Abstract
Despite the improved treatment of cardiovascular diseases, the population with end-stage heart failure (HF) is progressively growing. The scarcity of the gold standard therapy, heart transplantation, demands novel therapeutic approaches. For patients awaiting transplantation, ventricular-assist devices have been of great benefit on survival. To allow explantation of the assist device and obviate heart transplantation, sufficient and durable myocardial recovery is necessary. However, explant rates so far are low. Combining mechanical circulatory support with regenerative therapies such as cell (-based) therapy and biomaterials might give rise to improved long-term results. Although synergistic effects are suggested with mechanical support and stem cell therapy, evidence in both preclinical and clinical setting is lacking. This review focuses on advanced and innovative strategies for the treatment of end-stage HF and furthermore appraises clinical experience with combined strategies.
Keywords: heart failure, ventricular-assist device, mechanical circulatory support, regenerative therapies, cell therapy, cardiac recovery
Introduction
Heart failure (HF) is a progressive disease with an important economic burden on today’s healthcare. After initial injury, progressive worsening maladaptive (cellular and structural) changes result in a process called ventricular remodeling, eventually leading to diminished cardiac function (1, 2). According to the Framingham study, the incidence of HF has remained stable since the 1970s (3). Despite this unchanged incidence, the population of HF patients is growing, affecting up to around 23 million patients worldwide, due to various aspects. Improvement in the acute therapy of myocardial infarction (MI) has played a major role in survival rates. Other non-pharmacological treatment options such as ICD therapy have further decreased mortality. In addition, the widespread use of ACE-inhibitors, ATII-blockers, beta-blockers, and aldosterone-antagonists, but also cardiac resynchronization therapy further enhanced survival among HF patients. These developments in combination with an aging population translate into an increase in the prevalence of chronic “end-stage HF” (4, 5). Although not clearly defined, according to the guidelines for heart transplantation, heart transplantation should be considered in patients with severe symptoms of HF, intractable angina, or rhythm disturbances, without any alternative form of treatment available and with a poor prognosis (6). Concerning the guidelines for HF, there are different types of management approaches, which can be broadly subdivided in three groups, (1) general/non-pharmacological measures, (2) pharmacological therapy, and (3) devices and surgery (7, 8). The only current available therapy for end-stage HF is heart transplantation. Opposed to an increasing demand for donor hearts, the number of heart transplantations in Europe has diminished in recent years. In the Netherlands especially, decreasing mortality after traffic accidents, older donors, and shift from heart-beating donation to non-heart-beating procedures gave rise to a further decreasing amount of donors (6). To compensate for the shortcoming of donors, novel therapeutic strategies are inevitable. Experimental regenerative therapies, intended to restore functional cardiac cells and myocardial function are of great interest (9, 10). An overview of heart failure treatment is depicted in Figure 1. For some patients, mechanical circulatory support (MCS) with a ventricular-assist device (VAD) is an option. This review will focus on current and novel, advanced therapeutic strategies for end-stage HF.
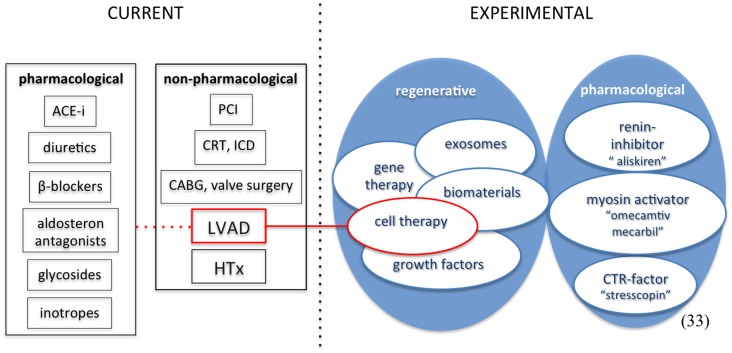
Figure 1.
Current and experimental heart failure therapy. ACE-i, angiotensin-converting-enzyme inhibitor; PCI, percutaneous coronary intervention; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; CABG, coronary artery bypass graft; HTx, heart transplantation; CTR-factor, cortico-trophin-releasing factor.
Current Therapies for End-Stage Heart Failure
Heart transplantation
In European countries that are represented by the European Society of Cardiology (ESC), there are estimated to be over 10 million patients with HF (7). For the Netherlands, this number is believed to be between 100.000 and 150.000 patients, and is expected to rise to approximately 195.000 in 2025 (11). These numbers are probably underestimated and lack accuracy due to the absence of a uniform definition for HF. Easier to determine is the number of patients waiting for a donor heart. Eurotransplant is the international collaborative framework responsible for allocation of donor organs in the Netherlands, Austria, Belgium, Croatia, Germany, Hungary, Luxembourg, and Slovenia. Annual statistics show a rising number of patients on the waiting list, with an actual number of 1250 patients at the end of December 2013, a 2.5-fold increase compared to 2000 (12). With a total of 563 heart transplantations in 2013, the scarcity of donor hearts is evident. In the Netherlands, the same trend is seen. Added up with the progressive decline in the amount of donors, heart transplantation will not relieve the burden of HF on healthcare.
Mechanical support
As briefly stated in the introduction, MCS with a VAD is a possibility for some patients. VADs can be used as a bridge to transplantation, recovery or decision, and as destination therapy. These mechanical pumps partially or completely take over ventricular function to support circulation. Either the left ventricle (LV), right ventricle (RV), or both ventricles can be unloaded. Predominantly, left ventricular-assist devices (LVADs) are implanted because of disappointing results of biventricular-assist device (BIVAD) support (13). Since the first successful implantation of a VAD in 1966 by DeBakey (14), mechanical support has shown to be of great value in survival of patients with advanced HF. The landmark REMATCH trial (15) compared the long-term use of the first generation, pulsatile LVADs with optimal medical therapy in end-stage HF and showed significantly improved survival with an absolute reduction in mortality rate of 27% at 1 year (16). Two major factors causing a low 2-year survival rate of 23% in the LVAD group were infection and mechanical device-failure (16). Since 2006, continuous-flow assist devices are implanted, with much better results (17). Lahpor et al. (18) explored the outcomes of more than 400 patients with this second generation, continuous-flow device and found no mechanical failure, a low incidence of neurological complications but still major infectious and bleeding complications. Although the mean duration of support was significantly higher due to the shortage of donor hearts, overall survival is comparable to other studies (17, 18). Permanent mechanical support, or LVAD as destination therapy, is an option for patients with contraindications for heart transplantation, but reimbursement differs per country (19). In addition to the financial aspects, durable LVAD support as a therapy for end-stage HF is still hampered by substantial bleeding (2.69 events/pt-year) and thromboembolic events (0.31 events/pt-year), as well as inflammatory complications (2.34 events/pt-year) (16, 18, 20–22). The fifth Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis demonstrated a sixfold increase of pump exchanges for pump thrombosis between 2011 and 2012, clinically concerning due to the associated higher mortality rates (23, 24). These findings emphasize the importance of restricting long-term support only for those who really need that and stimulating myocardial recovery and device explantation in as many patients as possible. While initially ventricular remodeling in end-stage HF was held to be irreversible, multiple analyses have shown high percentages of “reverse remodeling” but only low numbers of myocardial recovery (1, 2, 25). Although the influence on end-organ perfusion and unloading is similar with pulsatile versus continuous-flow support, conflicting literature exists regarding their influence on recovery (26). In the current clinical setting, LVADs infrequently lead to sufficient myocardial recovery to allow device explantation, i.e., function as a bridge to recovery (BTR). In a retrospective review with patients receiving MCS as a bridge to transplantation, recovery occurred in less than 5% of patients (27). An explantation rate of 9% was described by the LVAD working group, mostly in younger patients with recent onset HF of non-ischemic origin (2). These results correspond to prior data showing higher percentages of myocardial recovery in patients with non-ischemic cardiomyopathy (28). Yacoub et al. aimed at the process of “physiological hypertrophy” and “reverse remodeling” to maximize the rate of cardiac recovery by using LVAD support in combination with a specific sequence of pharmacological therapy, including beta-2-agonist Clenbuterol (29). A small cohort of 15 patients receiving MCS for non-ischemic cardiomyopathy without acute myocarditis, were treated with the particular sequence of medication that resulted in sufficient recovery to meet explantation criteria in 11 patients (73%). In most cases, improvement maintained for more than 4 years (30). Long-term outcomes of patients bridged to recovery versus bridged to transplantation were investigated (31) to review the results of an aggressive attempt at stimulating myocardial recovery. Particularly patients with non-ischemic cardiomyopathy profit from aggressively inducing reversal of HF. The rate of device explantation was 20.5%, much higher than other data so far (2, 31, 32).
Experimental Regenerative Therapies
A concise concept of different regenerative approaches, present experience, and associated hurdles for clinical application will be discussed. Experimental pharmacological therapies (33) are beyond the scope of this review.
Cell therapy
Various cell populations and delivery strategies have been examined for their cardiac repair and regenerative capacity in the last decades. Stem cells can be derived from blood, bone marrow, skeletal muscle, adipose tissue, embryonic sources, or cardiac tissue (34, 35). Initially, stem cells were presumed to replace damaged cardiomyocytes. Instead, currently, the major mechanism of action is assumed to be trough paracrine factors leading to decreased neurohormonal activation and apoptosis, better Ca2+-handling, stimulation of neovascularization, and activation of endogenous cardiac-resident cells (36–38). Most clinical experience with cell therapy is gained in ischemic heart disease with unselected bone marrow-derived mononuclear cells (BMMNCs). Results in clinical setting are modest but significant with greatest improvements in the lowest LV ejection fraction (EF) at baseline (34, 38–45). The discordance with preclinical data is not fully explained, but poor cell retention and survival plus possible malfunctioning of bone marrow-derived cells in patients with HF are alleged to play a role in these somewhat disappointing findings (34, 46–48). The discovery of so-called endogenous cardiac stem cells (CSCs) (49) and evidence that cardiomyocytes have renewal capacity (49–51) have provided a new therapeutic approach, to stimulate the endogenous CSCs since these cells are programed to reconstitute cardiac tissue. Encouraging results were shown in the SCIPIO trial (autologous CSCs) (52) and in the CADUCEUS trial (autologous cardiosphere-derived cells) (53). Compared to ischemic heart disease, limited clinical data is available regarding efficacy of cell therapy in dilated cardiomyopathy. Results in this population seem mostly positive, though heterogeneity of the population, procedures, and outcome parameters prohibit extrapolation (54). A novel cell-based technology in which somatic cells (all cells in the body except germ cells) are modified or reprogramed into a special type of stem cell, called induced pluripotent stem cell (iPS), is in development (35, 55). This technique is already applied for other purposes but is fairly unknown as therapeutic. To improve clinical success of cell therapy, better understanding of the primary mechanism and best cell type are fundamental (10, 36, 38, 41). In addition, knowledge about optimal timing, dosing, and delivery strategies, including better cell retention and survival, is essential (35, 46, 56).
Growth factors
Growth hormones (GHs) are essential for normal myocardial and endothelium development, and for the maintenance of function (57, 58). Endogenous CSCs can be activated by growth factors in the infarcted heart as shown in rodents (59) and in a porcine model (60) of acute MI (34, 59–61). Vascular endothelial growth factor (VEGF) and granulocyte–macrophage colony-stimulating factor (GM-CSF) augment levels of endothelial progenitor cells (EPCs) and improve neovascularization (34). Hepatocyt growth factor (HGF) promotes cell migration, insulin-like growth factor-1 (IGF-1) is mitogenic and antiapoptotic, stimulates myocyte formation, and reduces myocyte death after infarction (59). GH therapy in chronic setting seems rational since part of the neurohormonal disturbances in HF lies in the GH/IGF-1 signaling axis (58). Whereas animal studies demonstrate beneficial effects of growth factor therapy (34, 62), clinical data about the efficacy of growth factors in HF patients is conflicting (58). In a small group of patients (n = 13) with severe coronary artery disease and refractory angina, treatment with high doses of VEGF temporarily enhanced myocardial perfusion (63). A preliminary study by Fazio et al. (64) showed improved cardiac output in seven patients with dilated cardiomyopathy treated with GH, whilst other studies examining the effects of exogenous GH in HF yielded no beneficial effects on cardiac function (65–68). A more recent clinical trial (69) with granulocyte-colony-stimulating factor after MI, although appearing to improve LV function, was terminated because of high incidence of in-stent restenosis in this treated group. A safety and efficacy trial with IGF-1 is currently recruiting patients with acute MI (Clinical trial info: NCT 01438086).
Gene therapy
Interest for experimental gene therapy in cardiovascular disease has grown in the last 10 years. The most relevant systems targeted to restore function of failing cardiomyocytes are (1) the B-adrenergic system, (2) Ca2+ cycling proteins, (3) homing stem cells, and (4) cell death (70). The first clinical, phase 2A safety study (CUPID), with adeno-associated virus (AAV) type 1/sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) suggests a positive effect on LV function (71), but the therapeutic potential will become apparent as a phase 2b trial is ongoing (clinicaltrials.gov: NCT 01643330) (72). Other targets that have been taken forward toward clinical trials include adenylyl cyclase type 6 (clinicaltrials.gov: NCT 00787059) and stromal cell-derived factor-1 (SDF-1) (clinicaltrials.gov: NCT 01082094) (70). As more molecular targets associated with HF are discovered, more effective gene therapy is expected to emerge (70). Another concept in gene therapy for HF concerns microRNA (miRNA). These are small non-coding RNAs that bind to specific target mRNAs, thereby suppressing protein expression (73, 74). The capacity to manipulate miRNA expression and function, together with the fact that their function is heightened under pathophysiological conditions, make them attractive candidates for therapeutic manipulation. Either inhibitors (antimiR) or mimics of miRNA are of interest (73). Several small and large animal studies have targeted relevant miRNA (-families) (73). For example, miRNA-208a (cardiac remodeling), miRNA-21 (cardiac hypertrophy and fibrosis), miRNA-15 (cardiomyocyte apoptosis and regeneration), and miRNA-92a (angiogenesis and regeneration) have been found to play a role in cardiovascular pathology (74–77). Hinkel et al. demonstrated improved recovery after ischemia/reperfusion injury by inhibiting miR-92a by LNA-based miRNA inhibitor in a pig model (74). Challenges in miRNA therapy essentially concern the pleiotropy and multiplicity of miRNA that needs intensive research, since only target tissue is examined in all studies. Next to that, feasibility of adequate dosing has to be assessed (70).
Exosomes
These small membrane vesicles (40–100 nm), endosomal-derived and extracellularly released by many cells, are involved in intercellular communication (78, 79). Although discovered 30 years ago (80), major interest in exosomes and their function in regenerative medicine recently emerged. In response to injury, extracellular microvesicles are released from activated platelets and apoptotic endothelial cells, suggesting not only therapeutic but also diagnostic value (81). Special attention for exosomes derived from cardiac progenitor cells has originated from the postulated paracrine effects of cell-based therapy, mainly regarding the release of growth factors, cytokines, and chemokines (78, 81). Exosomes derived from cardiomyocyte progenitor cells are proposed to play a role in cardiac protection (78). In mice as well as in a porcine model of ischemia/reperfusion injury, mesenchymal stromal cell-derived exosomes reduced myocardial damage (78, 82). While acknowledged to target via transfer of proteins or genetic materials, the role of exosomes in cardiac injury is far from clear (79). Further research on the production and content sorting of exosomes and their effect on target (and non-target) cells is crucial (79, 81).
Biomaterials
Another recent topic in regenerative therapy for cardiovascular disease is the use of biomaterials. Multiple scaffolds, naturally derived and synthetic, are used. Therapeutic ability is suggested for MI, prevention of remodeling, and in consequence prevention of ischemic HF in small and large animal models (83–85). Whilst originally tissue-engineered cardiac patches were of interest, research in the area of injectable biomaterials is rapidly evolving (47, 61, 83, 86, 87). The prospective profit of biomaterials is two-sided, either to stimulate endogenous repair and regeneration or to provide a vehicle to support delivery of other therapeutics (e.g., cells, growth factors), generating greater cell retention and survival (47, 86). Gelatin microspheres have been shown to be a feasible carrier for cardiomyocyte progenitor cells and growth factors, resulting in improved engraftment and cell survival in mice (Feyen. Thesis: Strategies to improve cardiac cell therapy. Chapter 8: Gelatin microspheres as carriers for cardiac progenitor cell and growth factor to the ischemic myocardium, unpublished, 2014). Dai et al. studied the effect of non-cellular hydrogels versus cell therapy in a rat model of chronic ischemia and showed similar increases in EF and thereby potential of hydrogels alone (84). No clinical trials have yet been performed with biomaterials. The challenge of this therapy is the delivery, mainly relating to the solubility during the procedure while the hydrogel has to become gel-like after injection in the myocardium.
Combined Mechanical Support and Regenerative Therapies
Following unloading of the ventricle, a complex network of changes on molecular, cellular, tissue, and organ level arises (32, 88–94). Although the exact mechanism of reversal of HF during LVAD support is unclear, the effect of ventricular volume and pressure unloading together with improved neurohormonal and cytokine activation are thought to induce reverse remodeling (1, 92, 95). In a study comparing isolated human myocytes of failing hearts with and without prior LVAD showed increased contractile properties and beta-adrenergic responsiveness after LVAD support (96). Immunohistochemical analysis of the contractile myofilaments after LVAD implantation uncovered improved staining pattern of all thin contractile proteins and titin, however structural myocyte damage was persisting (89). Significant improvement of the proliferation/apoptosis balance by ventricular unloading has been shown in a mouse model of ischemic HF (95). Also, specific changes in gene expression of cytoskeletal proteins after LVAD support have been seen in recovered versus non-recovered myocardium (91). The beneficial effect on LV function appears to deteriorate over time (2), suggesting that combining mechanical support with other, regenerative, therapeutic strategies like GHs, gene therapy, or cell therapy might hold the key to better long-term results (92). The unloaded ventricle provides a less hostile milieu and thereby a potentially more appropriate platform for different regenerative therapies. Along the same lines, the combined approach of biventricular pacing and BMMNCs in ischemic HF indicated a significant and clinically relevant improvement in cardiac function in comparison with BMMNCs alone, while CRT showed no impact on perfusion (97). The rationale for this approach is that electrical stimulation might promote cell differentiation.
Preclinical experience
Up to date, a representative large animal model of chronic HF with myocardial unloading is lacking. The majority of LVAD studies was performed in healthy animals with only a few studies in chronically failing models (98). Preclinical experience consists of several (b)ovine ischemic HF models, induced by either coronary microembolization, coronary ligation, or ameroid constriction (99–103). Non-ischemic HF models include a pressure and volume overload model caused by aortic constriction, respectively, mitral regurgitation via chordae rupture (98, 104). Last-mentioned models have the disadvantage of required thoracotomy, undesirable in case of future device implantation. Other methods like pacing, pharmacotherapeutic induced (doxorubicin), direct shock, and cardiotoxins are not reflective of human HF (98). Recreating a model similar to human etiology remains a challenge. To advance innovative and clinically applicable strategies for cardiac regeneration, suitable preclinical research is inevitable. Not only to test combined unloading and regenerative therapies, but also to direct future mechanical support and treatment of earlier stage HF. Thereafter, different regenerative therapies must be evaluated in such a model.
Clinical experience
Combined mechanical unloading and regenerative therapy in clinical setting has only been examined with cell therapy. The results of these studies were systematically reviewed as shown in Table 1 (39, 40, 105–111). A total of 50 patients have been treated with the combinational strategy. The limited data illustrate that in all cases that LVAD was explanted, an extracorporeal device was used. Usually, percutaneous support is initiated when myocardial recovery is expected. However, Sawa et al. (105) describe a case where a patient with idiopathic dilated cardiomyopathy did not show enough improvement in LVEF for explantation 7 months after starting MCS. After additional cell transplantation, LV improved to a reasonable function that sustained for at least 1.5 years. All studies, except Ascheim et al., used autologous cells, either bone marrow-derived or skeletal myoblasts, mainly in patients with ischemic cardiomyopathy. The first and only randomized trial with allogeneic mesenchymal precursor cells in ischemic and non-ischemic HF shows encouraging results when it comes to efficacy, but safety regarding sensitization is concerning, especially when the aim is to increase the amount of cells in future studies (111). No results of the combination of SERCA gene therapy and MCS have yet been reported (clinicaltrials.gov: NCT 000534703). Accordingly, the combination of MCS and cell therapy is promising as both therapies share action mechanisms and might possess synergistic effects (39, 40, 92, 105, 107–111). Focusing on this combination provides not only a point of reference to gain more success in bridging to recovery but also the unique opportunity to analyze the myocardium in case of heart transplantation, which can broaden understanding in the process of ventricular reverse remodeling and myocardial recovery. In patients awaiting heart transplantation, allogeneic cell therapy should only be considered with great precaution because of immunologic sensitization (111).
Table 1.
Clinical experience of LVAD combined with cell therapy.
| Study type (Reference) | n | Etiology CMP | Cell type (and timing) | Clinical outcome | Measured effect |
|---|---|---|---|---|---|
| Phase I (111) | 20 | Ischemic and non-ischemic | Allogeneic MPCs (concomitant) | Increased weaning frequency and duration | Safe/efficacy |
| Case report (105) | 1 | Dilated | Autologous skeletal myoblasts (+16 months) | LVAD explantation | LVEF increased |
| Phase I (110) | 4 | Ischemic | Autologous skeletal myoblasts (concomitant) | 1 LVAD explantation, 3 non-cardiac deaths | n = 2 LVEF increased |
| Case series (108) | 2 | Ischemic | Autologous BMMNCs (concomitant) | 1 Improved perfusion, 1 unknown | Perfusion improved |
| Case report (107) | 1 | Ischemic | Autologous skeletal myoblasts (+3 months) | Death + 466 days (sepsis) | Increased EF |
| Case series (109) | 10 | Ischemic | Autologous BMMNCs (concomitant) | 1 LVAD explantation, 3 HTx, 2 deaths | n = 1 increased EF |
| Case report (106) | 1 | Ischemic | Autologous BMMNCs (+99 days) | LVAD explantation | Increased EF and perfusion |
| Phase I (40) | 6 | Ischemic | Autologous skeletal myoblasts (concomitant) | 4 HTx, 3 deaths | Safe/feasible |
| Phase I (39) | 5 | Ischemic | Autologous skeletal myoblasts (concomitant) | 3 HTx, 1 DT, 1 death | Safe/feasible |
CMP, cardiomyopathy; MPCs, mesenchymal progenitor cells; BMMNCs, bone marrow-derived mononuclear cells; HTx, heart transplantation; DT, destination therapy.
Systematic search LVAD/SCT: search detail: “heart-assist devices” [MeSH Terms] OR (“heart-assist devices” [MeSH Terms] OR (“heart-assist” [All Fields] AND “devices” [All Fields]) OR “heart-assist devices” [All Fields] OR (“heart” [All Fields] AND “assist” [All Fields] AND “device”[All Fields]) OR “heart-assist device” [All Fields]) AND (“cell- and tissue-based therapy” [MeSH Terms] OR (“cell-” [All Fields] AND “tissue-based” [All Fields] AND “therapy” [All Fields]) OR “cell- and tissue-based therapy” [All Fields] OR (“cell” [All Fields] AND “therapy” [All Fields]) OR “cell therapy” [All Fields]).
In total: 195 hits, excluding review/animal/Japanese/no combination → 9 included articles.
Future Perspective
The rapidly developing field of regenerative therapies enables various combinations with LVAD support (e.g., hydrogel loaded with exosomes or growth factors combined with microspheres). Considering the different etiologies of HF, the most pronounced effect of combined cell therapy, biomaterials, and mechanical unloading could be expected in patients with ischemic HF. The rationale is that the ischemic myocardium will benefit most from the paracrine effects leading to angiogenesis. The combination with biomaterials might positively enlarge efficacy by higher retention rates, and perhaps through a direct therapeutic effect of the biomaterial. Gene therapy in combination with (biomaterials and) MCS is more probable to enhance myocardial function of patients with dilated cardiomyopathy. The advancements in assist devices will help to uncover the most optimal technology to stimulate recovery and reduce adverse events. Cheng et al. suggest that pulsatile flow support might have better results with regard to recovery, due to the less affected vascular reactivity in the presence of a pulse pressure (26). The absence of arterial pulsatility leads to stiff unresponsive arteries (102). Moreover, the development of algorithms for continuous-flow-LVADs to generate a pulse pressure is very intriguing, also for the possible influence on adverse events (26). The increasing rate of permanent LVAD support will lead to more clinical data regarding recovery rates and adverse events. However, the small number of patients included in LVAD trials and the lack of an illustrative preclinical model, makes moving forward to clinical application time-consuming. Besides testing of combined therapeutic strategies, preclinical research is also inevitable to gain more understanding of the types of support in the setting of myocardial recovery.
Conclusion
Since heart transplantation, the gold standard therapy for end-stage HF, is not sufficiently available, other advanced therapeutic approaches are crucial. LVADs provide a bridge for patients awaiting heart transplantation or myocardial recovery. Rates of successful and durable recovery are very low, but this can be stimulated pharmacologically. Better-sustained results could be expected from combining LVADs with regenerative therapies such as gene therapy, biomaterials, and cell-based therapies. Especially, cell therapy for the treatment of heart disease has been extensively studied, showing promising results. The small number of LVAD patients does not allow clinical testing of the numerous potential combinations of therapies. A clinically relevant animal model of unloading should be established for preclinical testing of these regenerative approaches. Regarding current experience in the reversal of HF with combined LVAD and cell therapy, future clinical research should focus on placebo-controlled studies in patients undergoing LVAD implantation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by ICIN – Netherlands Heart Institute, www.icin.nl.
Abbreviations
BIVAD, biventricular-assist device; BMMNCs, bone marrow-derived mononuclear cells; CSCs, cardiac stem cells; EF, ejection fraction; ESC, European Society of Cardiology; GHs, growth hormones; HF, heart failure; HGF, hepatocyt growth factor; IGF-1, insulin-like growth factor-1; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LV, left ventricle or ventricular; LVAD, left ventricular-assist device; MCS, mechanical circulatory support; MI, myocardial infarction; miRNA, microRNA; VAD, ventricular-assist device.
References
- 1.Levin H, Oz M, Chen J, Packer M, Rose EA, Burkhoff D. Reversal of chronic ventricular dilation in patients with end-stage cardiomyopathy by prolonged mechanical unloading. Circulation (1995) 91(11):2717–20. 10.1161/01.CIR.91.11.2717 [DOI] [PubMed] [Google Scholar]
- 2.Maybaum S, Mancini D, Xydas S, Starling RC, Aaronson K, Pagani FD, et al. Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD working group. Circulation (2007) 115(19):2497–505. 10.1161/CIRCULATIONAHA.106.633180 [DOI] [PubMed] [Google Scholar]
- 3.Redfield M. Heart failure – an epidemic of uncertain proportions. N Engl J Med (2002) 347(18):1442–4 10.1056/NEJMe020115 [DOI] [PubMed] [Google Scholar]
- 4.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KKL, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med (2002) 347(18):1397–402. 10.1056/NEJMoa020265 [DOI] [PubMed] [Google Scholar]
- 5.De Jonge N, Vantrimpont PJ. Heart failure: chapter 8. Treatment of end-stage heart failure. Neth Heart J (2004) 12(12):548–54. [PMC free article] [PubMed] [Google Scholar]
- 6.De Jonge N, Kirkels JH, Klöpping C, Lahpor JR, Caliskan K, Maat AP, et al. Guidelines for heart transplantation. Neth Heart J (2008) 16(3):79–87 10.1007/BF03086123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J (2001) 22(17):1527–60 10.1053/euhj.2001.2783 [DOI] [PubMed] [Google Scholar]
- 8.Remme WJ, Swedberg K. Comprehensive guidelines for the diagnosis and treatment of chronic heart failure. Task force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Eur J Heart Fail (2002) 4(1):11–22 10.1016/S1388-9842(01)00231-8 [DOI] [PubMed] [Google Scholar]
- 9.Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med (2008) 3:1–5 10.2217/17460751.3.1.1 [DOI] [PubMed] [Google Scholar]
- 10.Du Pré BC, Doevendans PA, van Laake LW. Stem cells for cardiac repair: an introduction. J Geriatr Cardiol (2013) 10(2):186–97. 10.3969/j.issn.1671-5411.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koopman C, Van Dis I, Bots M, Vaartjes I. Feiten en cijfers Hartfalen. Nederlandse Hartstichting (2012). Available from: https://www.hartstichting.nl/downloads/factsheet-hartfalen
- 12.Eurotransplant International Foundation. Annual Report 2013. Rahmel A, editor. Leiden: Eurotransplant Foundation; (2013). [Google Scholar]
- 13.Cleveland JC, Naftel DC, Reece TB, Murray M, Antaki J, Pagani FD, et al. Survival after biventricular assist device implantation: an analysis of the interagency registry for mechanically assisted circulatory support database. J Heart Lung Transplant (2011) 30(8):863–9 10.1016/j.healun.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 14.Liotta D. Early clinical application of assisted circulation. Tex Heart Inst J (2002) 29(3):229–30. [PMC free article] [PubMed] [Google Scholar]
- 15.Rose EA, Moskowitz AJ, Packer M, Sollano JA, Williams DL, Tierney AR, et al. The REMATCH trial: rationale, design, and end points. Ann Thorac Surg (1999) 67(3):723–30. 10.1016/S0003-4975(99)00042-9 [DOI] [PubMed] [Google Scholar]
- 16.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med (2001) 345(20):1435–43. 10.1056/NEJMoa012175 [DOI] [PubMed] [Google Scholar]
- 17.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med (2009) 361(23):2241–51. 10.1056/NEJMoa0909938 [DOI] [PubMed] [Google Scholar]
- 18.Lahpor J, Khaghani A, Hetzer R, Pavie A, Friedrich I, Sander K, et al. European results with a continuous-flow ventricular assist device for advanced heart-failure patients. Eur J Cardiothorac Surg (2010) 37(2):357–61. 10.1016/j.ejcts.2009.05.043 [DOI] [PubMed] [Google Scholar]
- 19.Neyt M, Van den Bruel A, Smit Y, De Jonge N, Erasmus M, Van Dijk D, et al. Cost-effectiveness of continuous-flow left ventricular assist devices. Int J Technol Assess Health Care (2013) 29(3):254–60. 10.1017/S0266462313000238 [DOI] [PubMed] [Google Scholar]
- 20.Stulak JM, Lee D, Haft JW, Romano MA, Cowger JA, Park SJ, et al. Gastrointestinal bleeding and subsequent risk of thromboembolic events during support with a left ventricular assist device. J Heart Lung Transplant (2014) 33(1):60–4. 10.1016/j.healun.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 21.Kirklin JK, Naftel DC, Cantor RS, Myers SL, Clark ML, Collum SC, et al. Quarterly Statistical Report 2014 3rd Quarter. INTERMACS Interagency Registry for Mechanically Assisted Circulatory Support. Birmingham: The Data Collection and Analysis Center University of Alabama; (2014). [Google Scholar]
- 22.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant (2014) 33(6):555–64. 10.1016/j.healun.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 23.Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW, et al. Interagency registry for mechanically assisted circulatory support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant (2014) 33(1):12–22. 10.1016/j.healun.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 24.Mehra MR, Stewart GC, Uber PA, Pharm D. The vexing problem of thrombosis in long-term mechanical circulatory support. J Heart Lung Transplant (2014) 33(1):1–11. 10.1016/j.healun.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 25.Maybaum S, Kamalakannan G, Murthy S. Cardiac recovery during mechanical assist device support. Semin Thorac Cardiovasc Surg (2008) 20(3):234–46. 10.1053/j.semtcvs.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 26.Cheng A, Williamitis CA, Slaughter MS. Comparison of continuous-flow and pulsatile-flow left ventricular assist devices: is there an advantage to pulsatility? Ann Cardiothorac Surg (2014) 3(6):573–81. 10.3978/j.issn.2225-319X.2014.08.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancini DM, Beniaminovitz A, Levin H, Catanese K, Flannery M, DiTullio M, et al. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation (1998) 98(22):2383–9. 10.1161/01.CIR.98.22.2383 [DOI] [PubMed] [Google Scholar]
- 28.Simon MA, Kormos RL, Murali S, Nair P, Heffernan M, Gorcsan J, et al. Myocardial recovery using ventricular assist devices: prevalence, clinical characteristics, and outcomes. Circulation (2005) 112(9 Suppl):I32–6. 10.1161/CIRCULATIONAHA.104.524124 [DOI] [PubMed] [Google Scholar]
- 29.Yacoub MH. A novel strategy to maximize the efficacy of left ventricular assist devices as a bridge to recovery. Eur Heart J (2001) 22(7):534–40 10.1053/euhj.2001.2613 [DOI] [PubMed] [Google Scholar]
- 30.Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med (2006) 355(18):1873–84. 10.1056/NEJMoa053063 [DOI] [PubMed] [Google Scholar]
- 31.Birks EJ, George RS, Firouzi A, Wright G, Bahrami T, Yacoub MH, et al. Long-term outcomes of patients bridged to recovery versus patients bridged to transplantation. J Thorac Cardiovasc Surg (2012) 144(1):190–6. 10.1016/j.jtcvs.2012.03.021 [DOI] [PubMed] [Google Scholar]
- 32.Simon MA, Primack BA, Teuteberg J, Kormos RL, Bermudez C, Toyoda Y, et al. Left ventricular remodeling and myocardial recovery on mechanical circulatory support. J Card Fail (2010) 16(2):99–105. 10.1016/j.cardfail.2009.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valentova M, von Haehling S. An overview of recent developments in the treatment of heart failure: update from the ESC Congress 2013. Expert Opin Investig Drugs (2014) 23(4):573–8. 10.1517/13543784.2014.881799 [DOI] [PubMed] [Google Scholar]
- 34.Dimmeler S, Zeiher AM, Schneider MD. Review series unchain my heart: the scientific foundations of cardiac repair. J Clin Invest (2005) 115(3):572–83. 10.1172/JCI200524283.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature (2008) 451(7181):937–42 10.1038/nature06800 [DOI] [PubMed] [Google Scholar]
- 36.Menasché P, Hagège AA, Vilquin J-T, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol (2003) 41(7):1078–83. 10.1016/S0735-1097(03)00092-5 [DOI] [PubMed] [Google Scholar]
- 37.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol (2008) 28(2):208–16. 10.1161/ATVBAHA.107.155317 [DOI] [PubMed] [Google Scholar]
- 38.Menasché P. Cardiac cell therapy: lessons from clinical trials. J Mol Cell Cardiol (2011) 50(2):258–65. 10.1016/j.yjmcc.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 39.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge ASB, Jacoby DB, et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol (2003) 41(5):879–88. 10.1016/S0735-1097(03)00081-0 [DOI] [PubMed] [Google Scholar]
- 40.Dib N, Michler RE, Pagani FD, Wright S, Kereiakes DJ, Lengerich R, et al. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: four-year follow-up. Circulation (2005) 112(12):1748–55. 10.1161/CIRCULATIONAHA.105.547810 [DOI] [PubMed] [Google Scholar]
- 41.Strauer B-E, Yousef M, Schannwell CM. The acute and long-term effects of intracoronary stem cell transplantation in 191 patients with chronic heart failure: the STAR-heart study. Eur J Heart Fail (2010) 12(7):721–9. 10.1093/eurjhf/hfq095 [DOI] [PubMed] [Google Scholar]
- 42.Van der Spoel TI, Jansen Of Lorkeers SJ, Agostoni P, van Belle E, Gyongyosi M, Sluijter JP, et al. Human relevance of pre-clinical studies in stem cell therapy; systematic review and meta-analysis of large animal models of ischemic heart disease. Cardiovasc Res (2011) 91(4):649–58. 10.1093/cvr/cvr113 [DOI] [PubMed] [Google Scholar]
- 43.Koudstaal S. Stamceltherapie voor ischemische hartziekten. Cordiaal (2013) 2:40–4. [Google Scholar]
- 44.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation (2012) 126(5):551–68 10.1161/CIRCULATIONAHA.111.086074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Ramshorst J, Bax JJ, Beeres SL, Dibbets-Schneider P, Roes SD, Stokkel MP, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia. JAMA (2009) 301(19):1997–2004 10.1001/jama.2009.685 [DOI] [PubMed] [Google Scholar]
- 46.Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J (2011) 32(10):1197–206. 10.1093/eurheartj/ehr018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radisic M, Christman KL. Materials science and tissue engineering: repairing the heart. Mayo Clin Proc (2013) 88(8):884–98. 10.1016/j.mayocp.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurazumi H, Kubo M, Ohshima M, Yamamoto Y, Takemoto Y, Suzuki R, et al. The effects of mechanical stress on the growth, differentiation, and paracrine factor production of cardiac stem cells. PLoS One (2011) 6(12):e28890. 10.1371/journal.pone.0028890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science (2009) 324(5923):98–102 10.1126/science.1164680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smits AM, van Vliet P, Metz CH, Korfage T, Sluijter JP, Doevendans PA, et al. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc (2009) 4(2):232–43. 10.1038/nprot.2008.229 [DOI] [PubMed] [Google Scholar]
- 51.Senyo S, Wang M, Wu T, Lechene CP. Mammalian heart renewal by preexisting cardiomyocytes. Nature (2013) 493(7432):433–6. 10.1038/nature11682.Mammalian [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet (2011) 378(9806):1847–57. 10.1016/S0140-6736(11)61590-0 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol (2014) 63(2):110–22. 10.1016/j.jacc.2013.08.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gho JMIH, Kummeling GJM, Koudstaal S, Jansen Of Lorkeers SJ, Doevendans PA, Asselbergs FW, et al. Cell therapy, a novel remedy for dilated cardiomyopathy? A systematic review. J Card Fail (2013) 19(7):494–502. 10.1016/j.cardfail.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 55.Bellin M, Marchetto MC, Gage FH, Mummery CL. Induced pluripotent stem cells: the new patient? Nat Rev Mol Cell Biol (2012) 13(11):713–26. 10.1038/nrm3448 [DOI] [PubMed] [Google Scholar]
- 56.Janssens S. Stem cells in the treatment of heart disease. Annu Rev Med (2010) 61:287–300 10.1146/annurev.med.051508.215152 [DOI] [PubMed] [Google Scholar]
- 57.McElhinney DB, Colan SD, Moran AM, Wypij D, Lin M, Majzoub JA, et al. Recombinant human growth hormone treatment for dilated cardiomyopathy in children. Pediatrics (2004) 114(4):e452–8. 10.1542/peds.2004-0072 [DOI] [PubMed] [Google Scholar]
- 58.Castellano G, Affuso F, Di Conza P, Fazio S. The GH/IGF-1 axis and heart failure. Curr Cardiol Rev (2009) 5(3):203–15 10.2174/157340309788970306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res (2005) 97(7):663–73. 10.1161/01.RES.0000183733.53101.11 [DOI] [PubMed] [Google Scholar]
- 60.Ellison GM, Torella D, Dellegrottaglie S, Perez-Martinez C, Perez de Prado A, Vicinanza C, et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol (2011) 58(9):977–86. 10.1016/j.jacc.2011.05.013 [DOI] [PubMed] [Google Scholar]
- 61.Koudstaal S, Bastings MMC, Feyen DA, Waring CD, van Slochteren FJ, Dankers PYW, et al. Sustained delivery of insulin-like growth factor-1/hepatocyte growth factor stimulates endogenous cardiac repair in the chronic infarcted pig heart. J Cardiovasc Transl Res (2014) 7(2):232–41. 10.1007/s12265-013-9518-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cittadini A, Grossman JD, Napoli R, Katz SE, Strömer H, Smith RJ, et al. Growth hormone attenuates early left ventricular remodeling and improves cardiac function in rats with large myocardial infarction. J Am Coll Cardiol (1997) 29(5):1109–16. 10.1016/S0735-1097(97)00010-7 [DOI] [PubMed] [Google Scholar]
- 63.Giusti II, Rodrigues CG, Salles FB, Sant’Anna RT, Eibel B, Han SW, et al. High doses of vascular endothelial growth factor 165 safely, but transiently, improve myocardial perfusion in no-option ischemic disease. Hum Gene Ther Methods (2013) 24(5):298–306. 10.1089/hgtb.2012.221 [DOI] [PubMed] [Google Scholar]
- 64.Fazio S, Sabatini S, Capaldo B, Vigorito C, Giordano A, Guida R, et al. A preliminary study of growth hormone in the treatment of dilated cardiomyopathy. N Engl J Med (1996) 334(13):809–14. 10.1056/NEJM199603283341301 [DOI] [PubMed] [Google Scholar]
- 65.Isgaard J, Bergh CH, Caidahl K, Lomsky M, Hjalmarson A, Bengtsson B. A placebo-controlled study of growth hormone in patients with congestive heart failure. Eur Heart J (1998) 19(11):1704–11. 10.1053/euhj.1998.1123 [DOI] [PubMed] [Google Scholar]
- 66.Acevedo M, Corbalán R, Chamorro G, Jalil J, Nazzal C, Campusano C, et al. Administration of growth hormone to patients with advanced cardiac heart failure: effects upon left ventricular function, exercise capacity, and neurohormonal status. Int J Cardiol (2003) 87:185–91. 10.1016/S0167-5273(02)00249-8 [DOI] [PubMed] [Google Scholar]
- 67.Spallarossa P, Rossettin P, Minuto F, Caruso D, Cordera R, Battistini M, et al. Evaluation of growth hormone administration in patients with chronic heart failure secondary to coronary artery disease. Am J Cardiol (1999) 84(4):430–3. 10.1016/S0002-9149(99)00328-8 [DOI] [PubMed] [Google Scholar]
- 68.Smit JW, Janssen YJ, Lamb HJ, van der Wall EE, Stokkel MP, Viergever E, et al. Six months of recombinant human GH therapy in patients with ischemic cardiac failure does not influence left ventricular function and mass. J Clin Endocrinol Metab (2001) 86(10):4638–43. 10.1210/jcem.86.10.7832 [DOI] [PubMed] [Google Scholar]
- 69.Kang H-J, Kim H-S, Zhang S-Y, Park K-W, Cho H-J, Koo B-K, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical. Lancet (2004) 363(9411):751–6. 10.1016/S0140-6736(04)15689-4 [DOI] [PubMed] [Google Scholar]
- 70.Tilemann L, Ishikawa K, Weber T, Hajjar RJ. Gene therapy for heart failure. Circ Res (2012) 110(5):777–93. 10.1161/CIRCRESAHA.111.252981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2 + -ATPase in patients with advanced heart failure. Circulation (2011) 124(3):304–13. 10.1161/CIRCULATIONAHA.111.022889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greenberg B, Yaroshinsky A, Zsebo KM, Butler J, Felker GM, Voors AA, et al. Design of a phase 2b trial of intracoronary administration of AAV1/SERCA2a in patients with advanced heart failure: the CUPID 2 trial (calcium up-regulation by percutaneous administration of gene therapy in cardiac disease phase 2b). JACC Heart Fail (2014) 2(1):84–92. 10.1016/j.jchf.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 73.Van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov (2012) 11(11):860–72. 10.1038/nrd3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hinkel R, Penzkofer D, Zühlke S, Fischer A, Husada W, Xu Q-F, et al. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation (2013) 128(10):1066–75. 10.1161/CIRCULATIONAHA.113.001904 [DOI] [PubMed] [Google Scholar]
- 75.Van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A (2006) 103(48):18255–60. 10.1073/pnas.0608791103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science (2009) 324(5935):1710–3. 10.1126/science.1174381 [DOI] [PubMed] [Google Scholar]
- 77.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature (2008) 456(7224):980–4. 10.1038/nature07511 [DOI] [PubMed] [Google Scholar]
- 78.Vrijsen KR, Sluijter JPG, Schuchardt MWL, van Balkom BWM, Noort WA, Chamuleau SAJ, et al. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med (2010) 14(5):1064–70. 10.1111/j.1582-4934.2010.01081.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res (2014) 114(2):333–44. 10.1161/CIRCRESAHA.114.300639 [DOI] [PubMed] [Google Scholar]
- 80.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol (1983) 97(2):329–39 10.1083/jcb.97.2.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sluijter JPG, Verhage V, Deddens JC, van den Akker F, Doevendans PA. Microvesicles and exosomes for intracardiac communication. Cardiovasc Res (2014) 102(2):302–11. 10.1093/cvr/cvu022 [DOI] [PubMed] [Google Scholar]
- 82.Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res (2010) 4(3):214–22. 10.1016/j.scr.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 83.Johnson TD, Christman KL. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin Drug Deliv (2013) 10(1):59–72. 10.1517/17425247.2013.739156 [DOI] [PubMed] [Google Scholar]
- 84.Dai W, Kay GL, Kloner RA. The therapeutic effect of cell transplantation versus non-cellular biomaterial implantation on cardiac structure and function following myocardial infarction. J Cardiovasc Pharmacol Ther (2014) 19(4):350–7 10.1177/1074248413517746 [DOI] [PubMed] [Google Scholar]
- 85.Lam MT, Wu JC. Biomaterial application in cardiovascular tissue repair and regeneration. Expert Rev Cardiovasc Ther (2013) 10(8):1039–49 10.1586/erc.12.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol (2004) 44(3):654–60. 10.1016/j.jacc.2004.04.040 [DOI] [PubMed] [Google Scholar]
- 87.Bastings MMC, Koudstaal S, Kieltyka RE, Nakano Y, Pape ACH, Feyen DAM, et al. A fast pH-switchable and self-healing supramolecular hydrogel carrier for guided, local catheter injection in the infarcted myocardium. Adv Healthc Mater (2014) 3(1):70–8. 10.1002/adhm.201300076 [DOI] [PubMed] [Google Scholar]
- 88.Frazier OH, Benedict CR, Radovancevic B, Bick RJ, Capek P, Springer WE, et al. Improved left ventricular function after chronic left ventricular unloading. Ann Thorac Surg (1996) 62(3):675–82. 10.1016/S0003-4975(96)00437-7 [DOI] [PubMed] [Google Scholar]
- 89.De Jonge N, van Wichen DF, Schipper MEI, Lahpor JR, Gmelig-Meyling FHJ, Robles de Medina EO, et al. Left ventricular assist device in end-stage heart failure: persistence of structural myocyte damage after unloading. An immunohistochemical analysis of the contractile myofilaments. J Am Coll Cardiol (2002) 39(6):963–9. 10.1016/S0735-1097(02)01713-8 [DOI] [PubMed] [Google Scholar]
- 90.Terracciano CMN, Hardy J, Birks EJ, Khaghani A, Banner NR, Yacoub MH. Clinical recovery from end-stage heart failure using left-ventricular assist device and pharmacological therapy correlates with increased sarcoplasmic reticulum calcium content but not with regression of cellular hypertrophy. Circulation (2004) 109(19):2263–5. 10.1161/01.CIR.0000129233.51320.92 [DOI] [PubMed] [Google Scholar]
- 91.Birks EJ, Hall JL, Barton PJR, Grindle S, Latif N, Hardy JP, et al. Gene profiling changes in cytoskeletal proteins during clinical recovery after left ventricular-assist device support. Circulation (2005) 112(9 Suppl):I57–64. 10.1161/CIRCULATIONAHA.104.526137 [DOI] [PubMed] [Google Scholar]
- 92.Ibrahim M, Rao C, Athanasiou T, Yacoub MH, Terracciano CM. Mechanical unloading and cell therapy have a synergistic role in the recovery and regeneration of the failing heart. Eur J Cardiothorac Surg (2012) 42(2):312–8. 10.1093/ejcts/ezs067 [DOI] [PubMed] [Google Scholar]
- 93.Schipper MEI, van Kuik J, de Jonge N, Dullens HFJ, de Weger RA. Changes in regulatory microRNA expression in myocardium of heart failure patients on left ventricular assist device support. J Heart Lung Transplant (2008) 27(12):1282–5. 10.1016/j.healun.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 94.Lok SI, van Mil A, Bovenschen N, van der Weide P, van Kuik J, van Wichen D, et al. Post-transcriptional regulation of α-1-antichymotrypsin by microRNA-137 in chronic heart failure and mechanical support. Circ Heart Fail (2013) 6(4):853–61. 10.1161/CIRCHEARTFAILURE.112.000255 [DOI] [PubMed] [Google Scholar]
- 95.Suzuki R, Li T-S, Mikamo A, Takahashi M, Ohshima M, Kubo M, et al. The reduction of hemodynamic loading assists self-regeneration of the injured heart by increasing cell proliferation, inhibiting cell apoptosis, and inducing stem-cell recruitment. J Thorac Cardiovasc Surg (2007) 133(4):1051–8. 10.1016/j.jtcvs.2006.12.026 [DOI] [PubMed] [Google Scholar]
- 96.Dipla K, Mattiello JA, Jeevanandam V, Houser SR, Margulies KB. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation (1998) 97(23):2316–22. 10.1161/01.CIR.97.23.2316 [DOI] [PubMed] [Google Scholar]
- 97.Pokushalov E, Romanov A, Corbucci G, Prohorova D, Chernyavsky A, Larionov P, et al. Cardiac resynchronization therapy and bone marrow cell transplantation in patients with ischemic heart failure and electromechanical dyssynchrony: a randomized pilot study. J Cardiovasc Transl Res (2011) 4(6):767–78. 10.1007/s12265-011-9283-1 [DOI] [PubMed] [Google Scholar]
- 98.Monreal G, Sherwood LC, Sobieski MA, Giridharan GA, Slaughter MS, Koenig SC. Large animal models for left ventricular assist device research and development. ASAIO J (2014) 60(1):2–8. 10.1097/MAT.0000000000000005 [DOI] [PubMed] [Google Scholar]
- 99.Goldstein AH, Monreal G, Kambara A, Spiwak AJ, Schlossberg ML, Abrishamchian AR, et al. Partial support with a centrifugal left ventricular assist device reduces myocardial oxygen consumption in chronic, ischemic heart failure. J Card Fail (2005) 11(2):142–51. 10.1016/j.cardfail.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 100.Monreal G, Gerhardt MA. Left ventricular assist device support induces acute changes in myocardial electrolytes in heart failure. ASAIO J (2007) 53(2):152–8. 10.1097/MAT.0b013e3180302a8b [DOI] [PubMed] [Google Scholar]
- 101.Ghodsizad A, Kar BJ, Layolka P, Okur A, Gonzales J, Bara C, et al. Less invasive off-pump implantation of axial flow pumps in chronic ischemic heart failure: survival effects. J Heart Lung Transplant (2011) 30(7):834–7. 10.1016/j.healun.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 102.Bartoli CR, Giridharan GA, Litwak KN, Sobieski M, Prabhu SD, Slaughter MS, et al. Hemodynamic responses to continuous versus pulsatile mechanical unloading of the failing left ventricle. ASAIO J (2010) 56(5):410–6 10.1097/MAT.0b013e3181e7bf3c [DOI] [PubMed] [Google Scholar]
- 103.Geens JH, Jacobs S, Claus P, Trenson S, Leunens V, Vantichelen I, et al. Partial mechanical circulatory support in an ovine model of post-infarction remodeling. J Heart Lung Transplant (2013) 32(8):815–22. 10.1016/j.healun.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 104.Tuzun E, Bick R, Kadipasaoglu C, Conger JL, Poindexter BJ, Gregoric ID, et al. Modification of a volume-overload heart failure model to track myocardial remodeling and device-related reverse remodeling. ISRN Cardiol (2011) 2011:831062. 10.5402/2011/831062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sawa Y, Miyagawa S, Sakaguchi T, Fujita T, Matsuyama A, Saito A, et al. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today (2012) 42(2):181–4. 10.1007/s00595-011-0106-4 [DOI] [PubMed] [Google Scholar]
- 106.Gojo S, Kyo S, Nishimura S, Komiyama N, Kawai N, Bessho M, et al. Cardiac resurrection after bone-marrow-derived mononuclear cell transplantation during left ventricular assist device support. Ann Thorac Surg (2007) 83(2):661–2. 10.1016/j.athoracsur.2006.06.074 [DOI] [PubMed] [Google Scholar]
- 107.Miyagawa S, Matsumiya G, Funatsu T, Yoshitatsu M, Sekiya N, Fukui S, et al. Combined autologous cellular cardiomyoplasty using skeletal myoblasts and bone marrow cells for human ischemic cardiomyopathy with left ventricular assist system implantation: report of a case. Surg Today (2009) 39(2):133–6. 10.1007/s00595-008-3803-x [DOI] [PubMed] [Google Scholar]
- 108.Anastasiadis K, Antonitsis P, Argiriadou H, Koliakos G, Doumas A, Khayat A, et al. Hybrid approach of ventricular assist device and autologous bone marrow stem cells implantation in end-stage ischemic heart failure enhances myocardial reperfusion. J Transl Med (2011) 9(1):12. 10.1186/1479-5876-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nasseri B, Kukucka M, Dandel M, Knosalla C, Potapov E, Lehmkuhl H, et al. Intramyocardial delivery of bone marrow mononuclear cells and mechanical assist device implantation in patients with end-stage cardiomyopathy. Cell Transplant (2007) 16(9):941–9. 10.3727/096368907783338235 [DOI] [PubMed] [Google Scholar]
- 110.Fujita T, Sakaguchi T, Miyagawa S, Saito A, Sekiya N, Izutani H, et al. Clinical impact of combined transplantation of autologous skeletal myoblasts and bone marrow mononuclear cells in patients with severely deteriorated ischemic cardiomyopathy. Surg Today (2011) 41(8):1029–36. 10.1007/s00595-010-4526-3 [DOI] [PubMed] [Google Scholar]
- 111.Ascheim DD, Gelijns AC, Goldstein D, Moye LA, Smedira N, Lee S, et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary LVADs. Circulation (2014) 129:2287–96. 10.1161/CIRCULATIONAHA.113.007412 [DOI] [PMC free article] [PubMed] [Google Scholar]