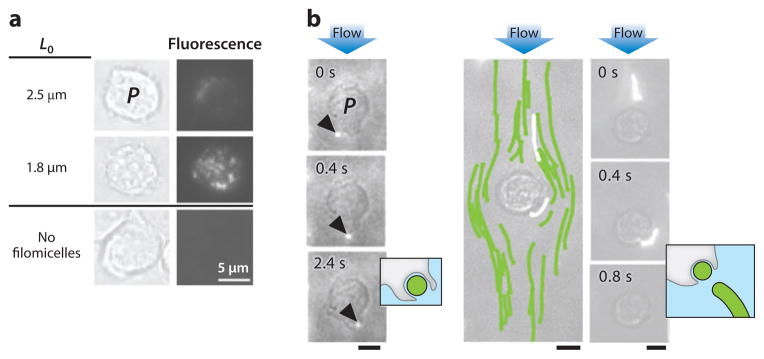
(a) Interaction of filomicelles of different lengths (L0) with phagocytes (P) in vitro. Fluorescence microscopy images show the degree of internalization of filomicelles. (b) An immobilized macrophage in a flow chamber interacts with a spherical micelle and internalizes it (left). Flexible filomicelles align with the flow and pass the macrophage, eventually breaking off a small fragment retained by the macrophage (right). Flow velocity is ~25 μm/s. Reprinted with permission from Reference 11 © 2007 Nat. Publ. Group.
Targeting
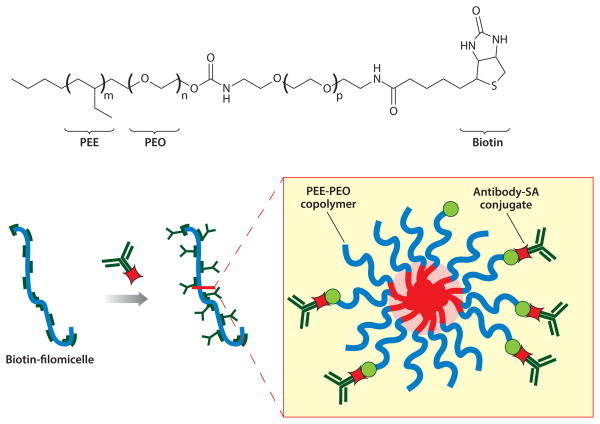
The EPR effect can promote accumulation of a nanocarrier in a leaky tumor site, and retention by tumor cells that such nanocarriers collide with can be enhanced by targeting units on the nanocarrier surface. Examples of targeted polymersomes and/or filomicelles are increasingly broad and now include peptides (45), antibodies (46), carbohydrates (47), small organic molecules (48), and oligonucleotides (49), although few have involved in vivo tests. One strategy for the introduction of targeting molecules onto the surface of preformed polymersomes and filomicelles is the biotin-streptavidin approach (50, 51). This allows for the incorporation of a wide variety of targeting molecules by conjugation to streptavidin and incubation with biotinylated nanoassemblies.
Biotinylated filomicelles have been obtained by self-assembly of biotin-modified PEG copolymers with nonfunctionalized block copolymers (Figure 5) (52, 53). Streptavidin-conjugated monoclonal antibodies against endothelial surface molecules were then successfully incorporated onto the surface. Such functionalized nanocarriers have shown effective targeting to desired molecules on pulmonary endothelium in vivo at different cellular locations, with ligand-anchoring able to overcome the opposing effect of blood flow.
Figure 5.
Antibody functionalization of biotinylated filomicelles by biotin-streptavidin interaction. Abbreviations: PEE, poly(ethyl-ethylene); PEO, poly(ethylene oxide) (which is equivalent to PEG); SA, streptavidin.
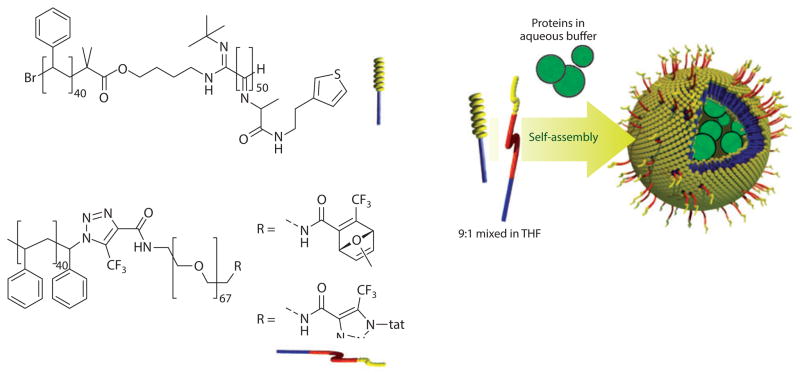
Other approaches for the functionalization of preformed assemblies involve metal coordination (54), adamantane-cyclodextrin interaction (55), and covalent attachment (56, 57). Targeting moieties can also be incorporated by end functionalization of the hydrophilic chain in the block copolymer before aggregates form. This strategy allows for purification and characterization of the conjugate and for control over the surface density in the assembly. In this case, however, the self-assembly can be affected by the nature and size of the targeting units. Following this strategy, van Hest and coworkers (58) synthesized a polystyrene-block-PEG copolymer covalently attached to tat, a cell-penetrating peptide that promotes cellular uptake. The peptide-containing block copolymer was assembled together with polystyrene-block-poly[L-isocyanoalanine(2-thiophen-3-yl-ethyl)amide] (PS-PIAT), resulting in a tat-polymersome (Figure 6). The tat-polymersome was loaded with the enzyme horseradish peroxidase (HRP) and incubated with HeLa cells. Results indicated intracellular activity of HRP as evidence of tat-polymersome internalization, and the system retained its activity as a nanoreactor.
Figure 6.
Tat-functionalized polymersome by coassembly of tat-poly(ethylene glycol)-block-polystyrene (tat-PEG-PS) and polystyrene-block-poly[L-isocyanoalanine(2-thiophen-3-yl-ethyl)amide] (PS-PIAT) containing the enzyme horseradish peroxidase. Reprinted with permission from Reference 58 © 2010 John Wiley & Sons. Abbreviation: THF, tetrahydrofuran.
Drug-Release Mechanism
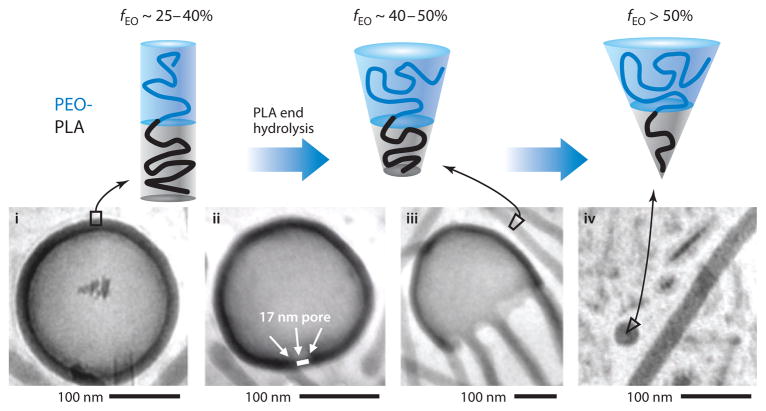
How a nanocarrier releases an encapsulated therapeutic when the target site is reached is an important aspect of the efficacy of a designed treatment. Slow release is possible from nanoparticles containing hydrolytically degradable polyesters like PLA and PCL. With block copolymers of PEG-PLA or PEG-PCL, a chain-end cleavage mechanism results in a systematic reduction of the hydrophobic chain length, altering the ratio between the hydrophobic and hydrophilic blocks in the molecule and inducing an overall morphological transition of the aggregates (Figure 7) (59). A gradual transition from polymersomes to wormlike micelles and finally to spherical micelles can be observed for aggregates of this nature. Importantly, the first pore in a polymersome causes loss of any hydrophilic drugs inside the vesicles (59), and the more highly curved aggregates that are generated through further degradation also have a disproportionately smaller compartment for hydrophobic drugs (60), so that drug release is intimately coupled to microphase transitions.
Figure 7.
Morphological transitions of assemblies from poly(ethylene glycol) (PEG)– poly(lactic acid) (PLA) resulting from PLA progressive degradation. Upon degradation of the hydrophobic chain, polymersomes convert into filomicelles and finally into spherical micelles. fEO denotes fraction of the hydrophilic block. Reprinted with permission from Reference 59 © 2006 Elsevier.
Degradation of polyesters can be affected by external factors like temperature and pH. At acidic pH, degradation is considerably enhanced and favors the morphological transition with the release of an encapsulant. This triggered release is a key factor in the delivery of drugs into tumor cells. The pH in those cells is acidic (~5.5) so that the circulating nanocarrier retains its structural integrity owing to the higher pH, but the acidic environments of tumors or inside cells will accelerate polymer degradation and produce a morphological change that releases the encapsulated therapeutics. The morphological transition from filomicelles to spherical micelles as a result of the polyester degradation has been observed for PEG-PCL assemblies (Figure 8) (60, 61). Fluorescence microscopy was used to track the contour length changes in dye-labeled filomicelles in time. Gradually the micelles shortened to spherical micelles. GPC confirmed generation of monomer product from hydrolysis of PCL (i.e., 6-hydroxycaproic acid), indicating that the degradation took place via chain-end rather than random cleavage (61). Cryo-TEM images confirmed the transitions, and molecular simulations also supported the experiments (62). Fluctuations in the core of the micelle increase along with the mass fraction of the hydrophilic block (fEO), inducing a strong probability of break-up for fEO ≥ 0.72. The instability initiates at the end of the micellar structure, propagating as fluctuations through the core and leading to eventual budding. This correlation between fEO and the molecular weight of the hydrophobic tail with the preferred morphology of the aggregates has been determined for PEG-PCL assemblies (26).
Figure 8.

Fluorescence microscopy images of PKH26-containing filomicelles in water over time. Insets depict cryotransmission electron microscopy images of the same assemblies in time (inset, scale bar is 100 μm). Reprinted with permission from Reference 61 © 2005 Am. Chem. Soc.
The influence of pH on release kinetics has been investigated using paclitaxel-loaded filomicelles (Figure 9), with release appearing to be enhanced at low pH (60). After an initial burst of release, relatively constant release was observed. The OCL designated copolymer [Mw (PEO or PEG) = 5,000, Mw (PCL) = 6,500] showed release of paclitaxel at pH 5 over three days, whereas eight days were necessary at pH 7.
Figure 9.
Paclitaxel release from poly(ethylene glycol)-block-poly(ε-caprolactone) filomicelles at different pH over time. Reprinted with permission from Reference 60 © 2006 Elsevier.
Besides release by hydrolytic degradation of the nanocarrier, other strategies have included stimuli to trigger liberation of encapsulants, taking advantage of specific environments of cancer cells (e.g., pH, redox potential) or of externally applied stimuli (e.g., temperature, ultrasound, light). Incorporation of moieties that respond to the specific stimulus can modify the permeability of the nanocarrier or disrupt it completely. Examples of stimuli-triggered release by modulating the membrane permeability include the use of polymers responsive to pH (63), CO2 (64), or temperature (65). Hubbell and coworkers (66) developed polymersomes from triblock copolymer PEG-poly(propylene sulfide)-PEG (PEG-PPS-PEG) that destabilize under oxidative conditions. The sulfide units on the PPS block are susceptible to oxidation to sulfoxides and ultimately to sulfones, thus increasing the hydrophilicity of the mid-block and leading to the disassembly of vesicles or other assemblies. Other examples include the use of thermoresponsive block copolymers composed of a hydrophilic and a hydrophobic polymer with variable hydrophilicity depending on the temperature (67). Along with redox environment (68) and pH (69), light has induced molecular cleavage with subsequent nanocarrier disruption (70). In these cases, the stimuli-triggered reaction on a specific site of the molecule leads to molecular cleavage that destabilizes the assembled structure. Multiresponsive nanocarriers also have been developed with the idea of being more robust in release (71).
APPLICATIONS
Throughout this review thus far, a few key considerations in the design of a typical drug delivery nanocarrier have been described. Polymers must be tuned according to application; therapeutic agents must be incorporated; and—in an iterative design cycle—the drug delivery system must be tested in a disease model.
Drug Encapsulation
Distinct features of polymersomes and filomicelles allow for the encapsulation or integration of therapeutics of a different nature, e.g., proteins, oligonucleotides, and hydrophobic drugs. The vesicular nature of polymersomes allows for the accommodation of both hydrophilic and hydrophobic molecules in the lumen and membrane, respectively. Filomicelles can only integrate hydrophobic molecules in their core. Depending on the nature and solubility of the therapeutic agents and the block copolymers, different encapsulation strategies are generally followed. Some of the strategies follow the aforementioned procedures for the formation of the aggregates, only in this case, the drug is dissolved in the polymer solution or added after assembly formation.
Direct dissolution is used when the hydrophobicities of the copolymer and the therapeutic agent are moderate, and the method involves the dissolution of the polymer and the drug in an aqueous solvent by heating and/or sonicating. Above the critical micelle concentration, the drug and the block copolymer self-assemble into drug-loaded micelles. When the solubility of the drug and/or the polymer in water is low, other techniques, such as the dialysis method, can be followed. This method involves the dissolution of both drug and block copolymer in a water-miscible solvent. Dialysis of this solution against water leads to the formation of the drug-loaded aggregates. Alternatively, the solvent evaporation or the thin-film hydration methods can be followed by dissolving the hydrophobic drug in the block copolymer solution. Hydrophobic drugs have also been incorporated into the membranes or cores of preformed polymersomes and micelles by diffusion. A solution of the hydrophobic drug in a water-miscible solvent is added to the preformed assemblies in water. Diffusion takes place over time, and the nonencapsulated drug can be removed by centrifugation or dialysis.
Entrapment of hydrophilic therapeutic agents in the aqueous lumen of polymersomes can be accomplished by directly injecting the polymer solution in a water-miscible solvent into an aqueous solution of the agent. An alternative method involves the use of a pH gradient (72). This method, initially developed for liposomes, has been successfully applied for the incorporation of doxorubicin into polymersomes (59). Incorporation of enzymes into polymersomes has also been achieved by lyophilizing samples and injecting them into water. HRP was successfully entrapped in polymersomes via this method with retention of enzymatic activity (68).
Delivery of Anticancer Drugs In Vivo
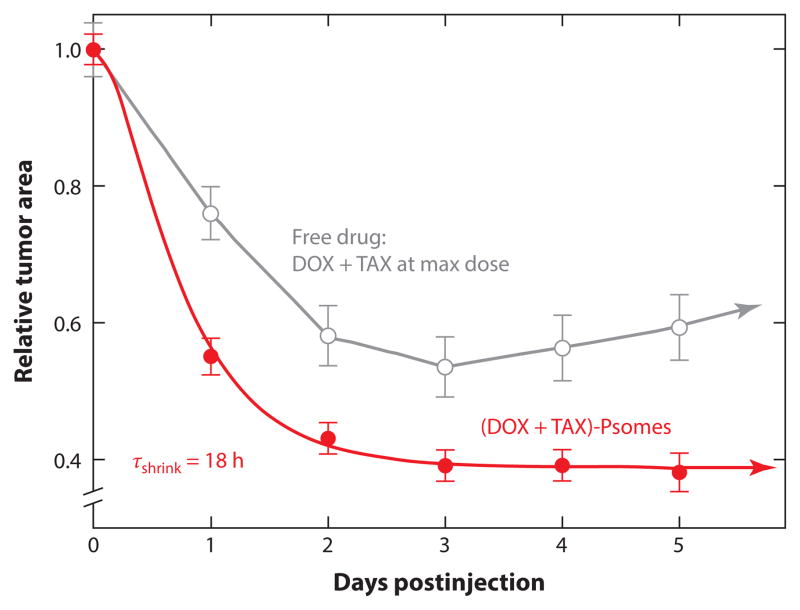
A few examples of in vivo delivery of hydrophilic and/or hydrophobic drugs using polymersomes have been reported (73, 74). Codelivery of hydrophobic paclitaxel and more hydrophilic doxorubicin (59) used polymersomes of PEG-PLA mixed with more-stable PEG-polybutadiene that were injected once into tumor-bearing nude mice. Treated mice showed considerable and sustained tumor shrinkage (below 40% of initial tumor size) (Figure 10). Coadministration of both drugs in free form at the maximum tolerated dose (MTD) did not perform as well as use of the polymersomes.
Figure 10.
Relative tumor area versus time for tumor-bearing mice injected with free doxorubicin (DOX) and paclitaxel (TAX) and with polymersomes (Psomes) loaded with both drugs. Reprinted with permission from Reference 59 © 2006 Elsevier.
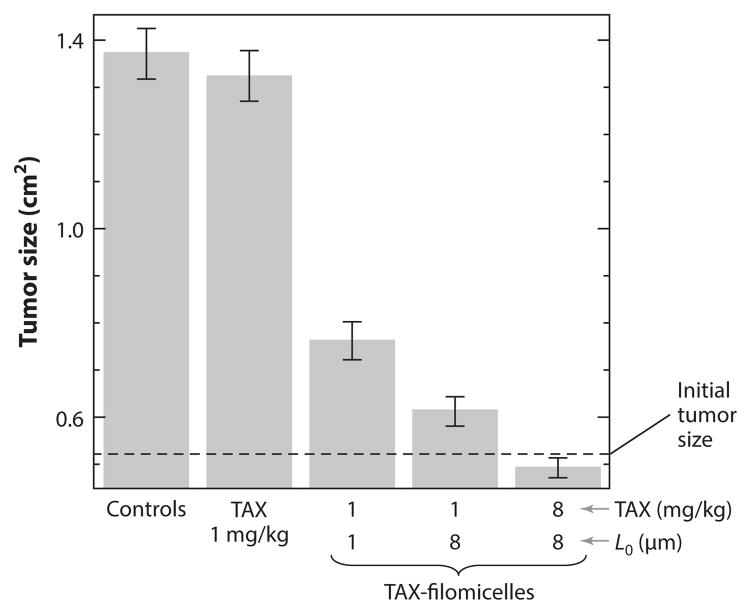
Degradable filomicelles have proven equally effective as drug delivery systems in vivo. Tumor-bearing mice injected with OCL filomicelles of different lengths and loaded with paclitaxel at different doses were compared with those that received single injections of either free drug or empty filomicelles (11). For the same drug dose, an eightfold increase in the length of the filomicelles had a therapeutic effect close to that of an eightfold increase in paclitaxel dosage (Figure 11).
Figure 11.
Measured tumor sizes in tumor-bearing mice injected with free paclitaxel (TAX) and paclitaxel-loaded filomicelles (TAX-filomicelles) of two lengths and at two different doses. Controls include saline injections and poly(ethylene glycol)-block-poly(ε-caprolactone) empty filomicelles. The error bars show the standard deviation. Reprinted with permission from Reference 11 © 2007 Nat. Publ. Group.
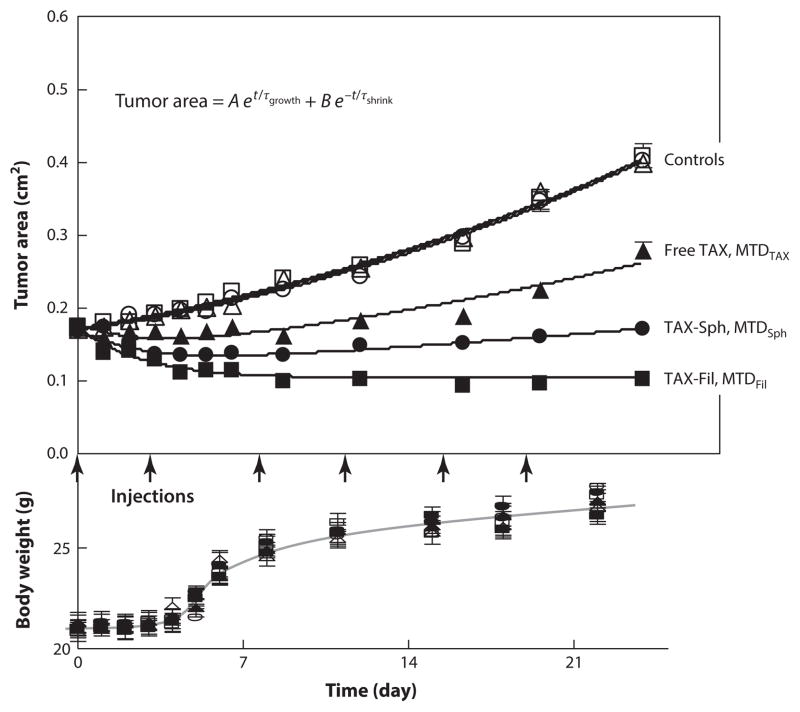
In a comparative study using paclitaxel-loaded filomicelles and spherical micelles from the same OCL, differences in the MTD in mice were observed (14). Whereas the MTD for free paclitaxel is 1 mg/kg, the MTD for filomicelles is ~18 mg/kg, and that for spherical micelles is ~10 mg/kg. The higher value for filomicelles is advantageous in drug delivery because higher dosages can be administered, resulting in an improved therapeutic effect. Studies were done on tumor-bearing mice treated with both loaded nanocarriers at concentrations just below the MTD. Although in all cases that paclitaxel was administered a suppression of tumor growth was observed, inhibition was highest for paclitaxel-loaded filomicelles (Figure 12). Moreover, only in this case did the treated tumors retain their smaller size after treatment.
Figure 12.
Tumor inhibition over a three-week treatment of twice-weekly injections of different formulations: free paclitaxel in phosphate-buffered saline (PBS) (free TAX, MTDTAX), paclitaxel-loaded OCL spherical micelles at MTD (~8 mg/kg) (TAX-Sph, MTDSph), paclitaxel-loaded OCL filomicelles at MTD (~16 mg/kg) (TAX-Fil, MTDFil), and controls. Controls include PBS alone, empty OCL spherical micelles, or filomicelles. t is time (in weeks), A and τgrowth are constants of the tumor growth phase, and B and τshrink are constants of the paclitaxel-inhibition phase. Reprinted with permission from Reference 14 © 2009 Am. Chem. Soc. Abbreviations: MTD, maximum tolerated dose; OCL, poly(ethylene glycol)-block-poly(ε-caprolactone).
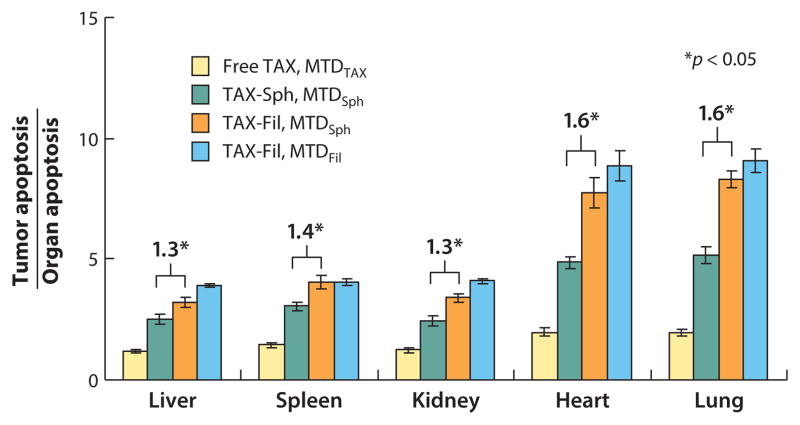
When mice were injected with paclitaxel-loaded filomicelles at MTDSph (maximum tolerated dose for spherical micelles), tumor shrinkage was similar to that observed with spherical micelles at MTDSph. The use of filomicelles, however, showed improved specificity of tumor cytotoxicity even at MTDSph, minimizing apoptosis in nontumor organs (Figure 13). These results illustrate the advantages of using filomicelles over spherical micelles.
Figure 13.
Cell apoptosis index ratio between tumors and nontumor major organs. Data points show (av ± SD) for four mice. Reprinted with permission from Reference 14 © 2009 Am. Chem. Soc.
Drug delivery from polymeric nanocarriers controlled by a specific stimulus has also been investigated. However, although there are numerous examples of stimuli-triggered release of therapeutic agents in vitro (75, 76), in vivo evidence of successful application is thus far lacking.
COMBINED THERAPIES
The treatment of some cancers is particularly challenging owing to the development of resistance to the treatment or to difficulties in accessing the tumor sites. In some of these cases, a combined therapy has proven to be effective (77). Combined therapies involve the use of several therapeutic agents or therapies simultaneously. Multiple anticancer drugs are usually codelivered to suppress drug resistance, which usually develops through selection within multicellular disease sites, such as tumors. As described above, Ahmed et al. (59) introduced codelivery of paclitaxel and doxorubicin against aggressive human breast tumors in nude mice using biodegradable polymersomes. Such codelivery from a single nanocarrier ensures hitting the same cell with both drugs to maximize cytotoxicity and minimize the chance of resistance emerging to individual drugs. Other approaches have been reported involving polymersomes loaded with combinations of chemotherapeutic drugs (doxorubicin) and siRNA (78) or a multidrug resistance inhibitor (tetrandrine) (79).
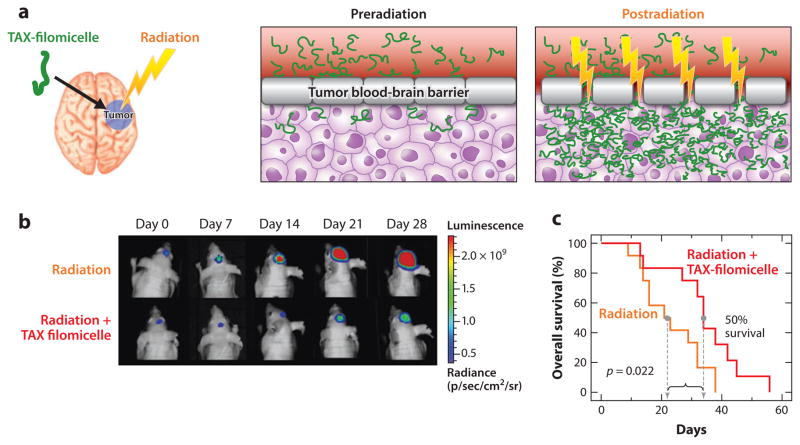
Combinations of distinct therapies for the treatment of difficult-to-access tumors, such as brain tumors, are also beginning to be explored. Brain tumors are difficult to access by conventional polymeric nanocarriers because the blood-brain barrier blocks the passage of small molecules and nanoparticles as it protects the brain from harmful agents. However, the permeability of such barriers can be disrupted by targeted radiotherapy common in the clinic, which enhances the permeation of nanocarriers loaded with anticancer drugs. A combination of radiotherapy with paclitaxel-loaded filomicelles has shown better therapeutic response when treating brain tumors in mice than use of radiotherapy or paclitaxel-loaded filomicelles alone (80) (Figure 14). The median survival period of mice treated with the combined therapy was more than doubled (125 days) compared with treatment with stereotactic radiotherapy alone (57 days).
Figure 14.
(a) Illustration of the combined modality therapy using paclitaxel (TAX)-loaded filomicelles (green) with localized radiotherapy (yellow). Penetration of drug-loaded nanocarriers (DLN) to the brain tumor from the blood circulation is prevented by the tumor blood-brain barrier (T-BBB). Upon radiation, the permeability of the T-BBB is altered and allows extravasation of the DLN into the tumor tissue. (b) Bioluminescence images of intracranial tumors in mice treated with radiation therapy (RT) and with a combination of RT and DLN. (c) Kaplan-Meier survival curves for mice treated with stereotactic RT (sRT) and with a combination of sRT and a high dose of DLN (hiDLN). Adapted from Reference 80.
CONCLUSIONS
The development of new nanocarrier systems to enhance drug delivery is a promising pursuit that requires the combined effort of scientists from many different disciplines. As illustrated here, drug formulations based on polymersomes and filomicelles from degradable block copolymers show some promise as drug delivery systems effective in cancer treatment with either single therapeutic agents or in combined therapies. Owing to the diversity in polymer composition, structure, and functionalization, considerable fine-tuning of the properties of such nanocarriers remains possible and important. The community has certainly learned from decades of work that circulation-enhancing polymers such as PEG have advantages and disadvantages, and this has motivated studies of alternative moieties, such as “self” peptides found on cells that specifically signal to the immune system. Further application, and perhaps discovery, of such molecules will undoubtedly intersect with the block copolymer–based and other nanocarriers reviewed in part here.
Acknowledgments
We greatly appreciate support from the FP7 Marie Curie Actions, the US National Institutes of Health (NIH R01HL062352, P01DK032094, R01EB007049, and NCATS-8UL1TR000003), the US National Science Foundation (the University of Pennsylvania Materials Research Science and Engineering Center and Nano Science and Engineering Center, Nano/Bio Interface Center), and the Stem Cell Xenograft Core (to A. Secreto, J. Glover, and G. Danet-Desnoyers).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Núria Sancho Oltra, Email: nurias@seas.upenn.edu.
Praful Nair, Email: prnair@seas.upenn.edu.
Dennis E. Discher, Email: discher@seas.upenn.edu.
LITERATURE CITED
- 1.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–52. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 2.Gregoriadis G, Ryman BE. Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases. Biochem J. 1971;124:58P. doi: 10.1042/bj1240058p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayhew E, Papahadjopoulos D, Rustum YM, Dave C. Inhibition of tumor cell growth in vitro and in vivo by 1-β-D-arabinofuranosylcytosine entrapped within phospholipid vesicles. Cancer Res. 1976;36:4406–11. [PubMed] [Google Scholar]
- 4.Juliano RL, Stamp D. Pharmacokinetics of liposome-encapsulated anti-tumor drugs. Studies with vinblastine, actinomycin D, cytosine arabinoside, and daunomycin. Biochem Pharmacol. 1978;27:21–27. doi: 10.1016/0006-2952(78)90252-6. [DOI] [PubMed] [Google Scholar]
- 5.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 6.Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4:95–99. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297:967–73. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 8.Brinkhuis RP, Rutjes FPJT, van Hest JCM. Polymeric vesicles in biomedical applications. Polym Chem. 2011;2:1449–62. [Google Scholar]
- 9.Blanazs A, Armes SP, Ryan AJ. Self-assembled block copolymer aggregates: from micelles to vesicles and their biological applications. Macromol Rapid Commun. 2009;30:267–77. doi: 10.1002/marc.200800713. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2012;64:37–48. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 11.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–55. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vriezema DM, Garcia PML, Sancho Oltra N, Hatzakis NS, Kuiper SM, et al. Positional assembly of enzymes in polymersome nanoreactors for cascade reactions. Angew Chem Int Ed. 2007;46:7378–82. doi: 10.1002/anie.200701125. [DOI] [PubMed] [Google Scholar]
- 13.Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee C-C, et al. pH-responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7:3912–25. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christian DA, Cai S, Garbuzenko OB, Harada T, Zajac AL, et al. Flexible filaments for in vivo imaging and delivery: Persistent circulation of filomicelles opens the dosage window for sustained tumor shrinkage. Mol Pharm. 2009;6:1343–52. doi: 10.1021/mp900022m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sancho Oltra N, Swift J, Mahmud A, Rajagopal K, Loverde SM, Discher DE. Filomicelles in nanomedicine—from flexible, fragmentable, and ligand-targetable drug carrier designs to combination therapy for brain tumors. J Mater Chem B. 2013;1:5177–85. doi: 10.1039/c3tb20431f. [DOI] [PubMed] [Google Scholar]
- 16.Nair LS, Laurencin CT. Polymers as biomaterials for tissue engineering and controlled drug delivery. Adv Biochem Eng/Biotechnol. 2006;102:47–90. doi: 10.1007/b137240. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Pan D, Luo K, Li L, Gu Z. Biodegradable and amphiphilic block copolymer-doxorubicin conjugate as polymeric nanoscale drug delivery vehicle for breast cancer therapy. Biomaterials. 2013;34:8430–43. doi: 10.1016/j.biomaterials.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Chan HF, Leong KW. Advanced materials and processing for drug delivery: the past and the future. Adv Drug Deliv Rev. 2013;65:104–20. doi: 10.1016/j.addr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seyednejad H, Ghassemi AH, van Nostrum CF, Vermonden T, Hennik WE. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J Control Release. 2011;152:168–76. doi: 10.1016/j.jconrel.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Dash TK, Konkimalla VB. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: a review. J Control Release. 2012;158:15–33. doi: 10.1016/j.jconrel.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 21.Albertsson A-C, Ljungquist O. Degradable polymers. I Synthesis, characterization, and long-term in vitro degradation of a 14C-labeled aliphatic polyester. J Macromol Sci A Pure Appl Chem. 1986;23:393–409. [Google Scholar]
- 22.Albertsson A-C, Ljungquist O. Degradable polymers. II Synthesis, characterization, and degradation of an aliphatic thermoplastic block copolyester. J Macromol Sci A Pure Appl Chem. 1986;23:411–22. [Google Scholar]
- 23.Nasongkla N, Shuai X, Ai H, Weinberg BD, Pink J, et al. cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angew Chem Int Ed. 2004;43:6323–27. doi: 10.1002/anie.200460800. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Zheng M, Renette T, Kissel T. Modular synthesis of folate conjugated ternary copolymers: polyethylenimine-graft-polycaprolactone-block-poly(ethylene glycol)-folate for targeted gene delivery. Bioconjugate Chem. 2012;23:1211–20. doi: 10.1021/bc300025d. [DOI] [PubMed] [Google Scholar]
- 25.Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm. 2007;65:259–69. doi: 10.1016/j.ejpb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Rajagopal K, Mahmud A, Christian DA, Pajerowski JD, Brown AEX, et al. Curvature-coupled hydration of semicrystalline polymer amphiphiles yields flexible worm micelles but favors rigid vesicles: polycaprolactone-based block copolymers. Macromolecules. 2010;43:9736–46. doi: 10.1021/ma101316w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kita-Tokarczyk K, Grumelard J, Haefele T, Meier W. Block copolymer vesicles—using concepts from polymer chemistry to mimic biomembranes. Polymer. 2005;46:3540–63. [Google Scholar]
- 28.Rajagopal K, Christian DA, Harada T, Tian A, Discher DE. Polymersomes and wormlike micelles made fluorescent by direct modifications of block copolymer amphiphiles. Int J Polym Sci. 2010;2010:379286. [Google Scholar]
- 29.Danino D. Cryo-TEM of soft molecular assemblies. Curr Opin Colloid Interface Sci. 2012;17:316–29. [Google Scholar]
- 30.Liaw J, Aoyagi T, Kataoka K, Sakurai Y, Okano T. Visualization of PEO-PBLA-pyrene polymeric micelles by atomic force microscopy. Pharm Res. 1998;15:1721–26. doi: 10.1023/a:1011908728838. [DOI] [PubMed] [Google Scholar]
- 31.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 32.Greenwald RB, Gilbert CW, Pendri A, Conover CD, Xia J, Martinez A. Drug delivery systems: water soluble taxol 2′-poly(ethylene glycol) ester prodrugs—design and in vivo effectiveness. J Med Chem. 1996;39:424–31. doi: 10.1021/jm950475e. [DOI] [PubMed] [Google Scholar]
- 33.Yang B-B, Lum PK, Hayashi MM, Roskos LK. Polyethylene glycol modification of filgrastim results in decreased renal clearance of the protein in rats. J Pharm Sci. 2004;93:1367–73. doi: 10.1002/jps.20024. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber S. Certolizumab pegol for the treatment of Crohn’s disease. Ther Adv Gastroenterol. 2011;4:375–89. doi: 10.1177/1756283X11413315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–37. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 36.Garay RP, Labaune JP. Immunogenicity of polyethylene glycol (PEG) Open Conf Proc J. 2011;2:104–7. [Google Scholar]
- 37.Ichihara M, Shimizu T, Imoto A, Hashiguchi Y, Uehara Y, et al. Anti-PEG IgM response against PEGylated liposomes in mice and rats. Pharmaceutics. 2011;3:1–11. doi: 10.3390/pharmaceutics3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown L, McArthur SL, Wright PC, Lewis A, Battaglia G. Polymersome production on a microfluidic platform using pH sensitive block copolymers. Lab Chip. 2010;10:1922–28. doi: 10.1039/c004036c. [DOI] [PubMed] [Google Scholar]
- 39.Murdoch C, Reeves KJ, Hearnden V, Colley H, Massignani M, et al. Internalization and biodistribution of polymersomes into oral squamous cell carcinoma cells in vitro and in vivo. Nanomedicine. 2010;5:1025–36. doi: 10.2217/nnm.10.97. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “self ” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–75. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci USA. 2011;108:10980–85. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Severi E, Hood DW, Thomas GH. Sialic acid utilization by bacterial pathogens. Microbiology. 2007;153:2817–22. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 43.Constantinou A, Epenetos AA, Hreczuk-Hirst D, Jain S, Deonarain MP. Modulation of antibody pharmacokinetics by chemical polysialylation. Bioconjugate Chem. 2008;19:643–50. doi: 10.1021/bc700319r. [DOI] [PubMed] [Google Scholar]
- 44.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008;16:1450–58. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pangburn TO, Georgiou K, Bates FS, Kokkoli E. Targeted polymersome delivery of siRNA induces cell death of breast cancer cells dependent upon Orai3 protein expression. Langmuir. 2012;28:12816–30. doi: 10.1021/la300874z. [DOI] [PubMed] [Google Scholar]
- 46.Lin JJ, Ghoroghchian PP, Zhang Y, Hammer DA. Adhesion of antibody-functionalized polymersomes. Langmuir. 2006;22:3975–79. doi: 10.1021/la052445c. [DOI] [PubMed] [Google Scholar]
- 47.Kim B-S, Yang W-Y, Ryu J-H, Yoo Y-S, Lee M. Carbohydrate-coated nanocapsules from amphiphilic rod-coil molecule: binding to bacterial type 1 pili. Chem Commun. 2005;15:2035–37. doi: 10.1039/b419258c. [DOI] [PubMed] [Google Scholar]
- 48.Yang X, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, et al. Multifunctional stable and pH-responsive polymer vesicles formed by heterofunctional triblock copolymer for targeted anticancer drug delivery and ultrasensitive MR imaging. ACS Nano. 2010;4:6805–17. doi: 10.1021/nn101670k. [DOI] [PubMed] [Google Scholar]
- 49.Brož P, Benito SM, Saw CL, Burger P, Heider H, et al. Cell targeting by a generic receptor-targeted polymer nanocontainer platform. J Control Release. 2005;102:475–88. doi: 10.1016/j.jconrel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 50.Diamandis EP, Christopoulos TK. The biotin-(strept)avidin system: principles and applications in biotechnology. Clin Chem. 1991;37:625–36. [PubMed] [Google Scholar]
- 51.Rigler P, Meier W. Encapsulation of fluorescent molecules by functionalized polymeric nanocontainers: investigation by confocal fluorescence imaging and fluorescence correlation spectroscopy. J Am Chem Soc. 2006;128:367–73. doi: 10.1021/ja056719u. [DOI] [PubMed] [Google Scholar]
- 52.Dalhaimer P, Engler AJ, Parthasarathy R, Discher DE. Targeted worm micelles. Biomacromolecules. 2004;5:1714–19. doi: 10.1021/bm049884v. [DOI] [PubMed] [Google Scholar]
- 53.Shuvaev VV, Ilies MA, Simone E, Zaitsev S, Kim Y, et al. Endothelial targeting of antibody-decorated polymeric filomicelles. ACS Nano. 2011;5:6991–99. doi: 10.1021/nn2015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nehring R, Palivan CG, Moreno-Flores S, Mantion A, Tanner P, et al. Protein decorated membranes by specific molecular interactions. Soft Matter. 2010;6:2815–24. [Google Scholar]
- 55.Felici M, Marzá-Pérez M, Hatzakis NS, Nolte RJM, Feiters MC. β-Cyclodextrin-appended giant amphiphile: aggregation to vesicle polymersomes and immobilisation of enzymes. Chem Eur J. 2008;14:9914–20. doi: 10.1002/chem.200801429. [DOI] [PubMed] [Google Scholar]
- 56.Opsteen JA, Brinkhuis RP, Teeuwen RLM, Löwik DWPM, van Hest JCM. “Clickable” polymersomes. Chem Commun. 2007;30:3136–38. doi: 10.1039/b704568a. [DOI] [PubMed] [Google Scholar]
- 57.Pang Z, Lu W, Gao H, Hu K, Chen J, et al. Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J Control Release. 2008;128:120–27. doi: 10.1016/j.jconrel.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 58.van Dongen SFM, Verdurmen WPR, Peters RJRW, Nolte RJM, Brock R, van Hest JCM. Cellular integration of an enzyme-loaded polymersome nanoreactor. Angew Chem Int Ed. 2010;49:7213–16. doi: 10.1002/anie.201002655. [DOI] [PubMed] [Google Scholar]
- 59.Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J Control Release. 2006;116:150–58. doi: 10.1016/j.jconrel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Geng Y, Discher DE. Visualization of degradable worm micelle breakdown in relation to drug release. Polymer. 2006;47:2519–25. [Google Scholar]
- 61.Geng Y, Discher D. Hydrolytic degradation of poly(ethylene oxide)-block-polycaprolactone worm micelles. J Am Chem Soc. 2005;127:12780–81. doi: 10.1021/ja053902e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loverde SM, Ortiz V, Kamien RD, Klein ML, Discher DE. Curvature-driven molecular demixing in the budding and breakup of mixed component worm-like micelles. Soft Matter. 2010;6:1419–25. doi: 10.1039/b919581e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeong IK, Gao GH, Li Y, Kang SW, Lee DS. A biodegradable polymersome with pH-tuning on-off membrane based on poly(β-amino ester) for drug delivery. Macromol Biosci. 2013;13:946–53. doi: 10.1002/mabi.201200468. [DOI] [PubMed] [Google Scholar]
- 64.Yan Q, Wang J, Yin Y, Yuan J. Breathing polymersomes: CO2-tuning membrane permeability for size-selective release, separation, and reaction. Angew Chem Int Ed. 2013;52:5070–73. doi: 10.1002/anie.201300397. [DOI] [PubMed] [Google Scholar]
- 65.Sanson C, Schatz C, Le Meins J-F, Soum A, Thévenot J, et al. A simple method to achieve high doxorubicin loading in biodegradable polymersomes. J Control Release. 2010;147:428–35. doi: 10.1016/j.jconrel.2010.07.123. [DOI] [PubMed] [Google Scholar]
- 66.Napoli A, Valentini M, Tirelli N, Müller M, Hubbell JA. Oxidation-responsive polymeric vesicles. Nat Mater. 2004;3:183–89. doi: 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]
- 67.Xu H, Meng F, Zhong Z. Reversibly crosslinked temperature-responsive nanosized polymersomes: synthesis and triggered drug release. J Mater Chem. 2009;19:4183–90. [Google Scholar]
- 68.Cerritelli S, Velluto D, Hubbell JA. PEG-SS-PPS: reduction-sensitive disulfide block copolymer vesicles for intracellular drug delivery. Biomacromolecules. 2007;8:1966–72. doi: 10.1021/bm070085x. [DOI] [PubMed] [Google Scholar]
- 69.Dan K, Pan R, Ghosh S. Aggregation and pH responsive disassembly of a new acid-labile surfactant synthesized by thiolacrylate Michael addition reaction. Langmuir. 2011;27:612–17. doi: 10.1021/la104445h. [DOI] [PubMed] [Google Scholar]
- 70.Cabane E, Malinova V, Menon S, Palivan CG, Meier W. Photoresponsive polymersomes as smart, triggerable nanocarriers. Soft Matter. 2011;7:9167–76. [Google Scholar]
- 71.Cheng R, Meng F, Deng C, Klok H-A, Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34:3647–57. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 72.Mayer LD, Bally MB, Cullis PR. Uptake of adriamycin into large unilamellar vesicles in response to a pH gradient. Biochim Biophys Acta. 1986;857:123–26. doi: 10.1016/0005-2736(86)90105-7. [DOI] [PubMed] [Google Scholar]
- 73.Upadhyay KK, Bhatt AN, Castro E, Mishra AK, Chuttani K, et al. In vitro and in vivo evaluation of docetaxel loaded biodegradable polymersomes. Macromol Biosci. 2010;10:503–12. doi: 10.1002/mabi.200900415. [DOI] [PubMed] [Google Scholar]
- 74.Ayen WY, Kumar N. In vivo evaluation of doxorubicin-loaded (PEG)3-PLA nanopolymersomes (PolyDoxSome) using DMBA-induced mammary carcinoma rat model and comparison with marketed LipoDox™. Pharm Res. 2012;29:2522–33. doi: 10.1007/s11095-012-0783-8. [DOI] [PubMed] [Google Scholar]
- 75.Song N, Liu W, Tu Q, Liu R, Zhang Y, Wang J. Preparation and in vitro properties of redox-responsive polymeric nanoparticles for paclitaxel delivery. Colloids Surf B. 2011;87:454–63. doi: 10.1016/j.colsurfb.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 76.Meng F, Zhong Z, Feijen J. Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules. 2009;10:197–209. doi: 10.1021/bm801127d. [DOI] [PubMed] [Google Scholar]
- 77.Hu C-MJ, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther Deliv. 2010;1:323–34. doi: 10.4155/tde.10.13. [DOI] [PubMed] [Google Scholar]
- 78.Kim H-O, Kim E, An Y, Choi J, Jang E, et al. A biodegradable polymersome containing Bcl-xL siRNA and doxorubicin as a dual delivery vehicle for a synergistic anticancer effect. Macromol Biosci. 2013;13:745–54. doi: 10.1002/mabi.201200448. [DOI] [PubMed] [Google Scholar]
- 79.Pang Z, Feng L, Hua R, Chen J, Gao H, et al. Lactoferrin-conjugated biodegradable polymersome holding doxorubicin and tetrandrine for chemotherapy of glioma rats. Mol Pharm. 2010;7:1995–2005. doi: 10.1021/mp100277h. [DOI] [PubMed] [Google Scholar]
- 80.Baumann BC, Kao GD, Mahmud A, Harada T, Swift J, et al. Enhancing the efficacy of drug-loaded nanocarriers against brain tumors by targeted radiation therapy. Oncotarget. 2013;4:64–79. doi: 10.18632/oncotarget.777. [DOI] [PMC free article] [PubMed] [Google Scholar]