Abstract
We examined the neural correlates of resting cardiac vagal activity in a sample of 432 participants (206 male; 61 African American; mean age 42 years). Pulsed arterial spin labeling was used to quantify whole brain and regional cerebral blood flow at rest. High-frequency heart rate variability (HF-HRV) was used to measure cardiac vagal activity at rest. The primary aim was to determine whether brain regions implicated in regulating cardiac vagal reactions were also related to cardiac vagal activity at rest, and whether these associations varied by sex or race. Brain areas previously related to vagal reactivity were related to resting HF-HRV. Directionality of relationships differed between overall and regional flows. Some relationships were only observed in women and African Americans. There appears to be communality between brain regions associated with task-induced vagal reactivity and those associated with resting cardiac vagal activity.
Descriptors: Central autonomic control, Heart rate variability, Vagal activity, Cerebral blood flow, Race, Sex
High-frequency heart rate variability (HF-HRV) is a purported noninvasive marker of postganglionic vagal traffic to the sinoatrial node and the final output of the so-called central autonomic network, which refers to a distributed group of cortical and subcortical brain regions that regulate autonomic outflow to the periphery (Akselrod et al., 1981; Benarroch, 1993; Berntson et al., 1997; Lewis, Furman, McCool, & Porges, 2012; Porges, 2007; Shoemaker, Wong, & Cechetto, 2012; Thayer & Lane, 2000). Human neuroimaging studies of the neural correlates of HF-HRV have largely employed reactivity paradigms and have commonly reported correlations between task-evoked reductions in HF-HRV, presumably reflecting “vagal withdrawal,” and corresponding reductions in regional cerebral blood flow (Gianaros, van der Veen, & Jennings, 2004; Napadow et al., 2008). However, no studies to our awareness have provided consistent evidence for a relationship between resting cardiac vagal activity and resting cerebral blood flow—which presumably reflects resting metabolic activity linked to neural function (Ye et al., 2000; cf. Cha et al., 2013). The distinction between rest and reactivity is important insofar as resting cardiac vagal activity has been the predominant vagal index used in epidemiological studies linking short-term estimates of HRV with diverse health outcomes and end points, such as all-cause mortality, cardiovascular disease, and inflammation (Britton et al., 2007; Y. W. Chang et al., 2012; Habib, 1999; Huston & Tracey, 2011; Marsland et al., 2007; Thayer, Yamamoto, & Brosschot, 2010). Thus, the present study extends prior work focused on reactivity to a focus on the neural correlates of resting cardiac vagal activity.
Neural Correlates of HF-HRV
Human evidence of the neural correlates of HF-HRV is largely based on neuroimaging studies using either functional magnetic resonance imaging (fMRI) or positron emission tomography (PET). One recent meta-analysis of neuroimaging studies of HF-HRV identified five brain regions in which changes in regional cerebral blood flow were related to task-evoked changes in HF-HRV (Thayer, Åhs, Fredrikson, Sollers, & Wager, 2012). Three brain regions (i.e., ventromedial prefrontal cortex, encompassing the perigenual and subgenual portions of the anterior cingulate cortex, as well as the left sublenticular extended amygdala) were related to HF-HRV across all studies included in the meta-analysis. Moreover, HF-HRV was related to regional cerebral blood flow during specific experimental paradigms, such as emotion (i.e., rostral medial prefrontal cortex) and cognitive/motor tasks (i.e., left posterior putamen).
A second recent meta-analysis of neuroimaging studies of HF-HRV differentiated brain regions associated with presumptive indicators of sympathetic and parasympathetic control (Beissner, Meissner, Bär, & Napadow, 2013). Brain regions generally associated with parasympathetic control included the hippocampal formation, amygdala, anterior insula, dorsal posterior cingulate cortex, primary motor cortex, superior temporal gyrus, and cerebellum. There is some overlap between these two meta-analyses in terms of the brain regions reportedly associated with cardiac vagal reactivity (e.g., left amygdala, regions of the cingulate), but it appears that these associations vary as a function of the domain of task during which the data were collected.
Limitations of Existing Neuroimaging Studies of Cardiac Vagal Function
While these meta-analytic results are informative, several characteristics of the studies included in these meta-analyses make the findings somewhat problematic to interpret and directly compare or integrate. For example, a wide variety of HF-HRV metrics were used, including spectral analysis, peak-to-valley, and an unorthodox method of orthogonalizing HF-HRV with respect to low frequency HRV (Allen, Chambers, & Towers, 2007; Critchley et al., 2003). As indicated above, it is also noteworthy that the majority of studies included in these meta-analyses identified brain regions in which task-evoked changes in neural activity (activation, deactivation) were correlated with task-evoked changes in HF-HRV (i.e., vagal withdrawal). Moreover, of these “reactivity” studies, several only presented task-evoked activation and deactivation patterns alongside task-evoked vagal reactions, rather than explicitly correlating HF-HRV with neuroimaging metrics (e.g., Goswami, Frances, & Shoemaker, 2011; Marci, Glick, Loh, & Dougherty, 2007). Thus, the findings outlined in these meta-analyses may only be relevant to domain-specific relationships with vagal reactivity, namely, vagal withdrawal, and not indicative of neural pathways generally related to resting cardiac vagal activity.
Furthermore, only eight studies were included in each meta-analysis with a median sample size of 12 subjects per study. Human neuroimaging studies are often characterized by small sample sizes (Button et al., 2013); however, this increases the probability of spurious correlations and limits the identification of between-subject effects (e.g., by sex, race). Moderating factors, such as sex and race, deserve attention for several reasons. For instance, sex differences have been reported in the neural correlates of HF-HRV (Nugent, Bain, Thayer, Sollers, & Drevets, 2011; Wong et al., 2007), and some evidence suggests that sex differences in HF-HRV may vary as a function of age (Kuo et al. 1999). Therefore, it is not clear how well the meta-analytic results reported above generalize across men and women.
An additional concern is that the participants in the studies included in these meta-analyses were predominantly of European descent. Race is an important issue regarding the neural correlates of cardiac vagal control given the consistent evidence of an increased risk for cardiovascular disease among African Americans (Karlamangla, Merkin, Crimmins, & Seeman, 2010). Some researchers have suggested that dysregulated autonomic functioning may be a potential mechanism underlying race differences in cardiovascular disease (Thayer et al., 2010). For example, some studies have reported significantly higher resting HF-HRV in African Americans compared to European Americans, despite racial differences in cardiovascular disease (Liao et al., 1995). These findings are paradoxical because higher HF-HRV is considered a cardioprotective factor, suggesting that resting HF-HRV may have different health implications for African and European Americans. Furthermore, racial differences in HF-HRV may also be paralleled by racial differences in neural correlates of resting cardiac vagal activity. Racial differences in the neural correlates of resting cardiac vagal control have yet to be examined.
Aims of the Current Study
Accordingly, we examined a relatively large sample of men and women of both African and European descent to determine the relationship between resting cardiac vagal activity, estimated via HF-HRV, and resting cerebral blood flow, estimated via pulsed arterial spin labeling (PASL). Whereas many prior reports of the neural correlates of HF-HRV reactivity have relied on change in the blood oxygenation level-dependent (BOLD) signal to estimate task-induced brain activation, PASL is a noninvasive brain imaging method capable of quantifying cerebral blood flow at rest, which is more tightly correlated with neuronal activity than the BOLD signal (Buxton & Frank, 1997; Pfeuffer et al., 2002). The majority of measures reported in brain imaging studies report relative measures rather than quantitative blood flow measures; hence, total cerebral blood flow was not reported in studies reviewed in the meta-analyses. Thus, one value of the current study was the comparison of total and proportional regional flows to resting HF-HRV.
We sought to examine both regional and whole brain resting cerebral blood flow, as whole brain cerebral blood flow has been related to numerous cardiometabolic risk factors (Jennings et al., 2013). Furthermore, neuronal signaling mechanisms (e.g., nitric oxide), which govern whole brain cerebral blood flow via vasodilation of cerebral arteries, also influence cardiac vagal activity. For example, experimentally induced increases in total cerebral blood flow also increase HF-HRV (Chowdhary et al., 2000). Thus, higher levels of resting total cerebral blood flow may also be related to relatively higher levels of resting HF-HRV.
Regarding the relationship between HF-HRV and regional cerebral blood flow, our analyses were largely guided by the two recent meta-analyses that identified specific brain regions commonly associated with task-induced vagal withdrawal (Beissner et al., 2013; Thayer et al., 2012). The vast majority of studies included in these meta-analyses reported that task-induced decreases in HF-HRV were related to task-induced reductions in regional neural activity (Gianaros et al., 2004; Napadow et al., 2008). Relatively higher resting HF-HRV is typically associated with greater task-induced reductions in HF-HRV (i.e., ↑ HF-HRVBaseline = ↓ HF-HRVTask; e.g., Salomon, 2005; cf. Park, Vasey, Van Bavel, & Thayer, 2014). Presuming communality between neural regulation of resting HF-HRV and task-induced reductions in HF-HRV (i.e., ↓ neural activityTask = ↓ HF-HRVTask), relatively lower levels of resting HF-HRV may be related to greater resting regional brain activation (↓ HF-HRVBaseline = ↑ regional cerebral blood flowResting). Thus, we examined the relationship between resting HF-HRV with resting total and regional cerebral blood flows. A secondary and exploratory aim of this paper was to examine the extent to which these relationships varied as a function of sex and race.
Method
Participants
The present study examined 432 participants (206 male; mean age 42 years, standard deviation = 7 years, age range = 30–54; 362 European American, 61 African American, 5 Asian, 2 multiracial, 2 other). Subjects were recruited via a mass mailing to residents of the Tri-County area in western Pennsylvania (Allegheny, Westmoreland, and Washington Counties) for participation in the Adult Health and Behavior study (see Table 1 for more sample characteristics; also see Gianaros et al., 2013). Mailing addresses were derived from lists obtained and purchased by the Department of Epidemiology, including public domain lists (e.g., Aldata, Info USA) and voter registration lists. A total of 177,415 residents were mailed the study advertisement, 8,957 (5%) responded to the advertisement, and 3,431 (38%) were reached for a screening interview. The age range recruited for this study was 30–55 years. Based upon laboratory and clinic assessments, participants were excluded if they had any of the following: history of cardiovascular disease/ heart surgery; prior stroke/history of cerebrovascular disease; a neurological disorder, prior convulsions, or a concussion involving a loss of consciousness; chronic kidney/liver disease; cancer; insulin-dependent diabetes/fasting glucose > 126mg/dL; high resting blood pressure (systolic/diastolic ≥ 160/110mmHg); use of psychotropic, lipid-lowering, glucocorticoid, or cardiovascular (e.g., antihypertensive) medications; or a reported history of schizophrenia or bipolar disorder. The study was approved by the University of Pittsburgh Institutional Review Board, and all participants gave informed consent.
Table 1.
Basic Characteristics of the Whole Sample and Subgroups
| Whole sample n=432 |
Men n=179 |
Women n=183 |
European Americans n=362 |
African Americans n=61 |
|
|---|---|---|---|---|---|
| Age (years) | 42 (7) | 41 (7) | 43 (6)* | 42 (7) | 44 (6)* |
| Body mass index | 26 (5) | 26 (4) | 25 (5)* | 26 (4) | 29 (5)*** |
| Systolic BP (mmHg) | 114 (11) | 116 (10) | 111 (10)* | 113 (11) | 119 (10)*** |
| Current smoker (%) | 14.8 | 13.4 | 13.7 | 13.5 | 23.0 |
| Ln HF-HRV | 6.10 (1.34) | 6.07 (1.32) | 6.12 (1.26) | 6.10 (1.29) | 6.28 (1.57) |
| Years of schooling | 16 (2) | 17 (2) | 16 (2) | 17 (2) | 15 (2)*** |
| Median household income (k) | 35 (6) | 36 (6) | 35 (6) | 36 (6) | 31 (6)*** |
| Kilocalories burned per week | 2,950 (2,441) | 3,392 (2,922) | 2,651 (1,898)** | 3,055 (2,422) | 2,795 (2,834)* |
Note. Means are reported with their standard deviation in parentheses. Mann-Whitney U tests were conducted to test for differences by sex and race. Age for the total sample ranged from 30 to 54. Smoking status was self-reported and subsequently converted into a binary scale, with 0 = nonsmoker/ex-smoker and 1 = current smoker/other smoker, other tobacco. Median household income was adjusted for number of occupants in the household (median income/√(# of occupants))3. Kilocalories burned each week were estimated with the Paffenbarger Physical Activity Questionnaire. Descriptive statistics for men and women are derived exclusively from subjects of European descent, to mirror the analyses of sex reported in the Results section. BP = blood pressure; Ln HF-HRV = natural log of high-frequency heart rate variability; k = $1,000 s.
p<.05;
p<.01;
p<.001.
HRV Protocol and Assessment
Heart rate variability (HRV) was derived from a continuous recording of a two-lead electrocardiogram (ECG) attached bilaterally to the wrists throughout a 5-min period of paced respiration (11 breaths/minute; ≈ 0.18 Hz). Pilot observations suggested that 11 breaths/minute was a comfortable rate for most people. Participants were instructed to breathe naturally in response to two auditory tones signaling them to inhale and exhale. Respiration was monitored using a thoracic strain gauge. During the paced respiration, participants were seated and asked to remain stationary in a temperature-controlled recording chamber to control for the effects of individual differences in breathing frequency and movement on HRV (Berntson et al., 1997). HRV was also collected during spontaneous breathing, though the results were not substantially different when unpaced HRV was used in the analyses, and HRV collected during paced breathing was more normally distributed (less skewed and kurtotic).
The ECG was collected in the morning, on average 2 weeks prior to the neuroimaging session. Participants were asked to not drink caffeine for 4 h, avoid alcohol or exercise for 12 h, and abstain from over-the-counter medications for 24 h. ECG signals were digitized at a sampling rate of 1000Hz (LabView acquisition software, National Instruments Corporation, Austin, TX). An interbeat-interval time series was derived from the ECG, corrected for artifacts in the R wave detection process, and the band-limited variance within the high frequency (0.12–0.40 Hz) was extracted using PhysioScripts (Christie & Gianaros, 2013).
Pulsed Arterial Spin-Labeling Acquisition
The neuroimaging session was collected under similar restrictions as the ECG session. Participants were asked to not drink caffeine for 4 h, avoid alcohol or exercise for 12 h, and abstain from over-the-counter medications for 24 h. The average scan time started at roughly 1 pm. Resting perfusion images were collected on a 3T Trio TIM whole-body scanner (Siemens, Erlangen, Germany) using a 12-channel phased-arrayed head coil and a PASL sequence. Interleaved perfusion images with and without arterial spin labeling were obtained over a 5-min, 28-s period using gradient-echo echo-planar imaging (EPI). The PASL sequence employed a modified version of the flow-sensitive alternating inversion recovery method (Kim, 1995), specifically applying a saturation pulse 700 ms after an inversion pulse. To reduce transit artifact, a 1,000-ms delay separated the end of the labeling pulse and the time of image acquisition. Resting perfusion image acquisition parameters were field of view (FOV) = 240 × 240mm, matrix = 64 × 64, repetition time (TR) = 4,000 ms, echo time (TE) = 18ms, and flip angle (FA) = 90°. Twenty-one slices (5mm thick, 1-mm gap) were acquired sequentially in an inferior-to-superior direction, yielding 80 total perfusion images (40 labeled, 40 unlabeled, 2 initial discarded images allowing for magnetic equilibration), and the acquisition time of each slice was 45 ms. A 24 s of equilibrium magnetization of brain (two sets of 21 slices; TR = 8,000ms; all other parameters are described as above) provided two images for baseline cerebral blood flow quantification.
Preprocessing of Perfusion Images
Resting perfusion images were preprocessed with computational routines implemented in Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK). For preprocessing, the MPRAGE (magnetization-prepared rapid gradient-echo) image was segmented into gray and white images. Resting perfusion images were realigned to the first image of the series. The baseline images were realigned to the first of the perfusion images, and one averaged baseline image was then calculated from the two realigned images for later use for cerebral blood flow imaging reconstruction. Each individual’s realigned gray image was coregistered to the respective mean perfusion image. The 80 realigned perfusion images and one averaged baseline image were then smoothed with a 12-mm full-width at half-maximum (FWHM) isotropic Gaussian kernel. Cerebral blood flow was then calculated. The realigned and smoothed 40 labeled and 40 unlabeled perfusion images were submitted to pairwise subtraction (e.g., the even-numbered unlabeled/control image is subtracted from odd-numbered labeled image). Subtraction images were converted to an absolute cerebral blood flow image series using a validated algorithm (Wang et al., 2003). This perfusion series was then averaged; generating for each individual a single resting voxelwise cerebral blood flow image and a global cerebral blood flow value, both in units of mL/100 g/min. After the perfusion reconstruction step, an individual’s mean cerebral blood flow images and structural gray image were then spatially normalized to the International Consortium for Brain Mapping 152 template (Montreal Neurological Institute; MNI) of gray image with voxel size of 3×3×3mm using a trilinear interpolation method. These steps resulted in a normalized mean cerebral blood flow image for each participant.
Regions of Interest
A total of 14 regions of interest (ROI) were selected with a spherical shape (10-mm radius) centered at MNI coordinates derived from two recent meta-analyses of human neuroimaging studies of central autonomic pathways (see Table 2; Beissner et al., 2013; Thayer et al., 2012). Labels for each ROI were derived by converting the MNI coordinates to Talairach coordinates using mni2tal, and then finding the nearest gray matter region using the Talairach client (Brett, 1999; Lancaster et al., 1997, 2000). Thus, the names for each ROI included in the present paper may differ somewhat from the labels reported in the meta-analyses upon which they were based. ROIs were normalized by dividing the resulting regional values by total cerebral blood flow, resulting in a proportional metric (see online supporting information Table S1 for means and standard errors of raw and proportional ROIs). While such a correction has been called into question in the literature regarding correlations between two resting state networks (Murphy, Birn, & Bandettini, 2013; Murphy, Birn, Handwerker, Jones, & Bandettini, 2009), we corrected for total cerebral blood flow because of its high correlation with regional flows. Furthermore, our analyses were aimed at the relationship between HF-HRV and regional cerebral blood flow, rather than the correlation between estimates of regional cerebral blood flow.
Table 2.
Relationship Between HF-HRV and Perfusion in Regions of Interest
| MNI coordinates |
βHF-HRV |
βHF-HRV×Race |
βHF-HRV×Sex |
|||
|---|---|---|---|---|---|---|
| Brain regions | x | y | z | (n=432) | (n=423) | (n=362) |
| Total cerebral blood flow | 0.149** | −0.275* | 0.046 | |||
| Left parahippocampal gyrus (BA 34) | −24 | 0 | −12 | −0.123*a | −0.043 | 0.294** |
| Left putamen | −26 | −8 | −2 | −0.138**a | −0.025 | 0.103 |
| Dorsal anterior cingulate (BA 32) | 2 | 46 | 6 | −0.043 | −0.365* | 0.100 |
| Ventral anterior cingulate (BA 24) | 2 | 22 | −2 | 0.026 | −0.320* | 0.129 |
| Medial frontal gyrus (BA 10) | 10 | 54 | 18 | −0.022 | −0.236† | −0.067 |
| Left amygdala | −20 | −6 | −18 | −0.123*a | −0.070 | 0.283** |
| Left insula (BA 13) | −40 | 0 | 12 | −0.154** | −0.094 | 0.035 |
| Right insula (BA 13) | 40 | 2 | 12 | −0.096*a | −0.077 | −0.118 |
| Cerebellar declive | −10 | −62 | −20 | −0.005 | 0.011 | −0.039 |
| Right hippocampus | 30 | −22 | −16 | −0.125** | 0.044 | 0.270* |
| Right superior temporal gyrus (BA 22) | 50 | −24 | 2 | −0.131**a | 0.068 | 0.120 |
| Dorsal posterior cingulate (BA 31) | −6 | −44 | 34 | 0.046 | 0.166 | 0.046 |
| Left precentral gyrus (BA 44) | −56 | 6 | 8 | −0.033 | 0.068 | 0.205 |
| Right superior temporal gyrus (BA 41) | 44 | −38 | 14 | −0.183*** | 0.076 | 0.046 |
Note. Brain regions consisted of a 10-mm radius sphere centered at MNI coordinates derived from two recent meta-analyses. Both men and women, as well as African American and European American participants, had similar levels of HF-HRV (all ps≥.310). There was only one main effect of race on cerebral blood flow values, with African American participants having relatively higher regional proportional cerebral blood flow in the left putamen (p<.05). Women had significantly higher total cerebral blood flow (p<.001), and significantly lower regional proportional cerebral blood flow in the ventral and dorsal anterior cingulate, left insula, right hippocampus, right superior temporal gyrus (BA 22), and left precentral gyrus (all ps<.05). Women also had significantly higher regional proportional cerebral blood flow in the cerebellar declive (p<.05). HF-HRV = high-frequency heart rate variability; MNI = Montreal Neurological Institute; β = beta weights (standardized); BA = Brodmann area.
Nonsignificant after controlling for age. Age was the only variable that significantly reduced any of the main effects.
p=.055;
p<.05;
p<.01;
p<.001.
Statistical Approach
To examine the relationship between HF-HRV and cerebral perfusion, linear regression models were computed with HF-HRV as the predictor and cerebral blood flow as the outcome. Separate regression models (15 total) were computed in SPSS 21 for total cerebral blood flow and each of the 14 ROIs. To test for sex and race differences in the relationship between HF-HRV and cerebral blood flow, a hierarchical regression approach was used. The Z score of HF-HRV and all cerebral blood flow values were first computed to allow for an accurate calculation of standardized (β) regression weights (Friedrich, 1982). First-order terms were entered into the initial step of the model (zHF-HRV and sex/race), and their multiplicative terms were added in the second step (i.e., zHF-HRV * race, zHF-HRV * sex; Aiken, & West, 1991). Significant interaction terms indicated that the relationship between HF-HRV and cerebral blood flow differed by sex/race, and were subsequently probed by computing simple slopes for the respective groups (men, women; European Americans, African Americans).
In addition to the ROIs outlined in Table 2, exploratory whole brain analyses were conducted using the same regression approach discussed above. The primary goal of these whole brain analyses was to determine whether HF-HRV was related to any additional brain regions not examined in the ROI analysis. In SPM 8, cerebral blood flow images were masked using an absolute threshold of zero and normalized using proportional scaling to conform to the ROI analyses. For the main effect of HF-HRV on brain perfusion, cerebral blood flow images were thresholded at p<.001, uncorrected, with a minimum cluster size of 10 contiguous voxels. A relaxed threshold (p<.005) was used when testing interactions between HF-HRV and race/sex. Familywise error-corrected p values are reported for each significant cluster. MNI coordinates at peak activity of each significant cluster were converted to Talairach coordinates using mni2tal (Brett, 1999). Labels from the resulting Talairach coordinates were determined by finding the nearest gray matter region using the Talairach client, similar to the ROI analyses explained above (Lancaster et al., 1997, 2000).
In the initial stages of the composition of this paper, we examined the effect of a number of variables potentially influencing the relationships between HF-HRV and cerebral blood flow: age, systolic blood pressure (mmHg), smoking status (nonsmoker/exsmoker vs. current smoker), and physical activity (Paffenbarger, Blair, Lee, & Hyde, 1993). Systolic blood pressure, smoking status, and physical activity had no appreciable effects on our findings, and were subsequently dropped from our statistical models. However, age was related to both HF-HRV (r = .40, p<.001) and cerebral blood flow in several regions. Controlling for age in our regression models reduced the magnitude of five of the reported main effects between HF-HRV and cerebral blood flow, which are noted in Table 2. We also controlled for demographic variables (see Table 1) that differed by group (sex/race) to see if any of our reported sex or race differences were accounted for by their statistical control. However, all sex and race by HF-HRV interactions remained significant and appear to be independent of the demographics we measured.
Results
Whole Sample Analyses: HF-HRV and Brain Perfusion
Table 2 shows the MNI coordinates and β weights representing the standardized relationships between perfusion in each brain region tested and resting HF-HRV. Overall, there were main effects and/ or interactive effects of resting HF-HRV on perfusion in the whole brain and 10 of the 14 ROIs examined (see Table 2).
Total cerebral blood flow was positively related to HF-HRV, β = .149, t(430) = 3.13, p = .002, R2 = .022. HF-HRV had a negative main effect on four ROIs in the left hemisphere: left parahippocampal gyrus (BA 34) [β=−.123, t(430) = 2.56, p = .011, R2 = .015], left putamen [β=−.138, t(430) = 2.88, p = .004, R2 = .019], left amygdala [β=−.123, t(430) = 2.56, p<.011, R2 = .015], and the left insula (BA 13) [β=−.154, t(430) = 3.22, p = .001, R2 = .024]. HF-HRV also had a negative main effect on four ROIs in the right hemisphere: right insula (BA 13) [β = 2.096, t(430) = 2.00, p = .046, R2 = .009], right hippocampus [β=−.125, t(430) = 2.60, p = .009, R2 = .016], right superior temporal gyrus (BA 22) [β=−.131, t(430) = 2.74, p = .006, R2 = .017], and the right superior temporal gyrus (BA 44) [β=−.183, t(430) = 3.85, p<.001, R2 = .033].
HF-HRV and Brain Perfusion as a Function of Race
In addition to the whole sample regression analyses reported above, race differences in the relationship between resting HF-HRV and brain perfusion were investigated. The interaction between race and HF-HRV was significantly related to total cerebral blood flow, as well as cerebral blood flow in two ROIs (see Table 2). The relationship between total cerebral blood flow and HF-HRV varied as a function of race (β=−.275, t(419) = 2.25, p = .025), with the interaction term significantly improving model fit (ΔR2 = .012, R2 = .034, F(3,419) = 4.98, p<.001). Simple slope testing revealed that higher resting HF-HRV was related to greater whole brain perfusion in European Americans (β = .206, t(419) = 3.80, p<.001), but not African Americans (β=−.069, t(419) = .628, p = .530). The relationship between HF-HRV and proportional cerebral blood flow in the dorsal anterior cingulate varied as a function of race (β=−.365, t(419) = 3.03, p = .003), with the interaction term significantly improving model fit (ΔR2 = .022, R2 = .022, F(3,419) = 3.20, p = .023). Simple slope testing indicated that higher resting HF-HRV was related to less perfusion in African Americans (β=−.321, t(419) = 2.98, p = .003), but not European Americans (β = .044, t(419) = .821, p = .412). Finally, the relationship between HF-HRV and proportional cerebral blood flow in the ventral anterior cingulate varied as a function of race (β=−.320, t(419) = 2.66, p = .008), with the interaction term significantly improving model fit (ΔR2 = .017, R2 = .019, F(3,419) = 2.77, p = .041). Simple slope testing showed that higher resting HF-HRV was related to less perfusion in African Americans (β=−.243, t(419) = 2.25, p = .025), but not European Americans (β=−.077, t(419) = 1.448, p = .148).
HF-HRV and Brain Perfusion as a Function of Sex
In light of the differential effects by race reported above and the relatively small number of African American males in the current sample (n = 21), sex differences in the relationship between HF-HRV and perfusion were investigated in European American subjects only (n = 362, 183 females). The interaction between sex and HF-HRV was significantly related to proportional cerebral blood flow in three ROIs (see Table 2). The relationship between HF-HRV and proportional cerebral blood flow in the left parahippocampal gyrus varied as a function of sex (β = .294, t(358) = 2.75, p = .006), with the interaction term significantly improving model fit (ΔR2 = .020, R2 = .036, F(3,358) = 4.45, p = .004). Simple slope testing revealed that higher resting HF-HRV was related to less perfusion in women (β=−.261, t(358) = 3.53, p<.001), but not men (β = .032, t(358) = .419, p = .676). The relationship between HF-HRV and proportional cerebral blood flow in the left amygdala also varied as a function of sex (β = .283, t(358) = 2.64, p = .008), with the interaction term significantly improving model fit (ΔR2 = .019, R2 = .034, F(3,358) = 4.19, p = .006). Simple slope testing indicated that higher resting HF-HRV was related to less perfusion in women (β=−.252, t(358) = 3.39, p<.001), but not men (β = .031, t(358) = .407, p = .685). Finally, the relationship between HF-HRV and proportional cerebral blood flow in the right hippocampus varied as a function of sex (β = .270, t(358) = 2.54, p = .012), with the interaction term significantly improving model fit (ΔR2 = .017, R2 = .045, F(3,358) = 5.66, p = .001). Simple slope testing showed that higher resting HF-HRV was related to less per-fusion in women (β=−.273, t(358) = 3.70, p<.001), but not men (β = .004, t(358) = .048, p = .962).
Exploratory Whole Brain Analyses
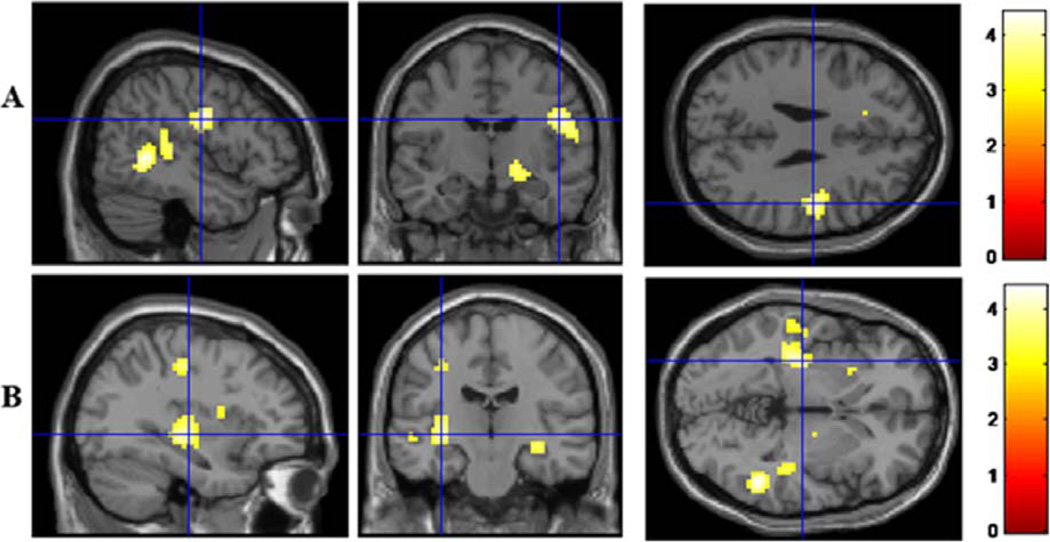
The relationship between HF-HRV and perfusion was further investigated using exploratory whole brain analyses to determine whether HF-HRV was related to any brain regions in addition to the 14 ROIs previously identified in the literature. For the whole sample, two additional clusters of voxels were negatively correlated with HF-HRV (see Table 3). These regions included a cluster of voxels centered at the left putamen and a cluster of voxels centered at the right precentral gyrus (BA 6) that also extended into the right postcentral gyrus (BA 43; see Figure 1). While the left putamen was included in the meta-analyses ROIs analyzed above, the region of the putamen found in these exploratory analyses was more posterior/left lateralized.
Table 3.
Exploratory Whole Brain Analyses of Perfusion and HF-HRV
| MNI coordinates of peak activity |
Cluster volume (mm3) |
Z value of peak activity |
FWE corrected cluster p-value |
|||
|---|---|---|---|---|---|---|
| Brain region | x | y | z | |||
| Putamen | −33 | −19 | −2 | 3753 | 3.97 | 0.04 |
| Precentral gyrus (BA 6) | 48 | −10 | 28 | 3591 | 3.99 | 0.05 |
Note. HF-HRV = high-frequency heart rate variability; MNI = Montreal Neurological Institute; FWE = familywise error; BA = Brodmann area.
Figure 1.
Exploratory whole brain analyses of the main effect of resting HF-HRV revealed a negative correlation between resting HF-HRV and perfusion in two clusters of voxels centered in the right precentral gyrus (BA 6) and left putamen. A: Precentral gyrus. B: Putamen. Blue crosshairs are centered at the voxel of peak activity.
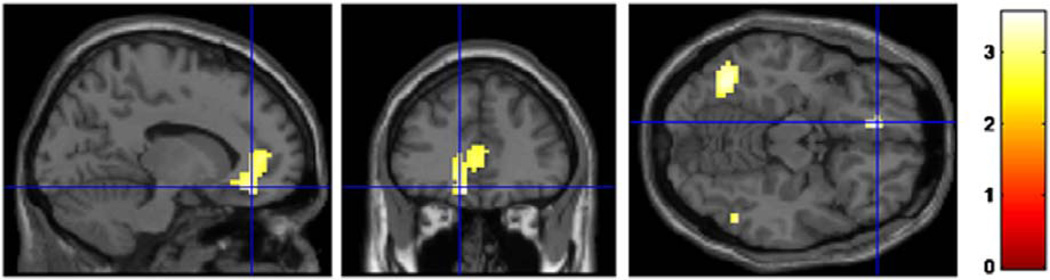
Similar exploratory whole brain analyses were conducted with the sample narrowed to only European and only African American subjects to determine whether the relationship between HF-HRV and perfusion varied as a function of race in any additional brain regions. The interaction between race and HF-HRV predicted one cluster of voxels centered in the left ventromedial prefrontal cortex (vmPFC), which revealed that HF-HRV was negatively correlated with cerebral blood flow in the vmPFC for African Americans, but not European Americans (see Figure 2). This region was centered in the vmPFC but also extended into the ventral and dorsal anterior cingulate. No additional brain regions were found in which HF-HRV correlated with perfusion as a function of sex.
Figure 2.
Exploratory whole brain analyses of the interaction between race and HF-HRV revealed a negative correlation for African Americans only between resting HF-HRV and perfusion in a cluster of voxels centered in the left ventromedial prefrontal cortex (BA 10). This cluster also extended into the ventral (BA 24) and dorsal anterior cingulate (BA 32). MNI coordinates of peak activity: x = −12, y = 38, z = −14; cluster size = 12,717mm3; Z value of peak activity = 3.529; FWE corrected cluster p value = .02. Blue crosshairs are centered at the voxel of peak activity.
Effect of Covariates
The role of several covariates was examined with respect to the relationship between HF-HRV and perfusion. Our main effects reported in Table 2 were independent of smoking status, blood pressure, and physical activity. However, five of the eight relationships between HF-HRV and region perfusion were no longer significant after controlling for age, suggesting that a mechanism related to aging may explain some of the reported relationships between HF-HRV and perfusion. The three regions robustly related to HF-HRV independent of age were the left insula (BA 13), right hippocampus, and right superior temporal gyrus (BA 41). In light of the racial differences in our sample characteristics noted in Table 1, we also determined whether the racial differences in the relationship between HF-HRV and perfusion were independent of age, body mass index (BMI), blood pressure, years of schooling, median household income, and physical activity. However, none of these covariates accounted for the racial differences reported herein.
Discussion
The aim of the present study was to examine the relationship between resting cardiac vagal activity, estimated via HF-HRV, with resting cerebral blood flow, estimated via pulsed arterial spin labeling. Based on previous neuroimaging studies of autonomic reactivity, we tested the hypothesis that higher resting HF-HRV would be related to lower resting regional cerebral blood flows. Specifically, we tested the correspondence between resting HF-HRV and whole brain perfusion, as well as several brain regions outlined in human neuroimaging studies of cardiac vagal reactivity (Beissner et al., 2013; Thayer et al., 2012). Our study offers empirical evidence connecting relatively higher resting cardiac vagal activity with greater total cerebral blood flow, and less cerebral blood flow in some, but not all, tested brain regions. Overall, we found at least partial support for common putative regulatory brain regions between resting HF-HRV and 10 of the 14 brain regions reported in the aforementioned meta-analyses of reactive vagal control. We found no support for the relationship between resting HF-HRV and resting perfusion in the cerebellar declive, dorsal posterior cingulate, or the left precentral gyrus. However, exploratory analyses did show that perfusion in the right pre- and postcentral gyrus was negatively related to resting HF-HRV. In addition, we found evidence of both race and sex differences in the relationship between resting HF-HRV and brain perfusion. Finally, controlling for age accounted for five of the eight regional relationships reported between HF-HRV and cerebral for the whole sample.
Resting Cardiac Vagal Activity and Whole Brain Perfusion: Possible Mechanisms
There are several mechanisms for the relationship between higher resting cardiac vagal activity and greater whole brain perfusion. Three possibilities can be briefly noted. First, regional cerebral blood flow is primarily determined by momentary metabolic demand and regulated via local signaling mechanisms, such as nitric oxide released from activated neurons (Attwell et al., 2010). Nitric oxide participates in the vasodilation of cerebral microvessels and the autoregulation of total cerebral blood flow (Kobari, Fukuuchi, Tomita, Tanahashi, & Takeda, 1994), and has been related to brainstem regions of autonomic cardiovascular control, including the nucleus ambiguus and the nucleus tractus solitarius (see review by Chowdhary & Townend, 1999). Nitric oxide has been shown to augment cardiac vagal activity in humans, as indexed via HF-HRV (Chowdhary et al., 2000). Thus, a coordinated physiological substrate of vasodilation of cerebral microvessels and autonomic cardiovascular control via the action of nitric oxide is consistent with our finding that total cerebral blood flow is associated with higher resting cardiac vagal activity.
Second, direct autonomic innervation of the cerebral vasculature is another potential pathway connecting total cerebral blood flow and cardiac vagal activity. Cerebral arteries are innervated by both sympathetic (superior cervical ganglion) and parasympathetic projections (otic and sphenopalatine ganglia; Goadsby & Edvinsson, 2002; Ter Laan, van Dijk, Elting, Staal, & Absalom, 2013), and cerebral arteries contain both adrenergic and cholinergic receptors, which modulate vascular tone and cerebral blood flow (Hamner, Tan, Lee, Cohen, & Taylor, 2010; Hamner, Tan, Tzeng, & Taylor, 2012; Ter Laan et al., 2013). Sympathetic modulation of vascular tone and cerebral blood flow may be inversely related to excitation of cardiac vagal neurons, and ultimately HF-HRV via sympathetic neuromodulating effects acting on the cholinergic vagal neurons in the nucleus ambiguous (Bateman, Boychuk, Philbin, & Mendelowitz, 2012; Boychuk, Bateman, Philbin, & Mendelowitz, 2011). Our finding that higher HF-HRV is related to greater whole brain perfusion may be indicative of diminished sympathetic adrenergic inhibition in the nucleus ambiguus and less sympathetic adrenergic vasoconstriction of cerebral arteries, resulting in higher resting cerebral perfusion.
Finally, total cerebral blood flow and resting cardiac vagal control may be related to a third, unknown variable. Emerging evidence suggests that total cerebral blood flow is a biomarker of cardiovascular risk (Jennings et al., 2013). Similarly, low levels of HF-HRV have been related to cardiovascular disease (Thayer et al., 2010). Experimental evidence is needed to decipher the extent to which the relationship between total cerebral blood flow and resting cardiac vagal activity can be explained by the aforementioned mechanisms.
Regional Perfusion and HF-HRV
In our whole sample analyses, we found evidence that resting cardiac vagal activity was related to resting perfusion in 8 of the 14 brain regions reported in meta-analyses of central autonomic regulation. Specifically, when assessed as a proportion of mean total cerebral blood flow, less relative perfusion was related to greater resting cardiac vagal activity in the left amygdala, left putamen, right hippocampus, left parahippocampal gyrus, left and right insula, and two subregions of the right superior temporal gyrus. Each of these areas showed regional cerebral blood flows lower than the mean total cerebral blood flow (see supporting information Table S1). Thus, the current negative correlations suggest that relatively more regional cerebral blood flow was associated with relatively less HF-HRV. This is generally suggestive of an inhibitory action of these cortical areas on medullary control of HF-HRV. Such control during the resting state contrasts with the control implied by the reactivity relationships in the studies reviewed by the two meta-analyses. Many of the studies included in these meta-analyses showed that task-induced reductions in neural activity were related to relative task-induced decreases in HF-HRV. In other words, task-induced neural activation was related to task-induced increases in HF-HRV. Thus, these results suggest that, while many regional brain structures associated with cardiac vagal withdrawal are also associated with resting HF-HRV, there may be functional differences between resting and reactive cardiac vagal control. However, this interpretation is speculative as our data did not include reactivity measures. Additionally, the proportional cerebral blood flow values in the current report are not directly comparable to the relative scores reviewed in the aforementioned meta-analyses.
The possible regulatory differences are interesting in the context of the relationship between resting HF-HRV and reactive HF-HRV. For example, prior work has shown that resting cardiac vagal activity is negatively correlated with cardiac vagal reactivity, such that higher cardiac vagal activity at rest is associated with greater task-induced vagal withdrawal (Demaree, Pu, Robinson, Schmeichel, & Everhart, 2006; Salomon, 2005). However, task-induced vagal withdrawal has been shown to be positively correlated with task-induced reductions in regional cerebral blood flow (Gianaros et al., 2004; Napadow et al., 2008). For example, greater vagal withdrawal during working memory tasks was positively correlated with decreases in regional cerebral blood flow in the left insula and left amygdala-hippocampal complex (Gianaros et al., 2004). Our results suggest that task-induced vagal withdrawal would be most pronounced among those individuals with relatively less resting blood flow in these areas relative to their mean total cerebral blood flow. Thus, we might impute that lower resting cerebral blood flow in these areas functionally maintains high levels of resting cardiac vagal activity, while subsequent inhibition of these areas induces cardiac vagal withdrawal. This inference is consistent with the notion that higher resting cardiac vagal activity is related to both lower regional perfusion at rest and greater task-induced regional activation (O’Connor, Gundel, McRae, & Lane, 2007).
In the meta-analysis from which many of the brain regions tested in the current study were derived, Beissner et al., (2013) posited that, independent of task domain, the core of the central autonomic network consists of the left amygdala, right and left insula, and the midcingulate. Conversely, the meta-analysis by Thayer et al. (2012) concluded that the brain regions associated with cardiac vagal reactivity across all tasks included the left amygdala, subgenual anterior cingulate, and pregenual anterior cingulate. The findings from the present study support the conclusion that the left amygdala is generally associated with cardiac vagal activity, even during a resting state. However, in line with the disagreement between the two meta-analyses in terms of which specific region(s) of the cingulate are related to parasympathetic control of the heart, our whole sample analyses failed to show an association between resting HF-HRV and perfusion in the anterior cingulate/medial prefrontal regions. Our analysis of race differences (discussed below), however, did show that in African Americans low resting cardiac vagal activity was related to greater perfusion in regions of the anterior cingulate and ventromedial prefrontal cortex (BA 10), suggesting that sample composition might contribute to differences between studies (for a discussion of dorsal/ventral brain regions and sympathetic/parasympathetic control, see Wager et al., 2009).
These discrepancies might also be an artifact of task-induced reciprocal sympathetic activation, resulting in a difficulty to statistically differentiate brain regions exclusively associated with parasympathetic and sympathetic activity (Berntson, Cacioppo, & Quigley, 1991). For example, the meta-analysis by Beissner et al. (2013) showed evidence that activity in the midcingulate and subgenual anterior cingulate was related to sympathetic reactivity (quantified via skin conductance), but not cardiac vagal reactivity (quantified via HF-HRV). Conversely, the meta-analysis by Thayer et al. (2012) only examined studies reporting HF-HRV, and thus couldn’t distinguish central regulation of different branches of the autonomic nervous system. Furthermore, it isn’t clear whether the meta-analytic techniques employed by these studies are capable of accurately distinguishing between brain regions associated with sympathetic and parasympathetic regulation. Moreover, skin conductance is not a measure of cardiac sympathetic activity, and comparison of the neural correlates of cardiac vagal activity and skin conductance is reminiscent of Cannon’s incorrect notion of nonspecific, diffuse sympathetic arousal (see review by Friedman, 2010). Thus, experiments involving autonomic blockade and neuroimaging methods may be required to provide more convincing evidence than statistical separation of central regulation of sympathetic and parasympathetic activation.
Race Differences in the HF-HRV/Perfusion Relationship
The present study found that in African Americans total cerebral blood flow was not related to resting cardiac vagal activity, yet relatively high resting cardiac vagal activity was related to less regional perfusion in regions of the midcingulate, with perfusion in the medial prefrontal cortex trending toward significance. Resting cardiac vagal activity was not related to perfusion in these regions for European Americans. Considering the majority of studies examined in the aforementioned meta-analyses consisted of people of predominantly European descent, these findings are relatively novel. Furthermore, current understanding of the role of cortical and subcortical structures in autonomic regulation may offer a potential explanation for these racial differences. For example, regions of the cingulate and medial prefrontal cortex are thought to tonically inhibit sympathoexcitatory neurons in the rostral ventrolateral medulla and disinhibition of the vagal efferents in the nucleus ambiguus (Charney, 2003). Thus, the present findings suggest that African Americans with lower resting cardiac vagal activity also have greater resting perfusion in brain regions associated with regulation of sympathetic activity and perhaps processing emotional salience (Kober et al., 2008). However, the present study did not include a metric of resting cardiac sympathetic activity, so this proposed explanation is speculative.
We also reported racial differences in several sample characteristics. Compared to European Americans, African Americans were relatively older, had higher BMI and blood pressure, were less physically active, and had fewer years of schooling and lower median household income. We added each of these variables as covariates to our regression models, but our reported race differences remained significant. Thus, the present data do not explain why these associations were only present for African Americans, but hopefully these findings inspire future research on racial differences in central autonomic control, which may have implications for health disparities in cardiovascular disease.
Sex Differences in the HF-HRV/Perfusion Relationship
Sex differences were found in three of the eight brain regions reported in the whole sample analyses in which a significant relationship was found between regional perfusion and resting cardiac vagal activity. Specifically, in women but not men relatively high resting cardiac vagal activity was related to lower resting cerebral blood flow in regions of the medial temporal lobe, namely, the left parahippocampal gyrus, right hippocampus, and left amygdala. These findings are somewhat consistent with prior reports of sex differences in the neural correlates of emotion processing and task-induced autonomic reactivity. For example, in a study of sex differences in regional brain activation during emotion regulation, women who exhibited greater responses in the left amygdala during an emotion regulation task also reported more negative emotion following the task (Mak, Hu, Zhang, Xiao, & Lee, 2009). In a study of sex differences in the neural correlates of autonomic arousal, task-induced regional cerebral blood flow in the amygdala was positively correlated with vagal reactivity in women, yet negatively correlated in men (Nugent et al., 2011). While we did not replicate the negative relationship in men, this discrepancy may be due to differences between neural regulation of resting autonomic function and task-induced reactivity.
Our findings are also somewhat consistent with prior reports of sex differences in the neural correlates of baroreflex cardiovascular control and cardiovascular responses to brief isometric exercise. For example, in a study of sex differences in forebrain neural patterns associated with baroreceptor unloading, women exhibited attenuated heart rate, sympathetic nerve, and amygdala responses during 35mmHg of lower body negative pressure (Kimmerly, Wong, Menon, & Shoemaker, 2007). In a study of sex differences in neural responses to an isometric handgrip task, women exhibited attenuated heart rate and blood pressure responses, as well as differential activation in a number of brain regions, including the right hippocampus and left parahippocampal gyrus (Wong et al., 2007). One speculative mechanism underlying sex differences in central autonomic control is estrogen. For example, intravenous injection of estrogen has been shown to attenuate cardiovascular and ventilatory responses to central command (Hayes, del Pino, & Kaufman, 2002). Furthermore, injection of estrogen into central autonomic nuclei reportedly reduce renal nerve outflow and increase cardiac vagal efferent activity (Saleh, Connell, & Saleh, 2000). Thus, a neurobiological basis of sex differences in central autonomic control may be at least partially explained by the influence of central and peripheral levels of circulating sex hormones, such as estrogen.
Limitations
While the present study extends prior work on the neural correlates of cardiac vagal reactivity to a focus on the neural correlates of resting cardiac vagal activity, there are certain limitations to the interpretation of our findings. For example, HF-HRV and cerebral blood flow were collected in separate laboratory sessions, roughly 2 weeks apart, which may have attenuated the strength of the reported associations by introducing random error. Furthermore, it is not exactly clear how to interpret resting cerebral blood flow as a metric of neural activity. While we offer speculation on the inverse association between resting HF-HRV and regional cerebral blood flow, for example, the absence of task-evoked changes in cerebral blood flow in our study limited our interpretations. In addition, our analysis of between-subject correlations with PASL estimates of cerebral blood flow differs from that taken by other recent research, which has examined within-subject correlations between resting-state fluctuations in the BOLD signal and spontaneous changes in HF-HRV (e.g., C. Chang et al., 2013), making comparisons between studies difficult. Given the observational nature of our study, in addition to currently available methods in human research, we were restricted in our ability to differentiate afferent versus efferent contributions to the associations reported herein. Thus, while brain-body relationships are often described from a top-down perspective, it is important to remember that afferent vagal fibers likely play a large role in brain-heart neural communication. Furthermore, the role of the sympathetic nervous system was not assessed in our study. Thus, it remains unclear whether the brain regions we examined are related to reciprocal sympathetic and parasympathetic control. Lastly, our examination of sex and race differences were conducted in independent analyses, thus the sex and race differences we report are potentially confounded with each other. While the sample size and composition limited our power to test for a three-way interaction, it is important to note that such an interaction between sex and race could exist and deserves future attention.
Conclusion
Recent neuroimaging studies of central autonomic pathways have often neglected to distinguish between task-induced cardiac vagal withdrawal and resting vagal activity. The goal of the present study was to determine whether brain regions reportedly associated with vagal reactivity were also associated with resting vagal activity, and examine the extent to which these associations varied as a function of sex and race. The results reported here show that many of the brain regions positively correlated with vagal reactivity during tasks involving cognitive, sensory/motor, and emotion processing are also negatively correlated with resting vagal function. Furthermore, some of these relationships may only be present in women and African Americans. Concurrent quantitative measures of regulatory brain regions associated with resting cardiac vagal/sympathetic activity and cardiac vagal withdrawal/sympathetic activation are critical to understand the excitatory/inhibitory relationships in these two functional states. While this study describes these relationships and their caveats, experimental manipulation of the potential mechanisms hypothesized herein are worthy of future attention.
Supplementary Material
Acknowledgments
We gratefully acknowledge the grant support from P01HL040962, RO1HL101959, T32HL007560, and R01HL089850. We also thank Dora Kuan for her assistance with the statistical procedures.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online verson of this article:
Table S1: Means and standard errors of raw and proportional (%) cerebral blood flow.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. New York, NY: Sage; 1991. [Google Scholar]
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Allen JJ, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biological Psychology. 2007;74:243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Boychuk CR, Philbin KE, Mendelowitz D. β adrenergic receptor modulation of neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2012;210:58–66. doi: 10.1016/j.neuroscience.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bär KJ, Napadow V. The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. Journal of Neuroscience. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Mayo Clinic Proceedings. 10. Vol. 68. Philadelphia, PA: Elsevier; 1993. The central autonomic network: Functional organization, dysfunction, and perspective; pp. 988–1001. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Boychuk CR, Bateman RJ, Philbin KE, Mendelowitz D. α1-adrenergic receptors facilitate inhibitory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2011;193:154–161. doi: 10.1016/j.neuroscience.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M. MNI2TAL software. 1999 Retrieved from http://www.mrccbu.cam.ac.uk/Imaging/mnispace.html.
- Britton A, Shipley M, Malik M, Hnatkova K, Hemingway H, Marmot M. Changes in heart rate and heart rate variability over time in middle-aged men and women in the general population (from the Whitehall II Cohort Study) American Journal of Cardiology. 2007;100:524–527. doi: 10.1016/j.amjcard.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. Journal of Cerebral Blood Flow & Metabolism. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Cha YHK, Jog MA, Kim YC, Chakrapani S, Kraman SM, Wang DJ. Regional correlation between resting state FDG PET and pCASL perfusion MRI. Journal of Cerebral Blood Flow & Metabolism. 2013;33:1909–1914. doi: 10.1038/jcbfm.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. Association between heart rate variability and fluctuations in resting-state functional connectivity. NeuroImage. 2013;68:93–104. doi: 10.1016/j.neuroimage.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YW, Lin JD, Chen WL, Yen CF, Loh CH, Fang WH, Wu LW. Metabolic syndrome and short-term heart rate variability in adults with intellectual disabilities. Research in Developmental Disabilities. 2012;33:1701–1707. doi: 10.1016/j.ridd.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatrica Scandinavica. 2003;108:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- Chowdhary S, Townend JN. Role of nitric oxide in the regulation of cardiovascular autonomic control. Clinical Science. 1999;97:5–17. [PubMed] [Google Scholar]
- Chowdhary S, Vaile JC, Fletcher J, Ross HF, Coote JH, Townend JN. Nitric oxide and cardiac autonomic control in humans. Hypertension. 2000;36:264–269. doi: 10.1161/01.hyp.36.2.264. [DOI] [PubMed] [Google Scholar]
- Christie IC, Gianaros PJ. PhysioScripts: An extensible, open source platform for the processing of physiological data. Behavior Research Methods. 2013;45:125–131. doi: 10.3758/s13428-012-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Dolan RJ. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Demaree H, Pu J, Robinson J, Schmeichel B, Everhart E. Predicting facial valence to negative stimuli from resting RSA: Not a function of active emotion regulation. Cognition & Emotion. 2006;20:161–176. [Google Scholar]
- Friedman BH. Feelings and the body: The Jamesian perspective on autonomic specificity of emotion. Biological Psychology. 2010;84:383–393. doi: 10.1016/j.biopsycho.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Friedrich RJ. In defense of multiplicative terms in multiple regression equations. American Journal of Political Science. 1982:797–833. [Google Scholar]
- Gianaros PJ, Marsland AL, Kuan DCH, Schirda BL, Jennings JR, Sheu LK, Manuck SB. An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biological Psychiatry. 2013;75:738–745. doi: 10.1016/j.biopsych.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, van der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. Neurovascular control of the cerebral circulation. Cerebral Blood Flow and Metabolism. 2002;2:172–188. [Google Scholar]
- Goswami R, Frances MF, Shoemaker JK. Representation of somatosensory inputs within the cortical autonomic network. Neuroimage. 2011;54:1211–1220. doi: 10.1016/j.neuroimage.2010.09.050. [DOI] [PubMed] [Google Scholar]
- Habib GB. Reappraisal of heart rate as a risk factor in the general population. European Heart Journal Supplements. 1999;1:2–10. [Google Scholar]
- Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke. 2010;41:102–109. doi: 10.1161/STROKEAHA.109.557132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner JW, Tan CO, Tzeng YC, Taylor JA. Cholinergic control of the cerebral vasculature in humans. Journal of Physiology. 2012;590:6343–6352. doi: 10.1113/jphysiol.2012.245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, Del Pino NBM, Kaufman MP. Estrogen attenuates the cardiovascular and ventilatory responses to central command in cats. Journal of Applied Physiology. 2002;92:1635–1641. doi: 10.1152/japplphysiol.00981.2001. [DOI] [PubMed] [Google Scholar]
- Huston JM, Tracey KJ. The pulse of inflammation: Heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. Journal of Internal Medicine. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Heim AF, Kuan DCH, Gianaros PJ, Muldoon MF, Manuck SB. Use of total cerebral blood flow as an imaging biomarker of known cardiovascular risks. Stroke. 2013;44:2480–2485. doi: 10.1161/STROKEAHA.113.001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001–2006. Annals of Epidemiology. 2010;20:617–628. doi: 10.1016/j.annepidem.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: Application to functional mapping. Magnetic Resonance in Medicine. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- Kimmerly DS, Wong S, Menon R, Shoemaker JK. Forebrain neural patterns associated with sex differences in autonomic and cardiovascular function during baroreceptor unloading. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2007;292:R715–R722. doi: 10.1152/ajpregu.00366.2006. [DOI] [PubMed] [Google Scholar]
- Kobari M, Fukuuchi Y, Tomita M, Tanahashi N, Takeda H. Role of nitric oxide in regulation of cerebral microvascular tone and autoregulation of cerebral blood flow in cats. Brain Research. 1994;667:255–262. doi: 10.1016/0006-8993(94)91503-2. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. American Journal of Physiology-Heart and Circulatory Physiology. 1999;277:H2233–H2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Mazziotta JC. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GF, Furman SA, McCool MF, Porges SW. Statistical strategies to quantify respiratory sinus arrhythmia: Are commonly used metrics equivalent? Biological Psychology. 2012;89:349–364. doi: 10.1016/j.biopsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr., Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability—the ARIC study. American Journal of Cardiology. 1995;76:906–912. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- Mak AK, Hu ZG, Zhang JX, Xiao Z, Lee T. Sex-related differences in neural activity during emotion regulation. Neuropsychologia. 2009;47:2900–2908. doi: 10.1016/j.neuropsychologia.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Marci CD, Glick DM, Loh R, Dougherty DD. Autonomic and prefrontal cortex responses to autobiographical recall of emotions. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(3):243–250. doi: 10.3758/cabn.7.3.243. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosomatic Medicine. 2007;69:709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Bandettini PA. Resting-state fMRI confounds and cleanup. NeuroImage. 2013;80:349–359. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: Combining heart rate variability with fMRI. NeuroImage. 2008;42:169–177. doi: 10.1016/j.neuroimage.2008.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Bain EE, Thayer JF, Sollers JJ, Drevets WC. Sex differences in the neural correlates of autonomic arousal: A pilot PET study. International Journal of Psychophysiology. 2011;80:182–191. doi: 10.1016/j.ijpsycho.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Gündel H, McRae K, Lane RD. Baseline vagal tone predicts BOLD response during elicitation of grief. Neuropsychopharmacology. 2007;32:2184–2189. doi: 10.1038/sj.npp.1301342. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr., Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Medicine and Science in Sports and Exercise. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- Park G, Vasey MW, Van Bavel JJ, Thayer JF. When tonic cardiac vagal tone predicts changes in phasic vagal tone: The role of fear and perceptual load. Psychophysiology. 2014;51:419–426. doi: 10.1111/psyp.12186. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Adriany G, Shmuel A, Yacoub E, De Moortele V, Hu X, Ugurbil K. Perfusion-based high-resolution functional imaging in the human brain at 7 Tesla. Magnetic Resonance in Medicine. 2002;47:903–911. doi: 10.1002/mrm.10154. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MC, Connell BJ, Saleh TM. Medullary and intrathecal injections of 17β-estradiol in male rats. Brain Research. 2000;867:200–209. doi: 10.1016/s0006-8993(00)02313-1. [DOI] [PubMed] [Google Scholar]
- Salomon K. Respiratory sinus arrhythmia during stress predicts resting respiratory sinus arrhythmia 3 years later in a pediatric sample. Health Psychology. 2005;24:68–76. doi: 10.1037/0278-6133.24.1.68. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Wong SW, Cechetto DF. Cortical circuitry associated with reflex cardiovascular control in humans: Does the cortical autonomic network “speak” or “listen” during cardiovascular arousal. Anatomical Record. 2012;295:1375–1384. doi: 10.1002/ar.22528. [DOI] [PubMed] [Google Scholar]
- Ter Laan M, van Dijk JMC, Elting JWJ, Staal MJ, Absalom AR. Sympathetic regulation of cerebral blood flow in humans: A review. British Journal of Anaesthesia. 2013;111:361–367. doi: 10.1093/bja/aet122. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, III, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Arterial spin labeling perfusion fMRI with very low task frequency. Magnetic Resonance in Medicine. 2003;49:796–802. doi: 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- Wong SW, Kimmerly DS, Masse N, Menon RS, Cechetto DF, Shoemaker JK. Sex differences in forebrain and cardiovagal responses at the onset of isometric handgrip exercise: A retrospective fMRI study. Journal of Applied Physiology. 2007;103:1402–1411. doi: 10.1152/japplphysiol.00171.2007. [DOI] [PubMed] [Google Scholar]
- Ye FQ, Berman KF, Ellmore T, Esposito G, van Horn JD, Yang Y, McLaughlin AC. H215O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magnetic Resonance in Medicine. 2000;44:450–456. doi: 10.1002/1522-2594(200009)44:3<450::aid-mrm16>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.