Abstract
Abnormalities in intracellular Ca2+ signaling have been proposed to play an essential role in the pathophysiology of atrial arrhythmias. However, a direct observation of intracellular Ca2+ in atrial myocytes during atrial arrhythmias is lacking. Here, we have developed an ex vivo model of simultaneous Ca2+ imaging and electrocardiographic recording in cardiac atria. Using this system we were able to record atrial arrhythmic intracellular Ca2+ activities. Our results indicate that atrial arrhythmias can be tightly linked to intracellular Ca2+ waves and Ca2+ alternans. Moreover, we applied this strategy to analyze Ca2+ signals in the hearts of WT and knock-in mice harboring a ‘leaky’ type 2 ryanodine receptor (RyR2-R2474S). We showed that sarcoplasmic reticulum (SR) Ca2+ leak increases the susceptibility to Ca2+ alternans and Ca2+ waves increasing the incidence of atrial arrhythmias. Reduction of SR Ca2+ leak via RyR2 by acute treatment with S107 reduced both Ca2+ alternans and Ca2+ waves, and prevented atrial arrhythmias.
Keywords: atrial arrhythmias, intracellular Ca2+ imaging, ex vivo model
1. INTRODUCTION
Atrial arrhythmias, including atrial tachyarrhythmia (AT), atrial flutter and atrial fibrillation (AF), are the most common arrhythmias and are associated with increased cardiovascular morbidity and mortality [1, 2]. The fundamental mechanisms underlying atrial arrhythmias involve triggers in the setting of a vulnerable substrate [3]. Indeed, electric remodeling of atrial tissue plays an essential role in the pathogenesis of atrial arrhythmias [4]. Remodeling increases the probability of generating multiple atrial wavelets by enabling rapid atrial activation and dispersion of refractoriness [5, 6]. Despite a wealth of information concerning the electrical abnormalities present in the working atrial muscle, a detailed understanding of the cellular events underpinning atrial arrhythmias is lacking.
Intracellular Ca2+ plays a central role in the action potential and excitation-contraction coupling in atrial myocytes [7]. Abnormal intracellular Ca2+ handling has been suggested to be a possible factor contributing to abnormality of cellular electricity [7]. Most recently, we and others have reported atrial arrhythmias in knock-in mice with “leaky” type 2 ryanodine receptors (RyR2), the major Ca2+ release channel on sarcoplasmic reticulum (SR) [8–10]. In most of these reports, intracellular Ca2+ activity was measured in isolated atrial myocytes, whereas atrial arrhythmias were detected in vivo or in Langendorff-perfused hearts. There are important limitations to these studies: (i) atrial arrhythmias and intracellular Ca2+ activities were measured under distinct stress conditions; (ii) cellular events without cell-to-cell communication likely differ from in vivo arrhythmias; (iii) no direct link has been established between altered intracellular Ca2+ signaling and atrial arrhythmias.
In the present study, we developed an ex vivo model using Langendorff-perfused murine hearts for simultaneous electrocardiographic (ECG) recording and confocal Ca2+ imaging in atrial myocytes. This new system enabled us to observe atrial intracellular Ca2+ during atrial arrhythmias for the first time. Our results show a direct association between dysregulated intracellular Ca2+ and atrial arrhythmias.
2. METHODS
Detailed methods, including imaging of atrial intracellular Ca2+ in Langendorff-perfused hearts, bipolar ECG recording and programmed electrical stimulation, are provided in the Online Data Supplement.
2.1 Acute S107 treatment
For acute S107 treatment, 10 μmol/L S107 was added to perfusion buffer and loading buffer. Langendorff hearts were perfused for 1.5 hours, then loaded with Quest Rhod-4 AM and washed out in the presence of S107. Vehicle treatment was performed according to the same time protocol.
2.2 Digital image processing and data analysis
All confocal digital images were processed using custom designed digital algorithms coded in Interactive Data Language (IDL). Data are presented as mean ± s.e.m. Student’s t test (for two groups) or one-way ANOVA analysis with Bonferroni post test (for more than 2 groups) was applied to determine statistical significance. A p value less than 0.05 was considered statistically significant.
3. RESULTS AND DISCUSSIONS
We used a Langendorff-perfusion system with heating coils, a glass-bottom perfusion chamber with an adjusted clamp to fix the perfusion tube and a micromanipulator to place the stimulating electrodes in the atria. The chamber and micromanipulator were placed on the stage of an inverted Zeiss 5 live confocal microscope (Fig. 1A). The right atrial recording electrodes were approximately 25-μm in diameter and tightly adhered to the glass coverslip in order to have minimal effect on the confocal imaging. We used a recently developed Ca2+ indicator, Quest Rhod-4 AM (AAT Bioquest, Sunnyvale, CA, USA), which is much brighter than the widely used Rhod-2 AM. To enhance the loading of Rhod-4 AM into atria 0.05% Pluronic F-127 was added to the loading perfusate. A 15-minute perfusion with 10 μM Rhod-4 AM achieved ideal loading in atrial myocytes as well as ventricular myocytes (Fig. S1). Using fast frame scan (Fig. S3) or linescan (Fig. 1B) we could simultaneously observe the intracellular Ca2+ from more than 20 atrial myocytes while recording the bipolar ECG from the atrium. Of note, other groups had previously attempted to use intact heart atria for electrophysiological examination. However, Ca2+ signals were measured in isolated atrial myocytes [11] or using optical mapping technologies for Ca2+ measurement [12]. Due to their low resolution (~1mm) these technologies allow imaging of “regional” Ca2+ but not Ca2+ at the cellular level. In our innovative model we used confocal imaging which allows the measurement of intracellular Ca2+ in intact heart atria and we simultaneously recorded a bipolar ECG, thereby detecting atrial intracellular Ca2+ activities during atrial arrhythmias.
Figure 1. Ex vivo model for simultaneous bipolar ECG recording and intracellular Ca2+ imaging in atrium.
A. A close-up of the model system. A Langendorff-perfused heart was placed on the imaging chamber with the right atrium downwards on the glass bottom. 1, a pair of silver electrodes for ECG recording of ventricle; 2, a pair of platinum electrodes for pacing the left atrium; 3, perfusion tube; 4, a pair of 25-μm-diameter platinum electrodes for bipolar ECG recording of right atrium; 5, objective of the confocal microscope. B. representative X-Y scan and X-T scan of the atrium. C, D. Bipolar ECG recording and intracellular Ca2+ imaging of a right atrium of RyR2-R2474S+/− mouse during burst atrial pacing induced atrial arrhythmic event. The upper, middle and lower traces show simultaneous atrial intracellular Ca2+ imaging, right atrial and ventricular bipolar ECG recording, respectively. The arrhythmic event has been split into initiation (C) and disappearance (D) part with some overlap labeled by the green lines above the linescan images. The red dotted line indicates the application of the 2-second burst pacing. E. Quantitative analysis of the relationship between arrhythmic atrial beating rate and the number of atrial myocytes exhibiting intracellular Ca2+ waves during atrial arrhythmias events. The arrhythmic events were divided into 2 groups according to the atrial beating rate ≤ or >1500 bpm.
We previously examined the properties of subcellular Ca2+ release using single atrial myocytes isolated from WT and knock-in mice harboring a ‘leaky’ RyR2 (RyR2-R2474S+/−) with a mutation linked to human catecholaminergic polymorphic ventricular tachycardia (CPVT) [8]. Here, we successfully recorded the atrial intracellular Ca2+ activity during an atrial arrhythmia. Fig. 1C and 1D display a representative complete burst pacing-induced atrial arrhythmic event with simultaneous linescan Ca2+ images (upper tracing) from the right atrium and the bipolar ECG from right atrium (middle tracing) and ventricle (lower tracing) from a RyR2-R2474S+/− mouse. During 2-second burst pacing, Ca2+ waves initialized in many myocytes and lasted after stopping pacing. The atrium was stimulated into arrhythmia with beating rate > 1600 bpm (Fig. 1C). After about 2 seconds, most of the Ca2+ waves gradually disappeared and the atrial beating rate slowed down. After lasting for about 4.8 seconds, the arrhythmic event stopped with a ~1-second pause in the atrium before sinus rhythm recovery (Fig. 1D). Fig. S4 depicts another atrial arrhythmia event with slower rate (~1000 bpm) at the beginning. Ca2+ transient alternans were observed in most atrial myocytes when atrial arrhythmias occurred. Typically the Ca2+ alternans resolved after ~2 seconds and arrhythmia resolved accordingly. We used fast X-Y scans at a speed of 100 frames/second to record intracellular Ca2+ in RyR2-R2474S+/− mice. Online videos S1a and S1b display Ca2+ fluorescence in a region from the right atrium before and during an atrial arrhythmia for which the corresponding atrial ECG is shown in Fig. S2a, b. When atrial burst pacing failed to stimulate the heart into an atrial arrhythmia there was an orderly Ca2+ response to the sinoatrial nodal signal. When the heart was stimulated into an atrial arrhythmia lasting for more than 10 seconds, chaotic Ca2+ waves occurred in atrial myocytes.
An important parameter of atrial arrhythmias is the atrial arrhythmic rate (i.e. atrial beating rate during atrial arrhythmias) [2]. We explored the relationship between atrial beating rate and atrial arrhythmic intracellular Ca2+ for 30 atrial arrhythmic events from the hearts of RyR2-R2474S+/− mice. We divided these events into 2 groups using as a cut-off the atrial rate of 1500 bpm (beat per minute). For each event, we counted the myocytes with Ca2+ waves in the first 2 second right after arrhythmia occurred. A higher percentage of atrial myocytes in the group with the high arrhythmic rate (> 1500 bpm) displayed obvious Ca2+ waves compared with the group with low arrhythmic rate (≤ 1500 bpm) (56% vs 31%, n = 14, 16 in high and low rate groups respectively, p<0.01, Fig. 1E).
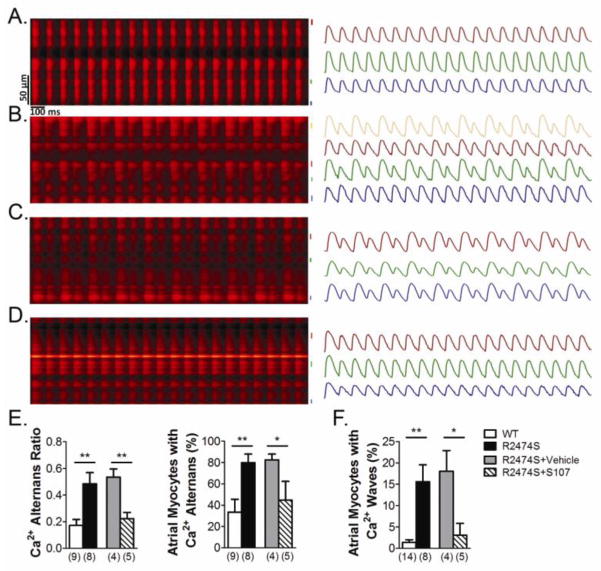
We quantitatively assessed the susceptibility to elicit Ca2+ waves and Ca2+ alternans in the right atria of RyR2-R2474S+/− mice compared to WT littermates. For Ca2+ alternans measurements, a series of 10 Hz electric field stimulation was applied to the left atrium via the platinum pacing electrodes which captured the cardiac rhythm. The Ca2+ alternans ratio (AR, AR = 1−AL/AH, where AL and AH denote the lower and higher Ca2+ transients amplitude) was used as a parameter to quantify the Ca2+ alternans in the different groups [13]. Upon pacing, as many as 80% of atrial myocytes in the hearts of RyR2-R2474S+/− mice displayed Ca2+ alternans (AR > 0.2) with an average AR of 0.49, while only one third of atrial myocytes in the hearts of WT mice exhibited Ca2+ alternans, with an average AR of 0.17 (n = 9 and 8 hearts in WT and RyR2-R2474S groups respectively, p<0.01) (Fig. 2A–E). To induce Ca2+ waves in atrial myocytes, we perfused the heart with 1 μmol/L isoproterenol for 30 minutes. We then randomly selected different regions to record the atrial intracellular Ca2+ activities with 3-second-duration fast frame imaging. Ca2+ waves occurred in more than 15% of atrial myocytes from RyR2-R2474S+/− mice compared to 2% of atrial myocytes from WT mice (n = 14 and 8 hearts in WT and RyR2-R2474S groups, respectively, p<0.01) (Fig. 2F and online videos S2a, b). No atrial arrhythmias occurred in RyR2-R2474S+/− hearts under these conditions.
Figure 2. Ca2+ alternans and Ca2+ waves in right atrial cardiomyocytes.
A–D, representative images of 10 Hz pacing-induced Ca2+ alternans in right atria of WT (A), RyR2-R2474S+/− (B) and Vehicle (C) or S107 treated (D) RyR2-R2474S+/− mice. The right panel displays the Ca2+ transients in atrial myocytes. E, Quantification (right: ratio, left: percent) of Ca2+ alternans in the indicated groups. F, Ca2+ waves in atrial myocytes in the indicated groups. Hearts were perfused with 1 μmol/L isoproterenol for 30 minutes to induce Ca2+ waves. The number of hearts examined in each group is labeled below each column. *: p< 0.05, **p< 0.01.
During atrial arrhythmias the atrial rate greatly increases. The propagation properties of Ca2+ waves during atrial arrhythmia are significantly different from those during non-atrial arrhythmias (Fig. S7). During atrial arrhythmias Ca2+ waves initiate at different sites in each atrial myocyte thus the average propagation distance is reduced. The increase in propagation velocity of Ca2+ waves further indicates increased propensity for Ca2+ waves development during atrial arrhythmias. However, the propagation properties of the Ca2+ waves during atrial arrhythmias are independent from atrial arrhythmic rate. Previous studies have shown distinct electric mechanisms for atrial arrhythmias including ectopic foci, micro- and macroreentry involving small or large activation areas [14, 15]. It has been shown that atrial arrhythmias based on macroreentrant mechanism usually display a faster rate than arrhythmias with ectopic foci and microreentry mechanism [14, 15]. Interestingly, our findings indicate that more atrial myocytes develop intracellular Ca2+ waves during atrial arrhythmias with faster rates. Taken together, our findings support the hypothesis that atrial intracellular Ca2+ waves are a contributing factor to atrial arrhythmias.
S107 is a RyR targeted compound that inhibits stress-induced dissociation of the stabilizing subunit calstabin 2 from RyR2, resulting in a reduction in diastolic SR Ca2+ leak [8]. We examined the effects of S107 on Ca2+ handling and atrial arrhythmias in the atria of perfused hearts. The hearts from RyR2-R2474S+/− mice were perfused with 10 μmol/L S107 for 2 hours before imaging. Compared to vehicle treatment, S107 treatment significantly decreased Ca2+ AR (Fig. 2C–E) and stimulation-induced Ca2+ waves (Fig. 2F, online video S2c) in the right atria of RyR2-R2474S+/− mice. Moreover, atrial burst pacing induced no atrial arrhythmias in four S107-treated hearts of RyR2-R2474S+/− mice, while 3 out of 4 hearts in vehicle group were stimulated into atrial arrhythmias. Our results support the hypothesis of a direct association between diastolic SR Ca2+ leak and atrial arrhythmias.
In summary, we have developed an ex vivo model of simultaneous Ca2+ imaging and bipolar ECG recording in cardiac atria which allows monitoring of arrhythmic intracellular Ca2+ signaling.
Supplementary Material
Highlights.
We present an ex vivo model to direct observe atrial arrhythmic intracellular Ca2+.
Atrial arrhythmias can be tightly linked to intracellular Ca2+ waves and alternans.
SR Ca2+ leak increases the susceptibility to Ca2+ alternans and Ca2+ waves in atria.
S107 treatment decreases Ca2+ alternans and waves, and prevents atrial arrhythmias.
Acknowledgments
SOURCES OF FUNDING
This work was supported by AHA (13POST16810041) to G.S. and NIH (R01HL102040) to A.R.M.
Footnotes
DISCLOSURES
A.R.M. is a consultant and member of the board of ARMGO Pharma, Inc., which is targeting RyR channels for therapeutic purposes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zoller B, Ohlsson H, Sundquist J, Sundquist K. High familial risk of atrial fibrillation/atrial flutter in multiplex families: a nationwide family study in Sweden. Journal of the American Heart Association. 2013;2:e003384. doi: 10.1161/JAHA.112.003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Lin YJ, Higa S, Tai CT, Chang SL, Lee KT, Lo LW, et al. Role of the right atrial substrate in different types of atrial arrhythmias. Heart Rhythm. 2009;6:592–8. doi: 10.1016/j.hrthm.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Santulli G, D’Ascia C. Atrial remodelling in echocardiographic super-responders to cardiac resynchronization therapy. Heart. 2012;98:517. doi: 10.1136/heartjnl-2012-301731. [DOI] [PubMed] [Google Scholar]
- 5.Yu WC, Chen SA, Lee SH, Tai CT, Feng AN, Kuo BI, et al. Tachycardia-induced change of atrial refractory period in humans: rate dependency and effects of antiarrhythmic drugs. Circulation. 1998;97:2331–7. doi: 10.1161/01.cir.97.23.2331. [DOI] [PubMed] [Google Scholar]
- 6.D’Ascia SL, D’Ascia C, Marino V, Lombardi A, Santulli R, Chiariello M, et al. Cardiac resynchronisation therapy response predicts occurrence of atrial fibrillation in non-ischaemic dilated cardiomyopathy. Int J Clin Pract. 2011;65:1149–55. doi: 10.1111/j.1742-1241.2011.02732.x. [DOI] [PubMed] [Google Scholar]
- 7.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 8.Shan J, Xie W, Betzenhauser M, Reiken S, Chen BX, Wronska A, et al. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111:708–17. doi: 10.1161/CIRCRESAHA.112.273342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, et al. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res. 2012;110:465–70. doi: 10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Fraser JA, Jeevaratnam K, Hao X, Hothi SS, Grace AA, et al. Acute atrial arrhythmogenicity and altered Ca(2+) homeostasis in murine RyR2-P2328S hearts. Cardiovasc Res. 2011;89:794–804. doi: 10.1093/cvr/cvq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Fraser JA, Schwiening C, Killeen MJ, Grace AA, Huang CL. Acute atrial arrhythmogenesis in murine hearts following enhanced extracellular Ca(2+) entry depends on intracellular Ca(2+) stores. Acta Physiol (Oxf) 2010;198:143–58. doi: 10.1111/j.1748-1716.2009.02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee P, Taghavi F, Yan P, Ewart P, Ashley EA, Loew LM, et al. In situ optical mapping of voltage and calcium in the heart. PLoS One. 2012;7:e42562. doi: 10.1371/journal.pone.0042562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aistrup GL, Kelly JE, Kapur S, Kowalczyk M, Sysman-Wolpin I, Kadish AH, et al. Pacing-induced heterogeneities in intracellular Ca2+ signaling, cardiac alternans, and ventricular arrhythmias in intact rat heart. Circ Res. 2006;99:e65–73. doi: 10.1161/01.RES.0000244087.36230.bf. [DOI] [PubMed] [Google Scholar]
- 14.Patel A, Markowitz SM. Atrial tachycardia: mechanisms and management. Expert Rev Cardiovasc Ther. 2008;6:811–22. doi: 10.1586/14779072.6.6.811. [DOI] [PubMed] [Google Scholar]
- 15.Saoudi N, Cosio F, Waldo A, Chen SA, Iesaka Y, Lesh M, et al. A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases; a Statement from a Joint Expert Group from The Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 2001;22:1162–82. doi: 10.1053/euhj.2001.2658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.