Abstract
Objective
We sought to evaluate the impact of a hygiene and sanitation intervention program among school-children to control active trachoma and intestinal parasitic infections.
Methods
This longitudinal epidemiologic study was conducted among 630 students in rural Ethiopia. Baseline and follow-up surveys were conducted to evaluate the impact of a three pronged intervention program i) constructing of ventilated improved pit latrines, ii) provision of clean drinking water, and iii) and hygiene education. Socio-demographic information was collected using a structured questionnaire. Presence of trachoma and intestinal parasitic infections were evaluated using standard procedures.
Results
At baseline 15% of students had active trachoma while 6.7% of them were found to have active trachoma post intervention (p<0.001). Similar improvements were noted for parasitic infections. At baseline 7% of students were reported to have helminthic infections and 30.2% protozoa infections. However, only 4% of students had any helminthic infection and 13.4% (p<0.001) of them were found to have any protozoa infection. Improvements were noted in students’ knowledge and attitudes towards hygiene and sanitation.
Conclusions
The results of our study demonstrated that provision of comprehensive and targeted sanitation intervention program was successful in reducing the burden of trachoma and intestinal parasitic infection among school children.
Keywords: Trachoma, Angolela, Ethiopia, School, Intervention, parasitic infection, water, sanitation, hygiene
Introduction
Trachoma is a leading cause of preventable infectious blindness in the world (WHO 2000). It is caused by infection with Chlamydia trachomatis bacterium, one of the most common human pathogens (Schachter et al. 1999). The World Health Organization (WHO) estimates that trachoma is endemic in 56 countries, most within Africa and the Middle East, and causes 3.6% of all blindness (Resnikoff et al. 2004). It is currently reported that close to 1.2 million individuals are blind from trachoma while about 21.4 million suffer from active trachoma. Factors most consistently associated with trachoma include inadequate access to water and sanitation facilities (Baggaley et al. 2006, Polack et al. 2006). Latrines have been proposed to play a critical role to the exposure of a mechanical vector of C. trachomatis, the eye seeking Musca sorbens, by reducing the amount of exposed human feces, the breeding media of M. sorbens (Emerson et al. 2000). To combat the burden of trachoma, the World Health Organization endorsed an integrated four components strategy known as SAFE: Surgery, Antibiotics, Face washing and Environmental sanitation (WHO 1997). Provision and improvement of latrines, access to clean water and encouraging the use of water for face washing are two of the important parts (‘F’ and ‘E’) of the WHO-recommended SAFE strategy for trachoma control (WHO 1996).
Intestinal parasitic infections, comorbid with trachoma, are the second most major causes of outpatient morbidity in Ethiopia (Alemu et al. 2011). Importantly, intestinal parasitic infections destroy the well-being and learning potentials of millions of school children in developing countries (WHO 2005). Although a number of local and international health organizations have implemented deworming programs in hardest hit areas, sustained benefits have been elusive, in part, because interventions were provided in isolation rather than in combination with sanitary improvement s and hygiene education programs (Knopp et al. 2011). Prior studies have shown that inadequate sanitary conditions and poor hygiene practices play a major role in the increased burden of gastro intestinal infections (WHO 2005).
Studies conducted in Ethiopia documented high levels of active trachoma (Berhane et al. 2007) and intestinal parasitic infections (Alemu et al. 2011, Belyhun et al. 2010) and signify the need for timely and sustainable efforts aimed at preventing new infections and treating prevalent cases. Despite the high magnitude of these problems, to the best of our knowledge, comprehensive sanitation intervention programs have not been systematically evaluated in Ethiopia. Control of active trachoma and intestinal parasitic infections alleviate suffering, reduce poverty, and support equal opportunities among young children. School based hygiene and sanitation program is one of the most widely used and effective approaches to control trachoma and parasitic infections (Lewallen et al. 2008). Notably, school enrolment is on the rise in most parts of the world (UNESCO 2007). Ethiopia is on track to achieve university primary education, one of the United Nations millennium development goals, with enrollment reaching up to 95.9 percent (MoFED 2010). With the vast majority of school-age children now enrolled, schools present an opportunity to reach thousands of children with safe water and hygiene and health messages. Targeting schools for water and sanitation improvement and hygiene training has multifaceted benefits including reaching children from households at all socio-economic levels (Nagpal 2012) as primary education is very highly subsidized or completely free (MoFED 2010, Nagpal 2012, UNESCO 2007). Instituting health promotion and disease prevention programs in schools benefits not only the students but also the family, community and country as a whole. Improved health among students will improve their school attendance, their learning potential and education achievement (WHO 2006). Therefore, in this intervention study we sought to evaluate the impact of hygiene and sanitation intervention program among Angolela Primary Education School students in controlling active trachoma and intestinal parasitic infections.
Methods and Materials
This longitudinal epidemiologic study was conducted in Angolela Woreda, North Showa zone during October 2008 and May 2009. Angolela Woreda is located at 140 Km from Addis Ababa (capital city) (Figure 1: created using the WHO health mapper software).
Figure 1.
Map of Study Setting
Baseline survey
The first survey was conducted in October 2008 to establish baseline prevalence estimates of active trachoma, intestinal parasitic infection as well as estimates of Knowledge Attitudes and Practices (KAP) of hand washing practices among students. The survey questionnaire consisted of: demographic information (grade, gender); mother and father literacy (no, yes); and frequencies of bathing, washing feet/hair, brushing teeth, and changing clothes. Students were queried as to whether their hands were washed during the day prior to interview (no, yes); reasons for washing hands (after defecation, before meals); materials used for hand washing (soap and water, water only). All 669 students attending grades 1–6 at Angolela Primary School were included in the survey (median age= 11 years old). Following the baseline survey, improvements in hygiene and sanitation were implemented by the school.
Intervention Components
The school implemented the following improvements in sanitation and hygiene.
A low cost 8 eight seat Ventilated Improved Pit (VIP) latrines were newly constructed for female students. Another existing eight seat pit latrine was refurbished; contents were emptied and became functional for use of male students.
Two fiberglass water tankers each with capacities of three cubic meters and with three taps were installed, separately for both males and females. Tankers were filled every other day with clean water from nearby protected spring water source using Jerry cans of 10 and 20 liters capacity to promote hand washing after toilet use as well to encourage students wash their faces. The protected spring had an effective protection around it. In addition set back distance, based on travel time, was established to protect against possible contaminant sources such as latrines.
A student led Health Club was formed to educate students every morning before class starts during morning parades and using special sessions about basic hygiene and sanitation. Members of the club and the teachers heading the club were oriented on proper hygiene and disease prevention. Different types of posters and printed materials on hygiene and sanitation were supplied. The health club’s main functions included: supervising the availability of water inside tankers; supervising the cleanliness of latrines; instructing students how to use latrines and water stands, and communicating health messages to students.
After baseline survey, students who were found to be positive for parasites and active trachoma (described below) were treated for free with appropriate doses of nationally recommended drugs; Albendazole for intestinal parasitic infection (a WHO recommended drug and noted to be highly efficacious against round- and hookworms) (Vercruysse et al. 2011) and Tetracycline eye ointment for active trachoma.
Post-intervention survey
Since October, 2008 all students had access to all the intervention components. A follow-up study of students enrolled in the baseline survey was conducted in May 2009 to evaluate the extent to which prevalence estimates of infections, as well as estimates of KAP are changed after interventions were implemented.
Socioeconomic and personal risk factors survey
A structured questionnaire (translated from English and printed in the local Amharic language) was used to collect data. The questionnaire included information on socioeconomic, sanitary, environmental, and demographic risk factors. . It also included questions concerning parental (mother and father) literacy, students’ gender, age, and grade in school. Prior to use, the questionnaire was pre-tested in Dalcha Elementary School in Basona Worena Woreda, Ethiopia to assess the suitability of the questionnaire with regard to duration, language appropriateness, and question comprehensibility. The questionnaire was administered by trained research personnel who performed in-person interviews of all participating students
Eye Examination
All students were examined for clinical signs of active trachoma using the WHO simplified clinical grading scheme by trained and standardized ophthalmic nurses. Each ophthalmic nurse was standardized against highly experienced ophthalmologist in trachoma diagnosis (gold standard). The same nurses were used for baseline and post-intervention surveys. In accordance with the WHO grading scheme (WHO 2008), active trachoma was defined as the presence of either trachomatous inflammation follicular (TF) or trachomatous inflammation-intense (TI). Each student was also examined for trachomatous trichiasis (TT) (defined as either in turned eyelashes rubbing on the eye or evidence of previously removed lashes) and trachomatous scarring (TS).
Parasitological examination
About 3 grams of fresh stool samples were collected in plastic cups and were labeled with the students’ unique study identification number. Following stool collection, samples were preserved in a tube containing 10% formalin in 0.85% saline. Samples were then taken to Debre Birhan Hospital, 10 km from the study site, for processing, using ether concentration technique for fecal examination to diagnose infections with intestinal worms and protozoa, following WHO standard operating procedures for the parasitological examination of feces (WHO 2001). To evaluate diagnostic accuracy, 5% of the fecal samples were randomly re-processed by a separate lab technician from Debre Birhan Hospital and the results were compared with the results made by the original lab technician. In accordance with the WHO recommendation, students who were found to harbor an intestinal parasite were treated with Albendazole for free at a single dose of 400mg orally (WHO 1995). All treatments were provided without charge to students or their families.
Ethical Consideration
Ethical approval for all study procedures was granted by the Institutional Review Board of Addis Continental Institute of Public Health (IRB of ACIPH) in Addis Ababa, Ethiopia. Approval from the Woreda Health Office and the Woreda Education Office was also granted prior to the commencement of this study. Verbal consent was obtained from parents or appropriate guardians of eligible children and assent was obtained from eligible children before they were included in the study in accordance with the principles of the declaration of Helsinki. Written consent was not deemed appropriate, given the low literacy rate in Angolela Woreda and the research involved no more than minimal risk to the subjects. Study procedures including verbal consenting process were approved by the IRB of ACIPH. Documentation of verbal assent was initiated and dated by the interviewers on data collection forms as approved by the IRB. Before analysis, personal identifiers were removed from each data set. The Human Subjects Division of the University of Washington, USA granted approval to use the de-identified and anonymised data set for analysis.
Statistical Analysis
Data were entered in the program EPI-INFO (Version 3.3.2), a public access software made available from the US Centers for Disease Control and Prevention (CDC Atlanta, GA, USA), the analysis was completed using SPSS (version 17.0, SPSS, Inc., Chicago, USA). Frequency distributions of students’ socio-demographic and behavioral characteristics according to gender were examined. Continuous variables were expressed as mean ± standard error of mean. Categorical variables were expressed as number (percentage, %). Since the same students were involved in the pre and post-intervention assessments, we used McNemar’s test for paired data to evaluate significance while accounting for correlations between the two time periods. All reported tests of statistical significance were two-sided set at α=0.05.
Results
The socio-demographic and personal characteristics of Angolela school children during baseline and post intervention surveys are presented in Table 1. At baseline a total of 669 students with a median age 11 years of age (interquartile range 9.8–13.0) participated in the study while 630 students participated during the post intervention survey a median age 11 years of age (interquartile range 9.7–13.0). There was no significant difference in the gender distribution, age, and parental literacy rates.
Table 1.
Personal and household characteristics
| Pre-intervention | Post-intervention | |||
|---|---|---|---|---|
| Characteristics | n (total =669) |
% | n (total =630) |
% |
| Grade | ||||
| 1 | 110 | 16.4 | 125 | 19.8 |
| 2 | 188 | 28.1 | 165 | 26.2 |
| 3 | 114 | 17.0 | 90 | 14.3 |
| 4 | 70 | 10.3 | 71 | 11.3 |
| 5 | 100 | 16.3 | 100 | 15.9 |
| 6 | 78 | 11.7 | 79 | 12.5 |
| Gender | ||||
| Female | 326 | 48.7 | 304 | 48.3 |
| Male | 343 | 51.3 | 325 | 51.7 |
| Mother Literate | ||||
| No | 399 | 59.7 | 345 | 54.8 |
| Yes | 263 | 39.3 | 269 | 42.7 |
| Don’t know | 7 | 1.0 | 16 | 2.5 |
| Father Literate | ||||
| No | 210 | 31.4 | 168 | 26.7 |
| Yes | 437 | 65.3 | 436 | 69.2 |
| Don’t know | 22 | 3.3 | 26 | 4.1 |
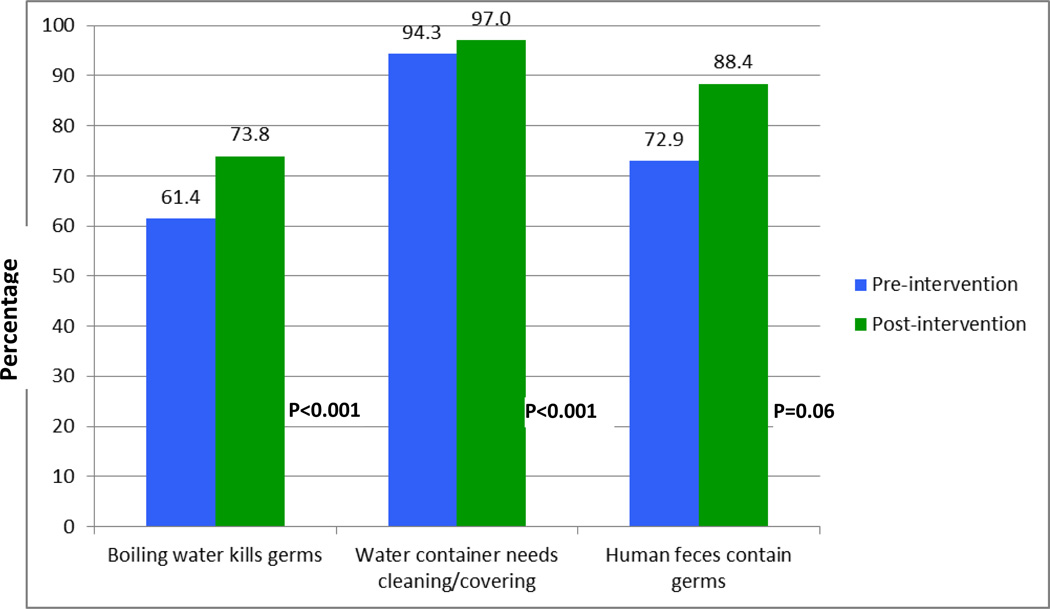
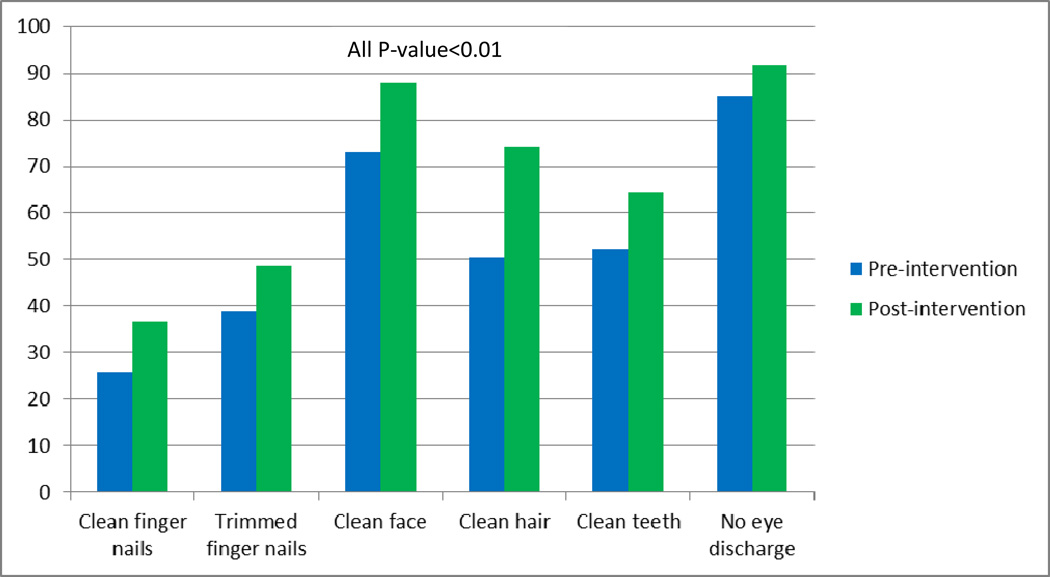
As shown in Figure 2, there was a marked improvement in knowledge, attitudes and practices toward sanitation among students after intervention. Approximately, 61% of students felt boiling water kills germs, while 74% of them reported this during post intervention. During baseline 73% of students thought human feces contain germs, whereas during follow up survey 88% of students reported this. Similarly, as shown in Figure 3 significant improvements were noted across all objectively observed personal hygiene characteristics (all p-value <0.01).
Figure 2.
Knowledge about sanitation and hygiene practices
Figure 3.
Objectively observed personal hygiene characteristics
As shown in Table 2, there was a significant improvement in the prevalence of active trachoma (p<0.001). At baseline 15% of students had active trachoma while, notably only 6.7% of students were found to have active trachoma post intervention.
Table 2.
Prevalence of Trachoma in study population
| Sign of trachoma | Pre-intervention | Post-intervention | P-value | ||
|---|---|---|---|---|---|
| n (total =669) |
% | n (total =630) |
% | ||
| Trachomatous intense(TI) | |||||
| No | 628 | 91.9 | 610 | 96.8 | 0.05 |
| Yes | 41 | 6.1 | 20 | 3.2 | |
| Trachomatous follicular (TF) | |||||
| No | 596 | 89.1 | 599 | 95.1 | <0.001 |
| Yes | 73 | 10.9 | 32 | 4.9 | |
| Trachomatous scarring (TS) | |||||
| No | 620 | 92.7 | 609 | 96.7 | 0.02 |
| Yes | 49 | 7.3 | 21 | 3.3 | |
| Any active trachoma | |||||
| No | 568 | 84.9 | 588 | 93.3 | <0.001 |
| Yes | 101 | 15.1 | 42 | 6.7 | |
Similar improvements were noted for intestinal parasitic infections (Table 3). At baseline 7% of students were diagnosed with any helminthic infection and 30.2% any protozoa infection. These prevalence estimates, however, were significantly reduced (p=0.04) post intervention. Only 4% of them had any helminthic infection and 13.4% of them were found to have any protozoa infection.
Table 3.
Prevalence of parasitic infections in study population
| Intestinal Parasitic Infection | Pre-intervention | Post-intervention | P-value | ||
|---|---|---|---|---|---|
| Number Infected |
% | Number Infected |
% | ||
| Ascaris lumbricoides | 23 | 3.6 | 11 | 1.7 | 0.15 |
| Hookworm | 3 | 0.5 | 3 | 0.5 | 0.81 |
| Hymenolopis nana | 10 | 1.5 | 1 | 0.2 | 0.01 |
| Hymnolepis dimunita | 2 | 0.3 | 0 | 0.0 | --* |
| Trichuris trichuira | 2 | 0.3 | 3 | 0.5 | 0.99 |
| Enterobius vermicularis | 11 | 1.6 | 10 | 1.6 | 0.60 |
| Any Helminithic infection | 47 | 7.0 | 27 | 4.3 | 0.04 |
| Giardia intestinalis | 49 | 7.6 | 10 | 1.6 | <0.001 |
| Entammoeba | 179 | 27.8 | 77 | 12.3 | <0.001 |
| Any protozoa infection | 202 | 30.2 | 85 | 13.4 | <0.001 |
McNemar test was not computed as no cases were found post intervention
Discussion
The results of our study demonstrated that provision of comprehensive and targeted sanitation intervention project was successful in reducing the burden of trachoma and intestinal parasitic infection among school children in Angolela, Ethiopia. To date, we are not aware of any published article that systematically evaluated the impact of access to water, latrines, and hygiene education on eye infection trachoma and intestinal parasitic infections in Ethiopia.
The findings from our study on the positive impact of access and use of latrines on trachoma are in general agreement with others (Rabiu et al. 2012). Because our study is one of the first to investigate impact of water and sanitation programs on trachoma and parasitic infection in school settings, our findings can only tentatively be compared with community based studies. For instance, in their study among Egyptian households, Courtright et al (Courtright et al. 1991) found that an absence of a latrine in a household was associated with a 3.3 fold increased odds of trachoma. Moreover, presence and use of a pit latrine in households, even when full and unscreened, was associated with a 14% reduction in trachoma prevalence (Courtright et al. 1991). Similarly in Ankober, Ethiopia Golovaty et al (Belyhun et al. 2010) using a community-based study of children ages 1–9 years, reported that those without access to a latrine were nearly 5 times more likely to have active trachoma. Other investigators have found protective effects of access and use of latrines on the incidence of trachoma among young children (Burton et al. 2010, Emerson et al. 2004, Montgomery et al. 2010). In contrast to prior findings, Stoller et al (Stoller et al. 2011) in their study among 24 communities in Northern Ethiopia found no significant reduction in eye infection trachoma among children due to latrine construction. Reasons for these differences are unclear. Additionally the prevalence of TS decreased from a baseline 7.3% to 3.3%. Scarring is not a sign of active infection, but rather it indicates that an individual had had repeated trachoma infection in the past. It is possible that some of the students had repeated infections since intervention (WHO 1996, 2000). Collectively the results of our study and those of others underscore the importance of latrine provision and use in trachoma prevention.
Our study results indicated an overall reduction in helminthic and protozoa infection rates. The lack of statically significant reduction in some of the helminthic infection is, in part, due to small sample size. At baseline the prevalence of any helminthic infection was 7% while any protozoa infection was found among 30% of students. These prevalence estimates, however, were significantly reduced (p=0.04) post intervention. Only 4% of them had any helminthic infection and 13.4% of them were found to have any protozoa infection. Although the effects of treating all school children at regular intervals with deworming drugs have been extensively studied, the knowledge base for the effects of screening and then treating infected children is limited (23152203). In their study among children in Bangladesh Hall and Nahar evaluated the effectiveness of a single dose of albendazole on Giardia. They noted that a single dose of albendazole treated 62% of infected children indicating the moderate efficacy of single doses on affected children (PID: 8465408). Given that a single dose of Albendazole may not be as efficacious as multiple dose for protozoa infections, provision of clean water, hand washing and other personal hygiene measures might have contributed to this reduction (Speich et al. 2013). Speich et al in their recent study among school-aged children in Tanzania evaluated the effect of a single dose albendazole, nitazoxanide or albendazole-nitazoxanide treatment. No significant effect was found in the in mean intensity of intestinal protozoa infection 3 weeks after treatment. The authors speculated that a single-dose albendazole or nitazoxanide or a combination of the two drugs do not have sufficient efficacy against pathogenic intestinal protozoa (Speich et al. 2013). In our study, it is possible that the changes in the attitudes and practices of personal hygiene among students might have contributed to the parasitic infection reduction. In comparison to baseline assessment, all objectively observed personal hygiene characteristics were significantly improved during post intervention assessment.
Studies have demonstrated that those who used open fields for defecation have markedly higher parasite infection rates than those who used some type of latrine although sustainable results can be achieved if coupled with hand washing practices (Alum et al. 2010). Most investigators, though not all, have shown previously that availability and use of sanitation facilities is associated with reduced intestinal parasitic infections. A recent meta-analysis by Ziegelbauer et al (Ziegelbauer et al. 2012) found that improved sanitation is associated with a reduced risk of transmission of parasitic infections. However, Yajima et al (Yajima et al. 2009) in their study among a rural agricultural community in Vietnam found no association between helminthic infections and presence and use of latrines. Intestinal parasitic infections occur at a critical time in life with a maximum intensity in the age range of 5 to 14. Their adverse health consequences are enormous including permanent organ damage, anemia, poor physical growth, poor intellectual development and impaired cognitive function (WHO 2005). School based deworming is one of the widely recommended strategies to combat intestinal parasitic infections among school children (Matthys et al. 2011). Although deworming programs are commendable; we and others recognized that sustained benefits can only be achieved if coupled with a multi-pronged approach of hygiene education and sanitation improvements (Knopp et al. 2011, Stoller et al. 2011).
Given that we have administered tetracycline treatments to those students who were found to be positive at baseline; it is difficult to delineate the exact contribution of each intervention component on trachoma infection. Although we don’t have reinfection rates among school children, studies have shown the moderate success of antibiotic treatment on active trachoma (Hu et al. 2010). Infection rates were initially falling immediately post-treatment, but increasing within 12 months of treatment (Hu et al. 2010). A study conducted in Egypt, The Gambia and Tanzania, noted that the prevalence of infection at 1 year after mass treatment was substantially lower than at baseline, but was higher than the prevalence at the 3-month follow-up (Schachter et al. 1999). Collectively, however, research thus far has shown that use of latrines block mechanical transmission of trachoma by reducing fly breeding (Kumie and Ali 2005, Rabiu et al. 2012, Stoller et al. 2011). This important environmental intervention thereby decreases the amount of contact between the reservoir of young children with active trachoma through contact with discharge from the eye and possibly the nose via the fly vector. It is important to note, however, that access to a latrine does not necessarily translate to increased latrine use (O'Loughlin et al. 2006). Furthermore improper use of latrines, particularly shared or communal latrines, when not properly maintained may exacerbate transmission.
The findings of our study should be interpreted in light of some study limitations. It is possible that the observed reduction in trachoma and parasitic infection may be due to the group treatment effect where there was no control comparison group. The concordance of our results with those from prior studies, however, serves to attenuate some of these concerns. In addition, it has been reported that there is seasonal variation in density of eye seeking flies in Ethiopia (Taye et al. 2007). In the month of May (when the post intervention survey was conducted), the eye seeking flies are expected to be the highest (Taye et al. 2007). As a result, the magnitudes of the reported effect of interventions on active trachoma are likely to be conservative. Additionally, it is possible that the students’ exposure to trachoma and parasitic infections might differ by place of residence. Future studies need to examine the effect of both school and residential exposures and family contacts for the identification and control of trachoma. Finally, it is possible that some educational efforts conducted at a national and regional level might have contributed to increased awareness about hand and face washing among students.
In conclusion, as evidenced in our study, provisions of latrines, water and hygiene education were found to be protective of trachoma and intestinal parasitic infections. In developed countries where major improvements in water, sanitation, and hygiene behaviors have occurred, trachoma and intestinal parasitic infections ceased to be public health problems. This change happened in the absence of any trachoma or parasitic infection-specific interventions such as antibiotic treatment (Burton et al. 2010). The solution for these problems remains simple, basic, and fundamental core of public health— improved sanitation. Schools are important environments to initiate and promote healthy behaviors and improved sanitation practices. Improving water and sanitation in schools will improve health, intellect, and school attendance. Consequently, school performance will improve, child mortality will decline, and economic productivity will increase (Taylor-Robinson et al. 2012). As illustrated in this study, improving sanitary infrastructure, provision of clean water and hygiene education is feasible with modest cost. The sustainability of these programs depends on health and economic benefits perceived by various stakeholders, commitment of teachers and school principals, involvement of community members, support of governmental and non-governmental organizations to promote and finance water and sanitation improvements (Hutton et al. 2007). Clearly, mobilizing local resource to improving school sanitation facilities should be actively sought by relevant stakeholders.
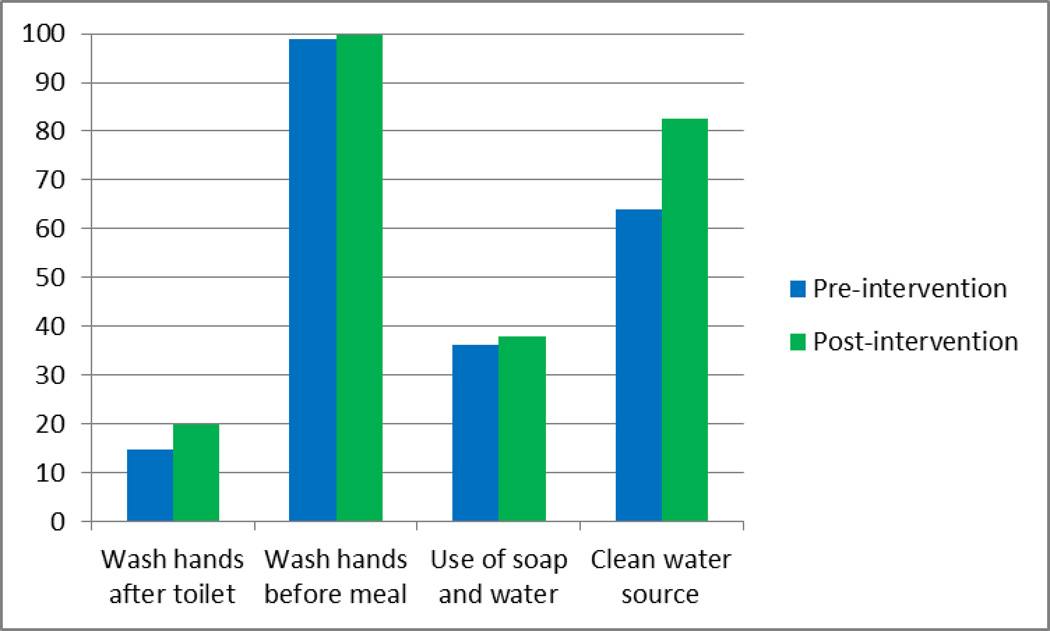
Figure 4.
Hand washing practices
Acknowledgment
The authors wish to thank Feed the Children Ethiopia, Angolela Primary School, and Addis Continental Institute of Public Health for providing facilities and logistics support throughout the research process. The authors would also like to thank Debre Birhan Hospital, the Woreda Health Office, and the Woreda Education Office for providing testing facilities and granting access to conduct the study.
Funding
Provision of VIP latrines and water tankers was made possible through the donation from Scott and Ann Marie Robertson, anonymous donor, and funds from the Brotman Award In partnership with Feed the Children (FTC) Ethiopia and Addis Continental Institute of Public Health (ACIPH). The research was supported in part by an award from the National Institutes of Health, National Institute of Minority Health and Health Disparities (T37-MD001449). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Footnotes
Declaration of interest: The authors declare that they have no competing interests.
Conflict of Interest
The authors have no competing interests to declare.
References
- Alemu A, Atnafu A, Addis Z, Shiferaw Y, Teklu T, Mathewos B, Birhan W, Gebretsadik S, Gelaw B. Soil transmitted helminths and schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia. BMC Infect Dis. 2011;11:189. doi: 10.1186/1471-2334-11-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alum A, Rubino JR, Ijaz MK. The global war against intestinal parasites--should we use a holistic approach? Int J Infect Dis. 2010;14(9):e732–e738. doi: 10.1016/j.ijid.2009.11.036. [DOI] [PubMed] [Google Scholar]
- Baggaley RF, Solomon AW, Kuper H, Polack S, Massae PA, Kelly J, Safari S, Alexander ND, Courtright P, Foster A, Mabey DC. Distance to water source and altitude in relation to active trachoma in Rombo district, Tanzania. Trop Med Int Health. 2006;11(2):220–227. doi: 10.1111/j.1365-3156.2005.01553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyhun Y, Medhin G, Amberbir A, Erko B, Hanlon C, Alem A, Venn A, Britton J, Davey G. Prevalence and risk factors for soil-transmitted helminth infection in mothers and their infants in Butajira, Ethiopia: a population based study. BMC Public Health. 2010;10:21. doi: 10.1186/1471-2458-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhane Y, Worku A, Bejiga A, Adamu A, Alemayehu W, Bedri A, Haile Z, Ayalew A, Adamu Y, Gebre T, Kebede TD, West E, West S. Prevalence of Trachoma in Ethiopia. Ethiopian Journal of Health Development. 2007;21(3):211–215. [Google Scholar]
- Burton MJ, Holland MJ, Makalo P, Aryee EA, Sillah A, Cohuet S, Natividad A, Alexander ND, Mabey DC, Bailey RL. Profound and sustained reduction in Chlamydia trachomatis in The Gambia: a five-year longitudinal study of trachoma endemic communities. PLoS Negl Trop Dis. 2010;4(10) doi: 10.1371/journal.pntd.0000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtright P, Sheppard J, Lane S, Sadek A, Schachter J, Dawson CR. Latrine ownership as a protective factor in inflammatory trachoma in Egypt. Br J Ophthalmol. 1991;75(6):322–325. doi: 10.1136/bjo.75.6.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson PM, Cairncross S, Bailey RL, Mabey DC. Review of the evidence base for the 'F' and 'E' components of the SAFE strategy for trachoma control. Trop Med Int Health. 2000;5(8):515–527. doi: 10.1046/j.1365-3156.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Emerson PM, Lindsay SW, Alexander N, Bah M, Dibba SM, Faal HB, Lowe KO, McAdam KP, Ratcliffe AA, Walraven GE, Bailey RL. Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet. 2004;363(9415):1093–1098. doi: 10.1016/S0140-6736(04)15891-1. [DOI] [PubMed] [Google Scholar]
- Hu VH, Harding-Esch EM, Burton MJ, Bailey RL, Kadimpeul J, Mabey DC. Epidemiology and control of trachoma: systematic review. Trop Med Int Health. 2010;15(6):673–691. doi: 10.1111/j.1365-3156.2010.02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton G, Haller L, Bartram J. Global cost-benefit analysis of water supply and sanitation interventions. J Water Health. 2007;5(4):481–502. doi: 10.2166/wh.2007.009. [DOI] [PubMed] [Google Scholar]
- Knopp S, Stothard JR, Rollinson D, Mohammed KA, Khamis IS, Marti H, Utzinger J. From morbidity control to transmission control: time to change tactics against helminths on Unguja Island, Zanzibar. Acta Trop. 2011 doi: 10.1016/j.actatropica.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Kumie A, Ali A. An overview of environmental health status in Ethiopia with particular emphasis to its organization, drinking water and sanitation: a literature survey. Ethiopian Journal of Health Development. 2005;19(2):89–103. [Google Scholar]
- Lewallen S, Massae P, Tharaney M, Somba M, Geneau R, Macarthur C, Courtright P. Evaluating a school-based trachoma curriculum in Tanzania. Health Educ Res. 2008 doi: 10.1093/her/cym097. [DOI] [PubMed] [Google Scholar]
- Matthys B, Bobieva M, Karimova G, Mengliboeva Z, Jean-Richard V, Hoimnazarova M, Kurbonova M, Lohourignon LK, Utzinger J, Wyss K. Prevalence and risk factors of helminths and intestinal protozoa infections among children from primary schools in western Tajikistan. Parasit Vectors. 2011;4:195. doi: 10.1186/1756-3305-4-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MoFED. Ethiopia: 2010 MDGs Report Trends and Prospects for Meeting MDGs by 2015; Ministry of Finance and Economic Development (MoFED) [Accessed August 14, 2013];2010 Available at: http://web.undp.org/africa/documents/mdg/ethiopia_september2010.pdf.
- Montgomery MA, Desai MM, Elimelech M. Comparing the effectiveness of shared versus private latrines in preventing trachoma in rural Tanzania. Am J Trop Med Hyg. 2010;82(4):693–695. doi: 10.4269/ajtmh.2010.09-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal T. Clean Start: Focusing on School Water, Sanitation, and Hygiene: A Reflection From GWC. [Accessed August 14, 2013];2012 Available at: http://www.globalwaterchallenge.org/resources/cleanstartschoolreflection.pdf.
- O'Loughlin R, Fentie G, Flannery B, Emerson PM. Follow-up of a low cost latrine promotion programme in one district of Amhara, Ethiopia: characteristics of early adopters and non-adopters. Trop Med Int Health. 2006;11(9):1406–1415. doi: 10.1111/j.1365-3156.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Polack S, Kuper H, Solomon AW, Massae PA, Abuelo C, Cameron E, Valdmanis V, Mahande M, Foster A, Mabey D. The relationship between prevalence of active trachoma, water availability and its use in a Tanzanian village. Trans R Soc Trop Med Hyg. 2006;100(11):1075–1083. doi: 10.1016/j.trstmh.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiu M, Alhassan MB, Ejere HO, Evans JR. Environmental sanitary interventions for preventing active trachoma. Cochrane Database Syst Rev. 2012;2 doi: 10.1002/14651858.CD004003.pub4. CD004003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- Schachter J, West SK, Mabey D, Dawson CR, Bobo L, Bailey R, Vitale S, Quinn TC, Sheta A, Sallam S, Mkocha H, Faal H. Azithromycin in control of trachoma. Lancet. 1999;354(9179):630–635. doi: 10.1016/S0140-6736(98)12387-5. [DOI] [PubMed] [Google Scholar]
- Speich B, Marti H, Ame SM, Ali SM, Bogoch II, Utzinger J, Albonico M, Keiser J. Prevalence of intestinal protozoa infection among school-aged children on Pemba Island, Tanzania, and effect of single-dose albendazole, nitazoxanide and albendazole-nitazoxanide. Parasit Vectors. 2013;6:3. doi: 10.1186/1756-3305-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller NE, Gebre T, Ayele B, Zerihun M, Assefa Y, Habte D, Zhou Z, Porco TC, Keenan JD, House JI, Gaynor BD, Lietman TM, Emerson PM. Efficacy of latrine promotion on emergence of infection with ocular Chlamydia trachomatis after mass antibiotic treatment: a cluster-randomized trial. Int Health. 2011;3(2):75–84. doi: 10.1016/j.inhe.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taye A, Alemayehu W, Melese M, Geyid A, Mekonnen Y, Tilahun D, Asfaw T. Seasonal and altitudinal variations in fly density and their association with the occurrence of trachoma, in the Gurage zone of central Ethiopia. Ann Trop Med Parasitol. 2007;101(5):441–448. doi: 10.1179/136485907X176544. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P. Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance. Cochrane Database Syst Rev. 2012;11 doi: 10.1002/14651858.CD000371.pub5. CD000371. [DOI] [PubMed] [Google Scholar]
- UNESCO. Education for all by 2015 : will we make it? Paris; Oxford; New York: UNESCO Publishing ; Oxford University Press; 2007. [Google Scholar]
- Vercruysse J, Behnke JM, Albonico M, Ame SM, Angebault C, Bethony JM, Engels D, Guillard B, Nguyen TV, Kang G, Kattula D, Kotze AC, McCarthy JS, Mekonnen Z, Montresor A, Periago MV, Sumo L, Tchuente LA, Dang TC, Zeynudin A, Levecke B. Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis. 2011;5(3):e948. doi: 10.1371/journal.pntd.0000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2nd edition. Geneva: 1995. World Health Organization Model Prescribing Information: Drugs used in Parasitic Diseases. [Google Scholar]
- WHO. World Health Organization. Report of the First Meeting of the WHO Alliance for the Global Elimination of Trachoma. [Accessed April 20, 2009];1996 Available at: http://whqlibdoc.who.int/hq/1996/WHO_PBL_96.56.pdf.
- WHO. World Health Organization. Report of the First Meeting of the WHO Alliance for the Global Elimination of Trachoma. [Accessed June 20, 2008];1997 Available at: http://wholibdoc.who.int/hq/1997/WHO/PBL/GET/97.1.pdf.
- WHO. Geneva: World Health Organization; 2000. Report of the Fourth Meeting of the WHO Alliance for the Global Elimination of Trachoma. [Google Scholar]
- WHO. Geneva: World Health Organization; 2001. Blood safety and clinical technology : 2000–2003 strategy, Dept. of Blood Safety and Clinical Technology. [Google Scholar]
- WHO. WHO, Millennium Development Goals, “The Evidence is in: Deworming Helps Meet the Millennium Development Goals,” 2005. 2005 http://whqlibdoc.who.int/hq/2005/WHO_CDS_CPE_PVC_2005.12.pdf.
- WHO. World Health Organization; 2006. A Guide: Trachoma Prevention through School Health Curriculum Development. [Google Scholar]
- WHO. World Health Organization. The Trachoma Grading Cards Slide 1/2 SAFE Documents. [Accessed June 20, 2008];2008 Available at: http://www.who.int/blindness/causes/trachoma_documents/en/index.html.
- Yajima A, Jouquet P, Do TD, Dang TC, Tran CD, Orange D, Montresor A. High latrine coverage is not reducing the prevalence of soil-transmitted helminthiasis in Hoa Binh province, Vietnam. Trans R Soc Trop Med Hyg. 2009;103(3):237–241. doi: 10.1016/j.trstmh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelbauer K, Speich B, Mausezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9(1):e1001162. doi: 10.1371/journal.pmed.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]