Abstract
Background
Hand transplantation has received international attention in recent years; however, the economic impact of this innovative treatment is uncertain. The aim of this study is to assess the utility and estimate the costs of hand transplantation and the use of hand prostheses for forearm amputations.
Methods
100 medical students completed a time trade-off survey to assess the utilities of single and double hand transplantation and the use of hand prostheses. Quality-adjusted life years (QALYs) were calculated for each outcome to create decision trees. Cost data for medical care were estimated based on Medicare fee schedules using the Current Procedural Terminology code for forearm replantation. The cost of immunosuppressive therapy was estimated based on the wholesale price of drugs. The incremental cost-utility ratio (ICUR) was calculated from the differences in costs and utilities between transplantation and prosthesis. Sensitivity analyses were performed to assess the robustness of the results.
Results
For unilateral hand amputation, prosthetic use was favored over hand transplantation (30.00 QALYS vs. 28.81 QALYs; p = 0.03). Double hand transplantation was favored over the use of prostheses (26.73 QALYs vs. 25.20 QALYs; p = 0.01). The ICUR of double transplantation when compared with prostheses was $381,961/QALY, exceeding the traditionally accepted cost-effectiveness threshold of $50,000/QALY.
Conclusion
Prosthetic adaption is the dominant strategy for unilateral hand amputation. For bilateral hand amputation, double hand transplantation exceeds the societally acceptable threshold for general adoption. Improvements in immunosuppressive strategies may change the ICUR for hand transplantation.
Keywords: cost-utility analysis, hand transplantation, outcomes, policy
Hand transplantation is the only reconstructive procedure available to treat hand amputation. Following the first successful case in 1998, 42 additional hand transplantations have been performed worldwide. All 43 have achieved graft survival at one year, and the eventual return of protective sensation and some intrinsic muscle function.(1–11) Although both sensory and motor function remain limited, it has been suggested that hand transplantation may be a viable option for managing hand amputation.(9) The risk-benefit tradeoff, however, remains uncertain.(12)
Immunosuppression is essential for the successful survival of the transplanted hand. Experience with solid organ transplantation has shown that long-term immunosuppressive therapy can cause serious complications, such as opportunistic infection, diabetes mellitus and development of malignancy.(13, 14) For this reason, prescribing continuous immunosuppressive medication for a non-life threatening condition, as is done in hand transplantation, is ethically controversial.(15–19) Before deciding to perform hand transplantation, the risks associated with immunosuppression must be weighed against the functional, cosmetic and psychological benefits to be gained.(13, 20) Unfortunately, due to the lack of evidence-based outcomes data, patients and physicians depend largely on their individual expectations of the outcome and their acceptance of the risks when deciding whether to undergo hand transplantation.
Generally, after hand amputation a prosthetic device is adopted to assist in activities of daily living. Although some patients use prostheses, others abandon them upon finding them uncomfortable or not particularly useful.(21–23) A direct comparison between the outcomes of hand transplantations and prostheses for hand amputation would be helpful in guiding future recommendations. However, it is both ethically and practically impossible to perform a randomized controlled trial to investigate the differences in outcomes between these treatments. Decision analysis, a technique for assessing the utility of, or preference for, potential treatment options, can be applied to evaluate treatment methods when clinical trials cannot be performed.
In the face of mounting financial constraints in healthcare, new treatments will be examined not only for their potential to enhance quality of life, but also for cost. Because hand transplantation has not yet been widely performed, the economic impact of this procedure remains unclear. Utilities derived from a decision analysis and cost data obtained from a financial assessment can be factored into a cost-utility model. A cost-utility analysis, which includes both direct and indirect costs, is particularly well-suited for objectively considering this difficult treatment choice.(24–27) The purpose of this study is to conduct a cost-utility analysis comparing unilateral and bilateral hand transplantation and prosthetics.
Materials and Methods
Study design
Forty-three cases of hand transplantation, all comprehensively reported by the International Registry on Hand and Composite Tissue Transplantation (IRHCTT), were examined to identify the most frequent outcomes and complications of the procedure.(9) The immunosuppressive regimen for hand transplantation is similar to that for solid organ transplantation.(14) Therefore, we assume that the risks of immunosuppression associated with hand transplantation would be similar to the risks associated with immunosuppression following kidney transplantation.(14, 18, 28)
We categorized complications of hand transplantation into four states: death, major complications, minor complications and graft loss due to rejection. Major complications included potentially life-threatening conditions and diseases requiring inpatient care such as opportunistic infection, diabetes mellitus, post-transplant lymphoproliferative diseases, leukopenia, and acute rejection. Minor complications included hypertension, elevated creatinine, anemia, and diarrhea. As is inherent to decision tree design, it was assumed that all outcomes are mutually-exclusive and as such a patient only experiences one complication category.(29) To calculate costs for graft loss, we assumed that the graft would be lost two weeks after transplantation and that the patient experienced no surgical or immunosuppressive complication during this time. In the event of patient death due to immunosuppression complications, it was assumed that immunosuppressive medication was taken for two weeks.
Utility Survey
Utility is the value assigned to a particular health state on a scale of 0 to 1, with 0 representing death and 1 representing perfect health.(30) In this study we derived utility using a time-trade off (TTO) survey, which offers respondents the choice of living for x years in perfect health or t years in a less desirable health state. Utilities are then calculated as x/t. For example, our survey asks the respondent to choose between living for 40 years with a prosthetic hand and living for x years with a healthy hand. The value of x is varied until the respondent feels that the choices are equivalent. If the respondent judges that living for 40 years with a prosthetic hand is equivalent to living for 20 years with a healthy hand, the utility of living with a prosthetic hand is calculated as 20/40, or 0.50.
In our survey, participants were asked to imagine as vividly as possible that they had experienced an amputation of the dominant hand or bilateral hand amputations. Each scenario was presented with an outcome, a potential complication and photographs of hand prosthesis and hand transplantation for unilateral and bilateral hand amputation. Function and complications of hand transplantation were based on data obtained from the literature.(9–11, 14) For the purposes of eliciting utility of prostheses, we selected a body-powered prosthesis, one of the most popular devices amongst hand amputees.(22) The function and complications of body-powered prostheses were based on a previously published systematic review article.(22) This study was approved by the Institutional Review Board at the University of Michigan.
Quality-Adjusted Life Years (QALYs)
QALYs represent a measure of utility following a particular procedure. The QALY calculation factors the utility of a health state and that state’s duration. To translate utilities to QALYs, we assumed a remaining life span of 40 years, based on the national Vital Statistics Expectation of Life data for a 35-year-old male.(31) The age and gender of the average hand transplant patients was previously determined to be a 34-year-old male.(9) Sample QALY calculation is shown in Figure 1
Figure 1.
QALY calculation
Costs
All cost calculations reflect the societal perspective and are based on Medicare fee schedules. Costs are assigned by the Current Procedural Terminology (CPT) code for a procedure and the 2009 conversion factor. (32)
Physician’s Fee Schedule
Surgeon and anesthesia fee schedules were obtained based on Medicare Resource Based Relative Value Scale (RBRVS).(33) For this analysis, we used the CPT code for forearm replantation (20805) because one for hand transplantation does not exist. The facility fee schedule was used in all cost calculations because it is a valid assumption that both hand transplantation and amputation after graft loss (25900) will be carried out at an inpatient hospital. For this cost analysis, we assume that unilateral and simultaneous bilateral transplantation would be performed by two and four surgeons, respectively, which may be an underestimation of the workforce requirement.
Calculating the anesthesia fee schedule was done in a manner that factors in both the base units assigned by the anesthesia CPT code (01840) and the time units that reflect the length of time the patient is under the supervision of anesthesiologists. We estimated average time units for the procedures at 48 time units (12 hours) for the hand transplantation and 4 time units (1 hour) for amputation of the rejected graft. Preoperative and postoperative care time was estimated at 2 time units (30 minutes) each. Preoperative psychiatric evaluation (90801) was included in the treatment course. Postoperative clinic visits (99212) were assumed to be performed on a monthly basis until death or graft loss, based on a recommendation for outpatient monitoring of kidney transplantation recipient.(34)
Hospital and Clinic Costs
Inpatient care cost was estimated following the Acute Inpatient Prospective Payment System (PPS) for Medicare using Medicare-Severity Diagnosis-Related Group (MS-DRG).(35) We utilized the MS-DRG of a forearm replantation (484) to estimate inpatient cost of hand transplantation. To estimate hospitalization cost for amputation of the rejected arm, we applied the MS-DRG for forearm amputation (476). Outpatient clinic costs were estimated based on the Hospital Outpatient PPS using Ambulatory Payment Classification (APC) (605) for Medicare.(36)
Prosthesis
The cost of a body-powered prosthesis with a terminal device was estimated using L-codes (L-6100, 6680, 6706, 7400, 7403, 8435) based on Medicare Durable Medical Equipment, Prosthetics, Orthotics and Supplies 2009 Fee Schedule for the State of Michigan. Based on expert opinions from the Orthotics & Prosthetics Center at the University of Michigan, we assumed that the prosthesis replacement would be required every four years.
Immunosuppressive Therapy
We estimated immunosuppressive medication costs using the wholesale prices of the medications.(37) We assumed that a maintenance immunosuppressive medication regimen used a triple drug combination of tacrolimus (4–8 mg/day), mycophenolate mofetile (MMF) (2g/day) and prednisolone (10mg/day) based published series (1, 7, 10). Immunosuppressive medication may change with better understanding of the unique requirements of hand transplantation. Maintenance immunosuppressive medication would be continued for the duration of the patient’s lifespan or graft survival.
Treatment of Complication
Calculating the cost of every potential treatment option for a variety of complications is unwieldy and impractical. Because diabetes mellitus and hypertension are common complications associated with immunosuppressive medication, we estimated the costs of treating these two complications. The treatment cost for diabetes attributable to maintenance immunosuppression regimens has been estimated to be $2,025 and $3,308 per patient at 1 and 2 years after transplantation, respectively.(38) We used the cost of treating immunosuppression–related diabetes as a proxy for the cost of treating major immunosuppressive complications. The wholesale price of losartan potassium (50mg/day, $2.12/50mg), for the treatment of hypertension, was used as the cost of minor immunosuppressive complications.(37)
Discounting and inflation
In economic analysis, the time value of money necessitates discounting future cost.(39) This is particularly important for studies that assess long-term cost due to the effect of compounding. In the present study, immunosuppressive therapy, treatment of complications and the cost of prostheses require discounting to calculate the future expenditures because these clinical states are assumed to be continued until graft loss or patient death. We discounted at 3% following the recommendation of the Panel on Cost-Effectiveness in Health and Medicine.(40) We adjusted the cost data of diabetes treatment, which were based on the financial record from 1994 to 1998 to project the cost in 2009 dollars using the CPI inflation calculator available from the Bureau of Labor Statistics.(41)
Productivity Loss
The cost of productivity loss is estimated using the average US hourly wage, as obtained from the US Bureau of Labor Statistics for May of 2007.(42) The average weekly earnings for all occupations are $782.40 (assuming a 40-hour work week). Our study assumed that patients returned to full-time work 1 year after hand transplantation and 12 weeks after amputation.
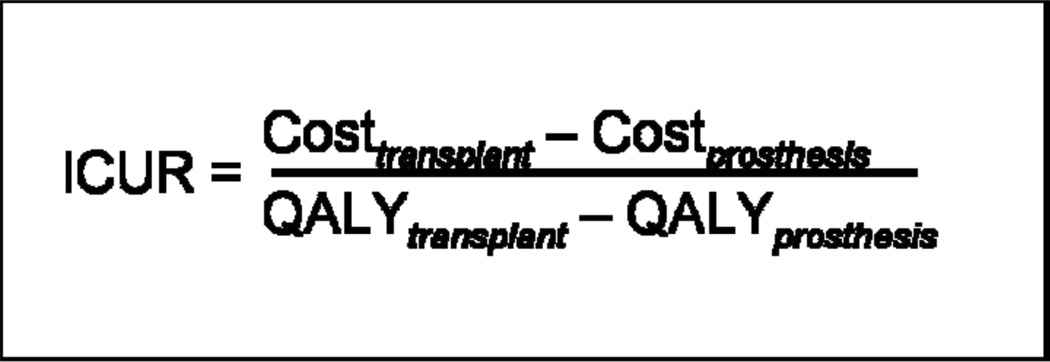
Incremental Cost-Utility Ratio (ICUR)
ICUR expresses the additional cost per QALY gained by using one treatment option versus another. This allows for the assessment of interventions that are preferred but are more costly than the alternatives. The ICUR of hand transplantation is derived from the differences of costs and QALYs between transplantation and prosthesis (Figure 2).
Figure 2.
ICUR calculation
Statistical Analysis
The data were analyzed using SAS 9.1 (SAS Institute, Cary, NC) statistical analysis software. Mean utility and QALY value with 95% confidence interval were calculated and differences between the utilities and QALYs of each health state were compared using Wilcoxon signed rank sum tests. Significance was set at p=0.05.
Sensitivity Analysis
Assumptions are inherent to cost-utility analyses. For this cost-utility analysis, we relied on risk estimates from published systematic reviews and cost data from Medicare reimbursement information. It would be nearly impossible to calculate the exact risk or exact cost in any scenario. Sensitivity analyses are used to test the assumptions used in the model in order to examine the effects of changes in variables such as complication risk rate, cost or complication time. We performed sensitivity analyses to determine the effect of varying the probability of complications and varying the expected utility of bilateral hand transplantation on QALYs.
Results
Utility Survey
After extensive pilot testing, 100 second, third and fourth-year University of Michigan Medical School students completed an anonymous, web-based TTO survey (Appendix 1). The student body at this institution is approximately 53% female. Nearly 13% of students are underrepresented minorities. The average age of students is 23.3 years. Students were paid $20 for their participation.
For both unilateral and bilateral hand amputation, participants assigned the highest utility to transplantation with minor complications (unilateral = 0.78; bilateral = 0.73) and the lowest utility to transplantation with major complications (unilateral = 0.59; bilateral = 0.53). For the use of prosthetic devices, there was a statistically significant difference between the utility assigned for unilateral prosthesis and bilateral prostheses (0.75 vs. 0.63; p<0.0001).
Mean utility values are presented in Table 1.
Table 1.
Mean utilities and QALYs with 95% Confidence Interval
| Scenarios | Utility* | QALYs# | ||
|---|---|---|---|---|
| Unilateral Hand Amputation | ||||
| No Hand Transplant | ||||
| Prosthesis | 0.75 (0.72–0.79) | 30.00 (28.66–31.64) | ||
| Hand Transplant | ||||
| Minor Immunosuppresion Complications | 0.78 (0.75–0.81) | 31.20 (29.83–32.32) | ||
| Major Immunosuppresion Complications | 0.59 (0.55–0.63) | 23.60 (21.55–25.02) | ||
| Graft Failure | 0.73 (0.69–0.76) | 29.20 (27.68–30.55) | ||
| Bilateral Hand Amputation | ||||
| No Hand Transplant | ||||
| Prosthesis | 0.63 (0.59–0.67) | 25.20 (23.54–26.72) | ||
| Hand Transplant | ||||
| Minor Immunosuppresion Complications | 0.73 (0.69–0.77) | 29.20 (27.66–30.60) | ||
| Major Immunosuppresion Complications | 0.53 (0.49–0.58) | 21.20 (19.70–23.06) | ||
| Graft Failure | 0.62 (0.58–0.66) | 24.80 (23.24–26.59) | ||
1.0 is perfect health
QALYs are based on 40 remaining healthy years
Decision Analytic Model
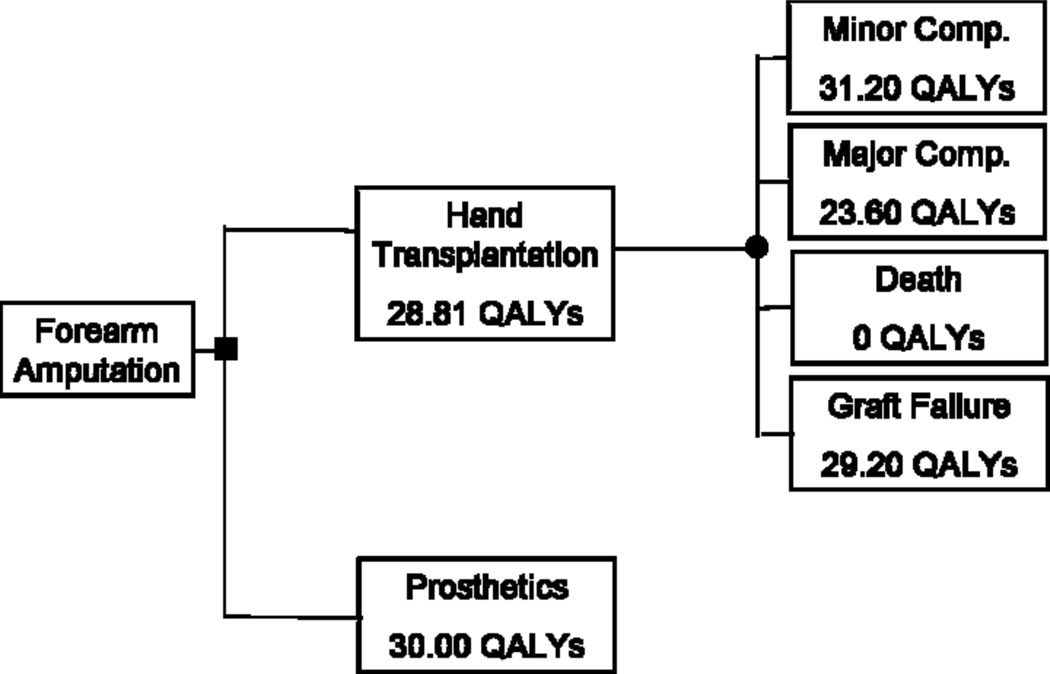
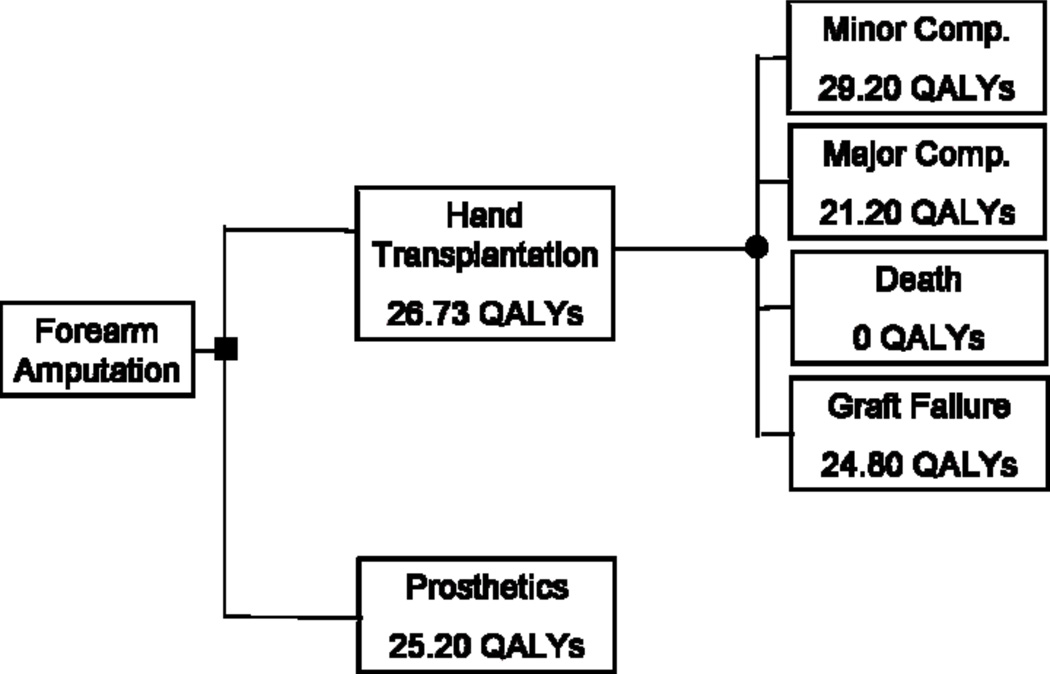
The probabilities of graft loss, minor complication, major complication and death were 0.02, 0.74, 0.23, and 0.01 respectively. Utility values were entered into TreeAge decision analysis software (TreeAge Software, Inc., Williamstown, Mass) to generate decision trees, which account for the utility assigned to each health state as well as the probability of each occurring. (Figures 3 & 4)
Figure 3.
Decision tree for unilateral hand amputation
Figure 4.
Decision tree for bilateral hand amputation
The decision tree demonstrated that for unilateral amputation, prosthesis usage was preferable to hand transplantation. Prosthesis usage was associated with a gain of 1.19 QALYs over hand transplantation (30.00 QALYS vs 28.81 QALYs; p = 0.03). For bilateral hand amputation, participants favored hand transplantation over prosthesis usage. Hand transplantation was associated with a gain of 1.53 QALYs over the use of prostheses (26.73 QALYs vs 25.20 QALYs; p = 0.01).
Costs
Total costs for each health states are shown in Table 2. Lifetime costs for single hand transplantation average $528,293, whereas costs for double hand transplantation average $529,315. Total costs of prosthesis adoption for unilateral and bilateral amputation are $20,653 and $41,305, respectively. The mean surgical cost, including preoperative evaluation, hospitalization and physician fee, are $13,796 for single hand transplantation and $14,608 for double hand transplantation. The cost of immunosuppressive therapy for 40 years, including drugs and clinic visit, is $433,283 ($362,894–503,672). The cost of productivity loss for hand transplantation and prosthetic adaptation are $42,265 and $9,753, respectively.
Table 2.
Total cost of hand transplantation and prosthetic adaptation
| Single | Double | |
|---|---|---|
| Hand transplantation Range | $528,293 ($457,904–598,682) | $529,315 ($458,926–$599,704) |
| Graft loss Range | $52,204 ($52,091–52,317) | $74,066 ($73,953–74,179) |
| Minor complication Range | $532,928 ($462,539–603,317) | $533,740 ($463,351–604,129) |
| Major complication Range | $578,757 ($495,621–661,894) | $579,570 ($496,433–662,706) |
| Death Range | $14,494 ($14,381–14,607) | $15,306 ($15,193–15,420) |
| Prosthesis | $20,653 | $41,305 |
ICUR
Because prosthesis usage is favored for unilateral hand amputation over hand transplantation, and it is the less costly option, there is no need to calculate the ICUR because prosthesis usage is the dominant strategy. For bilateral amputation, however, the preferred option is also the more costly one, so the ICUR needs to be calculated. If the incremental cost of one additional QALY using the preferred (and more expensive) intervention is below the cost-effectiveness threshold it is considered to be the optimal choice. If it is above this threshold, it is considered unacceptable. Traditionally, a cost-effectiveness threshold of $50,000/QALY has been employed based on the acceptance of kidney transplantation, although some recent studies indicate that a threshold of $100,000 per additional QALY may be more appropriate.(25, 43–48) The ICUR of double hand transplantation versus prostheses is $318,961/QALY, which exceeds accepted cost-effectiveness thresholds.
Sensitivity analysis
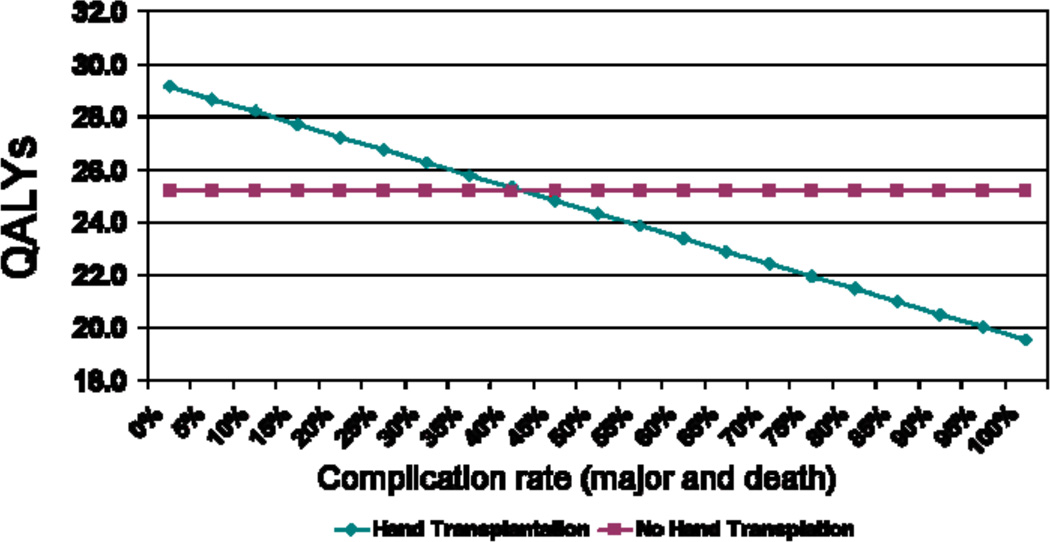
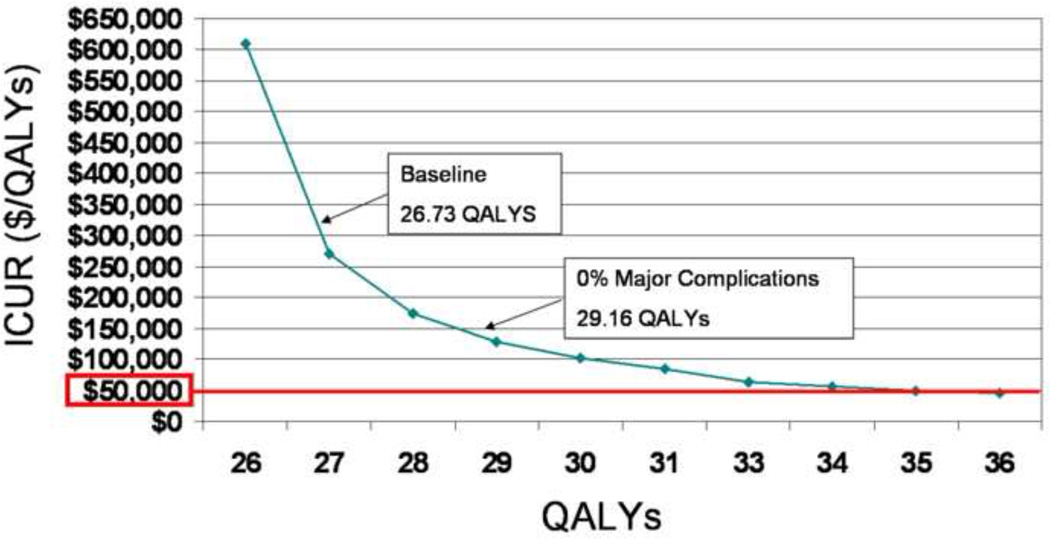
A sensitivity analysis was performed to assess the effect of changes in major complication rate (major immunosuppressive therapy complications and death) on utility of bilateral hand transplantation. The rate of major complications was lowered from its baseline of 25%, which resulted in a utility of 26.73 QALYs, to 0% (i.e. no major complications possible), raising the utility of double hand transplantation to 29.16 QALYs, a gain of 2.43 QALYs. (Figure 5)
Figure 5.
A sensitivity analysis varying the rate of major complications for bilateral hand transplantation
We also performed a sensitivity analysis varying the utility of bilateral hand transplantation. The goal was to find the utility necessary to bring the ICUR of bilateral hand transplantation versus prostheses into the acceptable range of $50,000–$100,000/QALY. We found that when the utility of bilateral hand transplantation reached 30.08 QALYs the ICUR versus prostheses was $100,002/QALY; when the utility reached 34.96 QALYs the ICUR was $50,001/QALY. (Figure 6) However, as the previous sensitivity analysis showed, even when there was no risk of major complications, the utility of bilateral hand transplantation was only 29.16 QALYs.
Figure 6.
A sensitivity analysis varying the expected utility of bilateral hand transplantation
Discussion
New, more expensive interventions and medical technology are always going to bring with them controversy. This is especially true when it is unclear if the new intervention is superior to its counterparts. Kidney transplantation, for example provides a much higher quality of life than the alternative, outpatient dialysis.(49, 50) But this increased quality of life comes at a high monetary cost. In this case, society has decided that the cost of kidney transplantation is “worth” the quality of life improvement and as such, this procedure is frequently performed. Other, even more expensive, transplant procedures such as liver and heart transplantation are widely accepted because there are no alternative treatments for these life-threatening conditions.(51) Hand amputation is not a life-threatening condition however, and prosthetic devices do provide a viable, less costly alternative. In this case, cost-utility analysis is absolutely necessary.(52)
Our cost-utility analysis found that the utility of single hand transplantation was lower than that of single prosthesis for unilateral forearm amputation. This supported the results of a previous decision analysis on single hand transplantation.(53) Double hand transplantation showed higher utility than double prosthetic adaptation; however, the ICUR far exceeded standard cost-effectiveness thresholds. These findings indicate that, from a societal perspective, prosthetic adaptation is the dominant strategy for unilateral amputation and the preferred strategy for bilateral forearm amputation at this time.
The scientific rationales for the indications for hand transplantation have not yet been established.(54) Currently, whether to attempt hand transplantation is subjectively decided upon based on the preferences and expectation of both physicians and patients. However, the toxicity of immunosuppressive medication brings about an ethical dilemma.(19) In solid organ transplantation, 40% of post-transplant deaths were attributed to infection;(55) transplant recipients have 7-fold 5-years risk over the general population of developing malignancies.(14) Although life-threatened complications associated with hand transplantation have not been reported at present, it can be expected that the incidence of malignancies in recipients of hand transplant may be similar to that of solid organ transplant recipients.(18) For hand transplantation, it is crucial to assess whether these probable negative outcomes are justified by the functional, cosmetic and psychological benefits that may result from the procedure.
Based on examination of four cases in the United States and China, functional outcomes of transplanted hands were nearly identical to those of replanted hands.(3, 10, 18, 56) This indicates that it is unlikely that further functional improvement of grafted hands will markedly increase the utility of this procedure in the future. As shown in our sensitivity analysis, even with no risk of major complications, the utilities of hand transplantation are insufficient for the ICUR to fall below the acceptable threshold. The perceived benefits of hand transplantation may not be able to offset the excessive cost until innovative immunosuppressive methods achieve not only a drastic reduction in complications, but also a marked decrease in cost.
Naturally, different populations will see any medical procedure, including hand transplantation, from different perspectives.(57) Assessing the general public is recommended for determining utilities from the societal perspective to reflect various needs and viewpoints and to compare cost-effectiveness across interventions for setting healthcare policy.(58) However, members of the general public may not be able to properly assess the function of grafted hands or prostheses nor the complications associated with immunosuppressive medication. Physicians are a narrow subset of general public who can more accurately assess these factors; however, physicians may overestimate the outcome, resulting in an inflated utility value.(52) Upper-extremity amputee patients may seem like an ideal population, but there is evidence that these patients are more risk adverse than healthy individuals, which also may result in unusually high utility values.(59) We considered medical students an appropriate population for eliciting utilities from the societal perspective because they are not engaged in direct medical care, yet can understand the complicated scenarios.
Because cost-utility analyses rely on numerous assumptions, there are limitations to our analytic model. First, in order to simplify the model, we divided the complications into four categories. It was assumed that complications were mutually-exclusive and continued for the remaining life span. In actuality, recipients are likely to experience several complications that may come and go throughout their lives. A second limitation of our study was that complications of solid organ transplantation were used to calculate the probabilities of hand transplantation complications. Kidney transplant recipients have overall poorer health than hand transplant recipients; they may experience more, and more serious, complications after transplant than a relatively healthy person receiving a hand transplant. The probabilities of complication have also been based on short-term results because these data are strictly derived from randomized controlled trials.(28) Long-term result would demonstrate higher rates of death and major complication such as malignancies and serious infections. Finally, hypertension and diabetes mellitus represented minor and major complications, respectively. It is impractical to individually estimate the cost of potential treatment options for all possible complications.
Respondents to our survey felt that double hand transplantation can result in benefits exceeding the risks for those with bilateral forearm amputation. But because of the necessity of life-long immunosuppressive therapy, it is not cost-effective. Currently, scientists are focusing their efforts on developing the ideal immunosuppression regimen to induce tolerance leading to allograft acceptance, or on minimizing the need for maintenance immunosuppression.(60) These advances may pave the way for strategies that can overcome allograft rejection without the serious complications and additional costs that current immunosuppressive regimens inflict. This may result in an increase in utility and decrease in cost that will allow hand transplantation to become a truly viable alternative treatment for forearm amputation.
Acknowledgement
The authors are grateful to Mr. Phil Clapham for his help developing and preparing this manuscript, and also to: Mr. Casey Crimmins, Manager in Third Party Reimbursement, University of Michigan Hospital and Health Centers; Ms. Alicia Davis, Residency Program Director in Orthotics & Prosthetics Center, University of Michigan Health Systems, Ms. Linda Miner, Upper Extremity Clinical Specialist in Hand Therapy, the University of Michigan Health System.
Supported in part by the National Endowment of Plastic Surgery and a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (to Dr. Kevin C. Chung).
References
- 1.Dubernard JM, Owen E, Herzberg G, et al. Human hand allograft: report on first 6 months. Lancet. 1999;353:1315–1320. doi: 10.1016/S0140-6736(99)02062-0. [DOI] [PubMed] [Google Scholar]
- 2.Jones JW, Gruber SA, Barker JH, et al. Successful hand transplantation. One-year follow-up. Louisville Hand Transplant Team. N Engl J Med. 2000;343:468–473. doi: 10.1056/NEJM200008173430704. [DOI] [PubMed] [Google Scholar]
- 3.Francois CG, Breidenbach WC, Maldonado C, et al. Hand transplantation: comparisons and observations of the first four clinical cases. Microsurgery. 2000;20:360–371. doi: 10.1002/1098-2752(2000)20:8<360::aid-micr4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Dubernard JM, Petruzzo P, Lanzetta M, et al. Functional results of the first human double-hand transplantation. Ann Surg. 2003;238:128–136. doi: 10.1097/01.SLA.0000078945.70869.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanzetta M, Nolli R, Borgonovo A, et al. Hand transplantation: ethics, immunosuppression and indications. J Hand Surg [Br] 2001;26:511–516. doi: 10.1054/jhsb.2001.0635. [DOI] [PubMed] [Google Scholar]
- 6.Margreiter R, Brandacher G, Ninkovic M, et al. A double-hand transplant can be worth the effort! Transplantation. 2002;74:85–90. doi: 10.1097/00007890-200207150-00015. [DOI] [PubMed] [Google Scholar]
- 7.Lanzetta M, Petruzzo P, Margreiter R, et al. The International Registry on Hand and Composite Tissue Transplantation. Transplantation. 2005;79:1210–1214. doi: 10.1097/01.tp.0000157118.28394.fa. [DOI] [PubMed] [Google Scholar]
- 8.Lanzetta M, Petruzzo P, Dubernard JM, et al. Second report (1998–2006) of the International Registry of Hand and Composite Tissue Transplantation. Transpl Immunol. 2007;18:1–6. doi: 10.1016/j.trim.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Petruzzo P, Lanzetta M, Dubernard JM, et al. The international registry on hand and composite tissue transplantation. Transplantation. 2008;86:487–492. doi: 10.1097/TP.0b013e318181fce8. [DOI] [PubMed] [Google Scholar]
- 10.Breidenbach WC, Gonzales NR, Kaufman CL, et al. Outcomes of the first 2 American hand transplants at 8 and 6 years posttransplant. J Hand Surg [Am] 2008;33:1039–1047. doi: 10.1016/j.jhsa.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Ravindra KV, Buell JF, Kaufman CL, et al. Hand transplantation in the United States: experience with 3 patients. Surgery. 2008;144:638–643. doi: 10.1016/j.surg.2008.06.025. discussion 643-634. [DOI] [PubMed] [Google Scholar]
- 12.Lee WP. Composite tissue transplantation: more science and patience needed. Plast Reconstr Surg. 2001;107:1066–1070. doi: 10.1097/00006534-200104010-00026. [DOI] [PubMed] [Google Scholar]
- 13.Brenner MJ, Tung TH, Jensen JN, et al. The spectrum of complications of immunosuppression: is the time right for hand transplantation? J Bone Joint Surg Am. 2002;84-A:1861–1870. [PubMed] [Google Scholar]
- 14.Baumeister S, Kleist C, Dohler B, et al. Risks of allogeneic hand transplantation. Microsurgery. 2004;24:98–103. doi: 10.1002/micr.20003. [DOI] [PubMed] [Google Scholar]
- 15.Foucher G. Prospects for hand transplantation. Lancet. 1999;353:1286–1287. doi: 10.1016/S0140-6736(99)00112-9. [DOI] [PubMed] [Google Scholar]
- 16.Herndon JH. Composite-tissue transplantation--a new frontier. N Engl J Med. 2000;343:503–505. doi: 10.1056/NEJM200008173430710. [DOI] [PubMed] [Google Scholar]
- 17.Cendales L, Hardy MA. Immunologic considerations in composite tissue transplantation: overview. Microsurgery. 2000;20:412–419. doi: 10.1002/1098-2752(2000)20:8<412::aid-micr12>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Hettiaratchy S, Randolph MA, Petit F, et al. Composite tissue allotransplantation--a new era in plastic surgery? Br J Plast Surg. 2004;57:381–391. doi: 10.1016/j.bjps.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Swearingen B, Ravindra K, Xu H, et al. Science of composite tissue allotransplantation. Transplantation. 2008;86:627–635. doi: 10.1097/TP.0b013e318184ca6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manske PR. Hand transplantation. J Hand Surg [Am] 2001;26:193–195. doi: 10.1053/jhsu.2001.24331. [DOI] [PubMed] [Google Scholar]
- 21.Gaine WJ, Smart C, Bransby-Zachary M. Upper limb traumatic amputees. Review of prosthetic use. J Hand Surg [Br] 1997;22:73–76. doi: 10.1016/s0266-7681(97)80023-x. [DOI] [PubMed] [Google Scholar]
- 22.Biddiss EA, Chau TT. Upper limb prosthesis use and abandonment: a survey of the last 25 years. Prosthet Orthot Int. 2007;31:236–257. doi: 10.1080/03093640600994581. [DOI] [PubMed] [Google Scholar]
- 23.Biddiss E, Chau T. Upper-limb prosthetics: critical factors in device abandonment. Am J Phys Med Rehabil. 2007;86:977–987. doi: 10.1097/PHM.0b013e3181587f6c. [DOI] [PubMed] [Google Scholar]
- 24.Chung KC, Walters MR, Greenfield ML, et al. Endoscopic versus open carpal tunnel release: a cost-effectiveness analysis. Plast Reconstr Surg. 1998;102:1089–1099. doi: 10.1097/00006534-199809040-00026. [DOI] [PubMed] [Google Scholar]
- 25.Brauer CA, Rosen AB, Olchanski NV, et al. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87:1253–1259. doi: 10.2106/JBJS.D.02152. [DOI] [PubMed] [Google Scholar]
- 26.Myers J, McCabe S, Gohmann S. Economic analysis in hand surgery. J Hand Surg [Am] 2006;31:664–668. doi: 10.1016/j.jhsa.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Davis EN, Chung KC, Kotsis SV, et al. A cost/utility analysis of open reduction and internal fixation versus cast immobilization for acute nondisplaced mid-waist scaphoid fractures. Plast Reconstr Surg. 2006;117:1223–1235. doi: 10.1097/01.prs.0000201461.71055.83. discussion 1236-1228. [DOI] [PubMed] [Google Scholar]
- 28.Cugno S, Sprague S, Duke A, et al. Composite tissue allotransplantation of the face: Decision analysis model. Can J Plast Surg. 2005;15:145–152. doi: 10.1177/229255030701500304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstein MC, Fineberg HV. Probabilities and clinical decisions. In: Weinstein MC, Fineberg HV, editors. Clinical Decision Analysis. Philadelphia: W.B. Saunders Company; 1980. [Google Scholar]
- 30.Thoma A, Sprague S, Tandan V. Users' guide to the surgical literature: how to use an article on economic analysis. Can J Surg. 2001;44:347–354. [PMC free article] [PubMed] [Google Scholar]
- 31.Arias E. United States life tables, 2003. Natl Vital Stat Rep. 2006;54:1–40. [PubMed] [Google Scholar]
- 32.Current Procedural Terminology. Chicago: American Medical Association; 2009. [Google Scholar]
- 33.Medicare RBRVS. Chicago: American Medical Association; 2009. [Google Scholar]
- 34.Hariharan S. Recommendations for outpatient monitoring of kidney transplant recipients. Am J Kidney Dis. 2006;47:S22–S36. doi: 10.1053/j.ajkd.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 35.Acute Inpatient Prospective Payment System. Centers for Medicare and Medicaid Services; 2009. [Google Scholar]
- 36.Hospital Outpatient Prospective Payment System. Centers for Medicare and Medicaid Services; 2009. [Google Scholar]
- 37.Red Book. Montvale: Thomson Healthcare; 2008. [Google Scholar]
- 38.Woodward RS, Schnitzler MA, Baty J, et al. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant. 2003;3:590–598. doi: 10.1034/j.1600-6143.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 39.Petitti DB. Meta-analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. 2 Ed. New York: Oxford University Press; 2000. pp. 196–199. [Google Scholar]
- 40.Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 41.Lidgren L. The Bone and Joint Decade 2000–2010. B World Health Organ. 2003;81:629. [PMC free article] [PubMed] [Google Scholar]
- 42.Ocupational Employment Statistics. Bureau of Labor and Statistics, United States Department of Labor; 2008. May 2008 National Occupational Employment and Wage Estimates United States. [Google Scholar]
- 43.Newby LK, Eisenstein EL, Califf RM, et al. Cost effectiveness of early discharge after uncomplicated acute myocardial infarction. N Engl J Med. 2000;342:749–755. doi: 10.1056/NEJM200003163421101. [DOI] [PubMed] [Google Scholar]
- 44.Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA. 2000;283:889–896. doi: 10.1001/jama.283.7.889. [DOI] [PubMed] [Google Scholar]
- 45.Braithwaite RS, Meltzer DO, King JT, Jr, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 46.Novak EJ, Silverstein MD, Bozic KJ. The cost-effectiveness of computer-assisted navigation in total knee arthroplasty. J Bone Joint Surg Am. 2007;89:2389–2397. doi: 10.2106/JBJS.F.01109. [DOI] [PubMed] [Google Scholar]
- 47.Larsen K, Hansen TB, Thomsen PB, et al. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91:761–772. doi: 10.2106/JBJS.G.01472. [DOI] [PubMed] [Google Scholar]
- 48.Lee CP, Chertow GM, Zenios SA. An empiric estimate of the value of life: updating the renal dialysis cost-effectiveness standard. Value in Health. 2009;12:80–87. doi: 10.1111/j.1524-4733.2008.00401.x. [DOI] [PubMed] [Google Scholar]
- 49.Garner TI, Dardis R. Cost-effectiveness analysis of end-stage renal disease treatments. Med Care. 1987;25:25–34. doi: 10.1097/00005650-198701000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Simmons RG, Abress L, Anderson CR. Quality of life after kidney transplantation. A prospective, randomized comparison of cyclosporine and conventional immunosuppressive therapy. Transplantation. 1988;45:415–421. doi: 10.1097/00007890-198802000-00034. [DOI] [PubMed] [Google Scholar]
- 51.Krueger H. Economic analysis of solid organ transplantation: a review for policy makers. Health Policy. 1989;13:1–17. doi: 10.1016/0168-8510(89)90107-3. [DOI] [PubMed] [Google Scholar]
- 52.Chen NC, Shauver MJ, Chung KC. A Primer on Use of Decision Analysis Methodology in Hand Surgery. J Hand Surg [Am] 2009 doi: 10.1016/j.jhsa.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 53.McCabe S, Rodocker G, Julliard K, et al. Using decision analysis to aid in the introduction of upper extremity transplantation. Transplant Proc. 1998;30:2783–2786. doi: 10.1016/s0041-1345(98)00808-2. [DOI] [PubMed] [Google Scholar]
- 54.Manske PR. The sound of one hand clapping. J Hand Surg [Am] 2008;33:1037–1038. doi: 10.1016/j.jhsa.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Kahan BD, Ghobrial R. Immunosuppressive agents. Surg Clin North Am. 1994;74:1029–1054. [PubMed] [Google Scholar]
- 56.Graham B, Adkins P, Tsai TM, et al. Major replantation versus revision amputation and prosthetic fitting in the upper extremity: a late functional outcomes study. J Hand Surg [Am] 1998;23:783–791. doi: 10.1016/s0363-5023(98)80151-2. [DOI] [PubMed] [Google Scholar]
- 57.Edgell SE, McCabe SJ, Breidenbach WC, et al. Different reference frames can lead to different hand transplantation decisions by patients and physicians. J Hand Surg [Am] 2001;26:196–200. doi: 10.1053/jhsu.2001.20152. [DOI] [PubMed] [Google Scholar]
- 58.Russell LB, Gold MR, Siegel JE, et al. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]
- 59.Majzoub RK, Cunningham M, Grossi F, et al. Investigation of risk acceptance in hand transplantation. J Hand Surg Am. 2006;31:295–302. doi: 10.1016/j.jhsa.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Ravindra KV, Wu S, Bozulic L, et al. Composite tissue transplantation: a rapidly advancing field. Transplant Proc. 2008;40:1237–1248. doi: 10.1016/j.transproceed.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]