Abstract
Retinol and vitamin A derivatives influence cell differentiation, proliferation, and apoptosis and play an important physiologic role in a wide range of biological processes. Retinol is obtained from foods of animal origin. Retinol derivatives are fundamental for vision, while retinoic acid is essential for skin and bone growth. Intracellular retinoid bioavailability is regulated by the presence of specific cytoplasmic retinol and retinoic acid binding proteins (CRBPs and CRABPs). CRBP-1, the most diffuse CRBP isoform, is a small 15 KDa cytosolic protein widely expressed and evolutionarily conserved in many tissues. CRBP-1 acts as chaperone and regulates the uptake, subsequent esterification, and bioavailability of retinol. CRBP-1 plays a major role in wound healing and arterial tissue remodelling processes. In the last years, the role of CRBP-1-related retinoid signalling during cancer progression became object of several studies. CRBP-1 downregulation associates with a more malignant phenotype in breast, ovarian, and nasopharyngeal cancers. Reexpression of CRBP-1 increased retinol sensitivity and reduced viability of ovarian cancer cells in vitro. Further studies are needed to explore new therapeutic strategies aimed at restoring CRBP-1-mediated intracellular retinol trafficking and the meaning of CRBP-1 expression in cancer patients' screening for a more personalized and efficacy retinoid therapy.
1. Retinol and Derivatives
1.1. Metabolism of Retinol and Its Derivatives
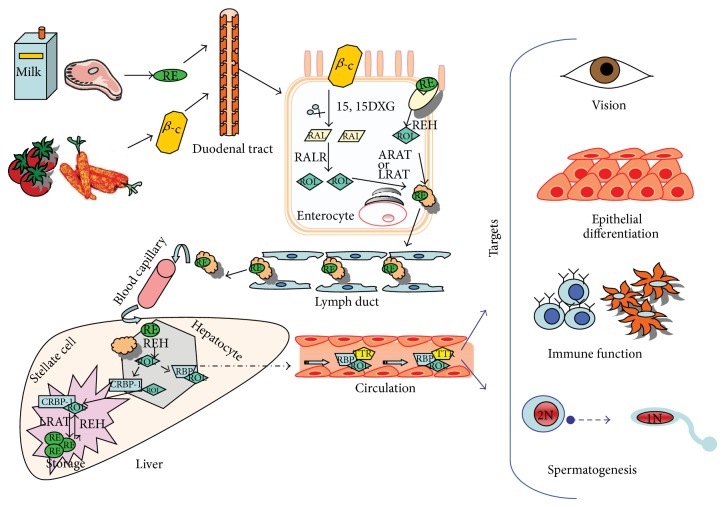
Vitamin A can be acquired from the diet either as preformed vitamin A (primarily as retinyl ester, retinol, and in much smaller amount as retinoic acid) or provitamin A carotenoids (Figure 1). Dietary retinyl esters are converted to retinol within the lumen of the small intestine or the intestinal mucosa and then reesterified to form retinyl ester (RE) within the enterocyte [1]. Provitamin A carotenoids, absorbed by the mucosal cells, are converted first to retinaldehyde and then to retinol [1]. After secretion of the nascent chylomicrons into the lymphatic system, the bulk of dietary vitamin A is taken up by hepatocytes and hydrolyzed again. The free retinol binds the epididymal retinoic acid binding protein (ERABP) and the retinol binding protein (RBP) [2] and into plasma transthyretin. Free retinol can be transferred to hepatic stellate cells for storage. Hepatocytes and hepatic stellate cells are very rich in retinyl ester hydrolases and in cellular retinol binding protein type 1 (CRBP-1). CRBP-1 is necessary to solubilize retinol in the aqueous environment of the cell [1].
Figure 1.
Absorption, transport and distribution of dietary retinoids.
1.2. Intracellular Trafficking of Retinoids
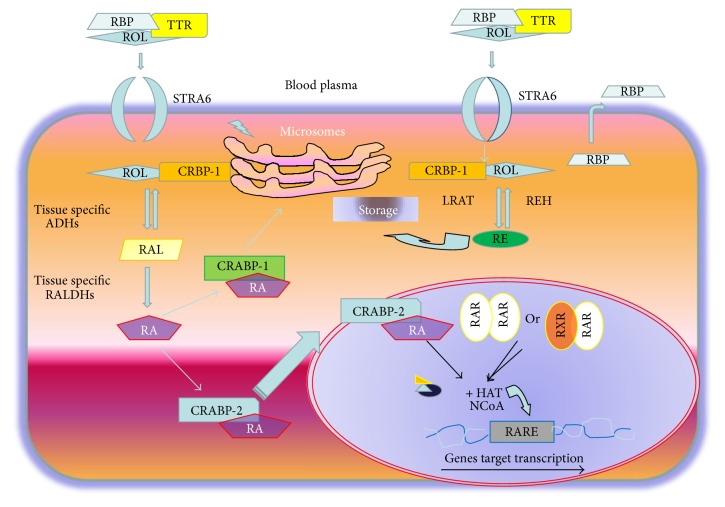
A cell-surface receptor named stimulated by retinoic acid 6 (STRA6) mediates vitamin A uptake from RBP [3]. Intracellular retinoid bioavailability is regulated by the presence of specific cytoplasmic retinol and retinoic acid binding proteins, CRBPs and CRABPs (Figure 2). In the cytoplasm vitamin A and derivatives are bound to cytoplasmic proteins: cellular retinol binding proteins (CRBPs) which comprised four isoforms, CRBP-1 and CRBP-2 and CRBP-3 and CRBP-4. CRBP-1, are the most represented isoform in many tissues. Cellular retinoic acid binding proteins (CRABPs) comprised two isoforms, CRABP-1 and CRABP-2. CRBPs specifically bind retinol, while CRABPs and well-characterized members of the fatty acid binding proteins (FABPs) bind retinoic acid (RA). These proteins control the availability of ligands and determine the physiological response of cells and tissues to vitamin A [4]. Cellular retinoic acid binding proteins may regulate the interactions between retinoic acids and their nuclear receptors by regulating the concentration of present retinoic acids [5]. Retinoids can activate gene expression by specific nuclear retinoid acid receptors. Two distinct classes of nuclear proteins, the retinoic acid receptors (RARs), and the retinoid X receptors (RXRs) have been identified. Each class consists ofα, β, and γ subtypes. RARs and RXRs form either homodimers or heterodimers and function as transacting nuclear transcriptional factors [6]. RAR can be activated by both all-trans and 9-cis RA, whereas RXR is only activated by 9-cis-RA. Heterodimerization of retinoid receptors is essential for the biological activity, although it should be noted that RXRs can heterodimerize with numerous other non-RA associated nuclear receptors to mediate alternative signaling pathways. In the presence of retinoids, nuclear receptors bind to their respective response elements RAREs and RXREs in regulatory regions of target genes and modulate gene transcription [7].
Figure 2.
Intracellular retinoid pathways.
1.3. Retinoids, Tissue Development, and Differentiation
Vitamin A and its derivatives are essential for biological processes such as vision, immune function, reproduction, maintenance of epithelial tissue, and differentiation. Vitamin A deficiency causes different pathological consequences such as night blindness, loss of vision, retardation, shortening and thickening of bones, atrophy of the testes, foetal reabsorption, and immunodeficiency [8]. Instead, an excess of vitamin A can cause teratogenic effects including major alterations in organogenesis [9]. So vitamin A is very important during embryonic development and adult tissue regeneration [10]. The best characterized bioactive metabolites of vitamin A are 11-cis retinal and all-trans retinoic acid (ATRA). The 11-cis retinal metabolite mediates photoreception by acting as the visual chromophore. Most of the nonvisual functions of vitamin A are mediated by ATRA, which regulates the expression of specific subsets of genes within target tissues via nuclear receptors. ATRA can be easily detected in many adult and embryonic tissues [11, 12]. A peculiar kind of retinol-storing cell can be found also in the lung [13]. These cells designated lipid interstitial cells are located in the alveolar wall and release ATRA synthesized from retinol into serum-free [13]. The release of ATRA induces changes in gene expression that initiate the formation of alveoli. It is noteworthy that, during alveolar formation, an increased expression of CRBP-1 is found in the pulmonary microvascular endothelial cells [13]. RA is not synthesized during all stages of development, but his production is restricted in a unique spatiotemporal pattern [14]. RA is expressed in a specific anterior-posterior pattern and its expression becomes more restricted during organogenesis [14]. In the adult RA levels are kept by retinol esterification through CRBP-1 in hepatic stellate cells [15]. Moreover, studies with RAR knockout mice indicate that RAR-α contributes to the regulation of alveolus formation after, but not during, the perinatal period [16], whereas RAR-β is an inhibitor of alveolus formation during, but not after, the perinatal period [17]. These receptors are important as developmental period-specific regulators of alveolus formation [13]. It has been known for more than 60 years that retinoids are involved in regulating the differentiation of various epithelia, promoting the formation of secretory epithelia and inhibiting the formation of highly keratinized, cornified epithelia. They also play a significant role in the control of endometrial growth and differentiation [18]. Given the dramatic shifts in differentiation that occurs in the rodent cervical epithelium from an early secretory phase to a later keratinizing phase, it is likely that retinoids play an important role in modulating the hormonal regulation of cellular differentiation in this tissue; the expression of retinoid binding proteins and nuclear receptors in a given tissue or cell type should be predictive of retinoid responsiveness itself. During pregnancy, in addition, spatial variations in the presence of cellular retinoid binding proteins have been observed, together with differences in patterns of expression between the functionally distinct upper and lower uterine segments [19]. Temporal variations were found with patterns of expression evolving through the three trimesters of pregnancy. This could be important for the regulation of myometrial proliferation in vivo. Moreover, RA is known to inhibit the binding of activator protein 1 to the cyclic AMP response element in the COX-2 promoter [20], so inhibiting phorbol-ester mediated induction of COX-2 gene transcription, whose intracellular levels consequently fall, reducing also prostaglandin production [20]. This process in human myometrium during pregnancy means that RA could contribute to the maintenance of uterine quiescence [19].
1.4. Retinoids and Vascular Pathology
Retinoids target numerous genes implicated in the pathological processes after vascular injury. The latter involves proliferation, differentiation, and migration of medial smooth muscle cells (SMCs) as well as matrix deposition [21–23]. Vascular postinjury, dedifferentiation, growth, and migration result in the formation of a neointima [24]. SMCs migrate to the luminal layer of the arterial wall after endothelial injury. Here growth factors and cytokines induce SMCs to enter into active phases of cell cycle [25]. SMCs proliferation plays also a critical role in the development of restenosis after coronary angioplasty and in the development of fatty streaks to the atherosclerotic plaques [23, 26]. A study shows two kinds of retinol metabolism in vascular SMCs of different phenotypes [24] and, more specifically, an increased uptake of retinol in intimal SMCs compared to medial SMCs, together with an increased expression of the retinoid metabolizing enzymes, such as retinol and retinal dehydrogenase. Consequently, an increased production of RA is found in intimal cells while a higher level of CYP26A1, the retinoic acid catabolizing enzyme, is observed in medial SMCs. Intimal SMCs show a dose- and time-dependent growth inhibition when treated with retinol in contrast to medial SMCs, in which retinol has a mitogenic effect. Moreover, intimal SMCs are more sensitive to RA than medial SMCs [22] and propyonil-L-carnitine increased apoptosis [27, 28]. In fact, systemic administration of RA significantly reduces arterial intimal thickening (IT) after endothelial injury in vivo [29] and induces apoptosis of CRBP-1 expressing intimal cells in vitro, but not of normal media SMCs [22]. Both in vivo and in vitro studies show that neointimal SMCs also display proinflammatory properties, such as higher expression of tumour necrosis factor-α (TNF-α) [30], so contributing to atherosclerotic process and restenosis. The different retinol metabolism in the two SMCs phenotypes supports the role of retinoids in preventing vascular proliferative disorders [31].
1.5. Retinoids and Cancer Therapy
Epidemiological studies have suggested an inverse correlation between cancer development and dietary consumption of vitamin A. Pharmacological concentrations of vitamin A decrease the incidence of chemically induced experimental tumours [32]. Natural and synthetic retinoids have been demonstrated to inhibit the growth and the development of different types of tumours, including skin, breast, oral cavity, lung, hepatic, gastrointestinal, prostatic, and bladder cancers [33–37]. Moreover, the addition of RA or synthetic retinoids to human cancer cell lines or human tumour xenografts in nude mice result in growth arrest, apoptosis, or differentiation [32]. It is noteworthy that natural retinoids act as chemotherapeutic agents for the treatment of acute promyelocytic leukemia (APL). APL is a subset of acute myeloid leukaemia characterized by uncontrolled expansion of leukemia blast cells, blocked at promyelocytic stage of hematopoiesis in the bone marrow [38]. It is characterized by a reciprocal translocation between the long arms of chromosomes 15 and 17 [t(15; 17)] [39–42]. This aberration leads to the fusion between the promyelocytic leukemia (PML) gene located on chromosome 15q21, normally responsible for the formation of the nuclear bodies and regulation of the stem cells self-renewal [43, 44] and the (RAR-α) gene from the chromosome 17q21, with the formation of a chimeric oncoprotein PML-RARA [41, 42, 45]. The fusion transcript is detectable in more than 95% of APL patients with t(15; 17) and acts as an oncogene, causing an enhanced DNA hypermethylation, proliferation, and inhibition of terminal differentiation of hematopoietic cells [46, 47]. ATRA-induced degradation of the fusion product is a basic therapeutic mechanism in APL cells [41, 48]. Recent results reveal several mechanisms leading to the destruction of the fusion oncogene, such as ubiquitination [49], sumoylation [50], or autophagy [51]. High concentrations of ATRA induce postmaturation apoptosis of APL-blasts through the induction of the tumour-selective death ligand TNF-related apoptosis-inducing ligand [52]. Effectiveness of ATRA in the treatment of APL observed in more than 90% of the patients leading to the complete remission [53–55]. Moreover, in animal models several studies establish an inhibitory role of retinoids in breast cancer. It was reported a 52% reduction in the incidence of mammary cancer in animals treated with retinyl acetate [56]. In vitro studies show that retinoids, in particular 9-cis-RA, inhibit the growth of oestrogen receptor- (ER-) positive through blocking cell cycle [57], but not ER-negative human breast cancer cells [25, 58]; ER-negative cells have been demonstrated to express lower RAR-β levels compared to their ER-positive matched cells [59] and they exhibit retinol-induced growth inhibition when transfected with RAR-β [60]. Preclinical studies demonstrate that ATRA induces cell cycle and proliferation arrest in breast cancer cells [61] through the modulation of cyclin-dependent kinase inhibitors p21WAF1 and p27KIP1, with dephosphorylation of retinoblastoma protein [62]. Primary brain tumours are among the top ten causes of cancer-related deaths in the USA. Gliomas, in particular, may result from an imbalance in retinoid receptor expression initiated by environmental factors that increase the endogenous production of RA in the glial cells [63]. It is proposed that this imbalance is characterized by excessive expression of RAR-α and reduced expression of RAR-β. The combined use of RAR-α antagonist and RAR-β agonist is suggested as potential new treatment strategy for gliomas, possibly even at a late stage of the disease. According to this hypothesis, the RAR-α antagonist would be expected to inhibit RAR-α-induced gliomas, while the RAR-β agonist would suppress tumour growth and possibly contribute to the regeneration of normal glia [63]. Moreover, vitamin A reduces the induction of carcinoma of the stomach by polycyclic hydrocarbons [64] and vitamin A-deficient rats are more susceptible to induction of colon tumours by aflatoxin B than normal animals [65]. The combination treatment of histone deacetylase inhibitors SL142 or SL325 with retinoic acids exerts a significant antitumour activity and is a promising therapeutic candidate to treat human lung cancer and show antitumour effect in neuroblastoma [66]. While synthetic retinoids are generally promising for cancer treatment, only few of them are FDA-approved or currently undergoing clinical trials for cancer therapy. Preclinical studies show that synthetic retinoids inhibit human cancer growth. Fenretinide (4-HPR) is one of the most promising clinically tested retinoids. 4-HPR demonstrated a significant cytotoxic activity of tumour cells through the induction of apoptotic and nonapoptotic cell death [67] in breast [68, 69], prostate, bladder, skin [52, 70–74], colon-rectal [75], head and neck [76], ovarian cancers [77, 78], both small cell and non-small cell lung cancer [79–81], neuroblastoma [82–84], and leukaemia cell lines [85, 86]. 4-HPR has activity against tumours by generating reactive oxygen species [76, 87, 88], increasing dihydroceramide production [87, 89–91] and natural killer cell activity [92, 93] and inhibiting angiogenesis [89, 94]. TAC-101 has shown efficacy in inhibiting liver tumour growth in particular of hepatocellular carcinoma [95, 96]. Synthetic retinoids, approved from FDA for dermatological purposes, have a potential antitumour activity. Bexarotene is a retinoid that specifically binds retinoid X receptors and has numerous effects on cellular growth and differentiation. It is approved for the treatment of cutaneous T-cell lymphoma both topically and systemically [97, 98]. A preliminary clinical experience suggests tazarotene, a new acetylenic retinoid, as an effective alternative topical treatment of basal cell carcinomas. Tazarotene demonstrated a good efficacy in the therapy of basal cell carcinoma [99, 100].
2. Cellular Retinol Binding Proteins
2.1. The Specific Role of CRBP-1 in Retinol Metabolism
CRBP-1 is a small cytosolic protein of 15 KDa. CRBP-1 is widely expressed and evolutionarily conserved in many tissues and acts as chaperone protein that regulates the uptake and subsequent esterification of retinol and its bioavailability. CRBP-1 gene is located on 3q21 chromosome. CRBP-1 is required for the efficient synthesis of ATRA [101]. CRBP-1 binds retinol and interacts with enzymes involved in esterification of retinol with long chain fatty acids and in hydrolysis of retinyl esters into retinol. In vitro, CRBP-1 also channels either retinol towards microsomal dehydrogenases that catalyze the oxidation of retinol into RAL or its addressing towards cytosolic dehydrogenases for oxidation into RA. Several microsomal enzymes, termed retinol dehydrogenases types I, II, and III, have high specificity for CRBP-1-bound retinol. Retinal dehydrogenases oxidize CRBP-1-bound retinal [102]. Retinal-CRBP-1 formed through oxidation of retinol-CRBP-1 by the microsomal retinol dehydrogenase might be directly oxidized to RA by retinal dehydrogenases. CRBP-1 may also delivery retinol to newly synthesized RBP for secretion from the liver into the circulation. In the mouse, CRBP-1 deficiency decreases the capacity of hepatic stellate cells to take up incoming retinol and to maintain RE stores, because of an accelerated rate of RE turnover, indicating that CRBP-1 is essential for efficient retinol storage during postnatal life [4].
2.2. Role of CRBP-1 during Development
It has been shown that a lack or an excess of RA during embryonic development results in congenital abnormalities. As a matter of fact, RA regulates genes that specify body axis pattern and may help to program limb formation in the developing embryo. In mature vertebrates, RA maintains epithelial tissues, contributes to bone remodelling and sustains reproductive processes, such as the oestrous cycle, spermatogenesis, and placental growth [103]. As RA synthesis previously seemed to involve intricate metabolic pathways, carried out by multiple enzymes recognizing both liganded (holo-CRBP-1) and nonliganded forms (apo-CRBP-1) of CRBP-1, the role of this protein in organ development has deserved to be deepened. So in mice, the absence of CRBP-1 does not show to be life-threatening during development, at least under conditions of maternal vitamin A dietary sufficiency [4]. However, retinol and RE contents were lower in CRBP-1−/− embryos and foetuses than in WT [4]. Researchers hypothesized that this depends on a reduced transfer of retinol from the maternal circulation to the foetus through the placenta, where it is normally expressed, as CRBP-1 enhances retinol uptake by placental membranes in vitro [104]. It could also depend on an altered retinol uptake by the foetal cells themselves [4]. As a matter of fact, tritiated retinol, administered to pregnant WT mice, specifically accumulates in regions of the embryo expressing CRBP-1 [105]. These lower ER contents could finally depend on a reduced RE half-life, as in adult liver [106] or to an impaired delivery of retinol to specific enzymes for esterification. The normal embryonic development of CRBP-1 null mice suggests that, even though involved in RA homeostasis, CRBP-1 is not critically required for development as its ablation does not change, at least under conditions of maternal vitamin A sufficiency, the expression of RA-target genes during prenatal life. Even if no embryonic, foetal, or placental abnormality could be detected in CRBP-1-null mice (indicating the dispensability of this protein during intrauterine development), CRBP-1-null mice, fully exhausted their RE stores within 5 months and developed abnormalities characteristic of postnatal hypovitaminosis A, as also described in rats [107], and, namely, consisted in vision defects, testicular degeneration, and squamous keratinizing metaplasia. The decrease capacity to store incoming retinol in CRBP-1 null liver could depend on a higher hydrolysis of hepatic stores, due to a decrease of RE half-life and higher amounts of retinol in blood. This hydrolysis could be attributed to an impaired delivery of retinol to esterifying enzymes, above all LRAT, that requires CRBP-1-bound retinol as a substrate [108]. The morphological appearance of CRBP-1 null testes after 14 weeks under the vitamin A deficient diet represents a clear phenocopy of the RAR-α-null mutation, while the squamous keratinizing metaplasia observed in CRBP-1-null mice fed with the vitamin A deficient diet is similar to those observed in old adult mice lacking RAR-γ [109].
2.3. Expression of CRBP-1 in Normal and Adult Tissues
CRBP-1 expression has been studied at histological distinct stages of the rat oestrus cycle. CRBP-1 was detected during the early proliferative phase and after the formation of a mucinous cell layer during proestrus [18]. CRBP-1 expression closely follows the state of differentiation of the cervical epithelial cells. High levels were found in both columnar cells and incompletely differentiated epithelial cells [110]. Decreased CRBP-1 expression coincides with the loss of retinol responsiveness in rat cervical epithelial cells [18], whileCRBP-1 expression was higher in the atrophied epithelium and both CRBP-1 mRNA and protein levels decreased when animals were treated with oestrogen. During proestrus high CRBP-1 expression indicates that high local levels of retinol or retinoic acid are either directly required for the formation of these secretory cells or indirectly required to inhibit keratinization. CRBP-1 levels subsequently decline and the epithelium extensively keratinizes, this might limit retinol uptake and metabolism [18]. It is worth noting that the pattern of CRBP-1 expression in the human endocervical epithelium is identical to that reported for the rat [111]. Moreover, in human, nonpregnant myometrium CRBP-1 is easily available, together with the CRABP proteins, thus suggesting a role for ATRA in the control of myometrial proliferation in vivo, but CRBP-1 is downregulated in both the upper and the lower uterine segments during the first and the second trimester of pregnancy [19]. By the end of the third trimester, CRBP-1 is upregulated in upper segment myometrium, together with CRABP-2 [19]. CRBP-1 expression characterizes endometrial stromal cells at eutopic and ectopic sites and appears to be more specific than CD10 [112]. The level of CRBP-1 varies in intensity according to hormonal variations, with an increase during the secretory phase compared with those of proliferative endometrium. The highest CRBP-1 immunodetection was observed in predecidual and decidual tissue. CRBP-1 expression in the endometrial stroma was similar to that of CD10. Thus, immunodetection of CRBP-1 may help to elucidate the physiopathological changes which occur in endometrial stroma and can also be applied as an adjuvant stromal marker [112].
2.4. Role of CRBP-1 in Tissue Remodelling
One of the key events in the wound repair process is the infiltration of fibroblasts into the damaged area where they proliferate and differentiate into myofibroblasts expressing features of SMCs. These fibroblasts present in the granulation tissue originate from subcutaneous tissue fibroblasts [113]. CRBP-1 expression strongly increased during wound healing in rat skin, suggesting that it plays a role in the evolution of granulation tissue [114]. Liver myofibroblasts derived from hepatic stellate cells or from portal fibroblasts express CRBP-1. HSC express CRBP-1 both in normal liver and during liver fibrosis, as these cells are involved in myofibroblast differentiation [115]. Aberrant CRBP-1 expression has shown to be accompanied by parallel variations ofα-smooth muscle actin. Theα-smooth muscle actin is mainly controlled by TGF-β1, a fibrogenic mediator involved in both myofibroblast differentiation and extracellular matrix deposition. Surprisingly, TGF-β1 induces CRBP-1 gene and protein expression in primary cultures of HSC and in portal fibroblasts. The myofibroblast differentiation of HSC is associated with a decrease in total hepatic levels of retinyl palmitate [116], the predominant storage form of vitamin A in rat HSC, whereas levels of retinol are increased together with CRBP-1 levels [117]. So, it is possible that the stable expression of CRBP-1 in HSC during liver fibrosis is under the control of free retinol level [115]. The pattern of CRBP-1 expression in portal fibroblasts is similar to that observed in subcutaneous fibroblasts or in arterial SMCs that, after injury, rapidly acquire CRBP-1 expression [118]. The expression of CRBP-1 in arterial SMC during arterial repair, in myofibroblasts during skin wound healing and in portal fibroblasts during liver reaction to bile duct ligation, strongly supports that CRBP-1 plays a role in multiple tissue repair phenomena [118]. As a matter of fact, distinct rat aortic SMC subpopulations and clones express CRBP-1 and a CRBP-1-containing SMC subpopulation appears transiently in vivo during the evolution of the experimental aortic IT produced by endothelial injury. CRBP-1 expression in cultured SMCs has shown to be regulated by ATRA and retinol. Moreover, the presence of CRBP-1 in cultured SMCs appears to be correlated with the expression of cytokeratin 8 [119]. CRBP-1 has also shown to be constitutively expressed in aortic endothelial cells and, occasionally, in SMCs of the normal media of adult and old rats but not in newborn rats [118]. It is conceivable that the scattered CRBP-1-positive SMCs of the media participate in the formation of IT after endothelial injury and a proportion of CRBP-1-negative SMCs becomes positive, as the large majority of the IT SMCs express CRBP-1 at 7 days. These results suggest that a subset of medial SMCs becomes rapidly CRBP-1 positive after injury, undergoes replication during the early phases of IT development, and then disappears, possibly through apoptosis, when reendothelialization takes place. So, CRBP-1 is not simply a marker of SMC activation but participates in this process [118].
2.5. CRBP-1 and Cancer Progression: New Therapeutic Perspectives
Because of the complexity of vitamin A metabolism, the precise role of CRBP-1 in retinoid signaling remains controversial, despite numerous studies conducted over the past three decades on its binding properties, three-dimensional structure, tissue localization, regulation of expression, involvement in retinal metabolism, and null mutation [120]. The function of the CRBP-1 gene in controlling the cell bioavailability of vitamin A suggests that it can have a special relevance in the inhibition of early steps of cancer transformation. Nevertheless, in human cancer, the presence and role of the specific binding proteins for retinol and RA have not been extensively investigated. Downregulation or loss of CRBP-1 expression occurs in a series of tumors: breast, ovarian, endometrial, prostate, renal cancer, astrocytic gliomas [112, 121, 122], cervical cancer, larynx cancer, nasopharyngeal carcinoma, lymphoma, and gastrointestinal carcinomas [123]. Furthermore, the CpG island hypermethylation of CRBP-1 is responsible for its inactivation in some cancer cell [124–127]. So, epigenetic disruption of CRBP-1 is a common event in human cancer and may have important implications for cancer prevention and retinoid therapy. What are the biological consequences of the methylation-mediated silencing of CRBP-1? The loss of CRBP-1 may compromise retinoic acid metabolism by reducing retinol transport and blocking the formation of retinyl esters and RAR activity, leading to loss of cellular differentiation and tumor progression [123, 128–130]. CRBP-1 downregulation was observed in stage I as well as in stage II and III patients and also reported in ovarian cancer precursors, suggesting that interruption of CRBP-1 signaling may occur at all stages of cancer progression [120]. Several studies highlighted the role of CRBP-1 signaling in cancer progression during the last years [131], but the mechanisms by which it affects carcinogenesis are far from being fully elucidated. Uterine and gastrointestinal leiomyosarcomas express high levels of CRBP-1, whereas its expression is weak in leiomyoma, symplastic leiomyoma, borderline tumours, and nontumour smooth muscle tissue [27]. Accumulation of CRBP-1 in leiomyosarcoma likely supports the conversion of retinol into RA and its biological effects through RAREs induction, which influence the expression of many genes through the interaction with RAREs regions, inducing the increase of CRBP-1 expression in epithelioid SMCs as well as fibroblasts [29]. These results support the possibility that the expression of CRBP-1 represents a target for pharmacological strategies aimed at influencing sarcoma growth through the control of RA availability. About 30% of colorectal cancers did not present any apparent lesion in the retinoid pathway and this subset of tumors may be extremely sensitive to treatment with retinoids [123]. With regard to liver pathologies, CRBP-1 expression is downregulated in almost all hepatocellular carcinoma specimens [132]. Some studies show that CRBP-1 is strongly expressed in the cytoplasm of hepatic stellate cells in normal liver and in myofibroblasts, with only low CRBP-1 levels in hepatocytes. Patients with high CRBP-1 expression in myofibroblasts show a significantly higher 2-year survival as compared with patients with low CRBP-1 expression [132]. The loss of CRBP-1 expression in intratumoral myofibroblasts likely disturbs retinoid homeostasis, thereby potentially leading to more aggressive liver tumour growth. These studies also highlight a strong positive association between nuclear CRBP-1 expression and nuclear β-catenin accumulation [132, 133]. This cotransactivation complex exerts several regulatory effects on a growing number of downstream target genes, many of which have been implicated in tumorigenesis [134]. In a case-controlled CRBP-1 study in gynaecological cancers, significant differences were found between the concentrations of CRBP-1 in the dysplastic cervical lesions and the normal cervix [122]. CRBP-1 was detected at a reduced level in the carcinoma poorly differentiated of endometrium, ovary (Figure 3), and breast compared with normal tissue aliquots. CRBP-1 immunodetection in simple hyperplasia was weakly and similar to that of proliferative endometrial cells and increased in atypical hyperplasia and in G1 endometrioid carcinomas [122]. Importantly, a progressive decrease of CRBP-1 immunoreactivity was observed in less differentiated endometrial tumours [122]. The striking overall difference in the expression of CRBP-1 in type I and type II endometrial carcinomas reflects the differences in their risk factors and molecular pathogenesis [122]. The low or absent CRBP-1 expression in serous and clear cell carcinomas further supports the presence of distinct molecular carcinogenetic pathways [122].
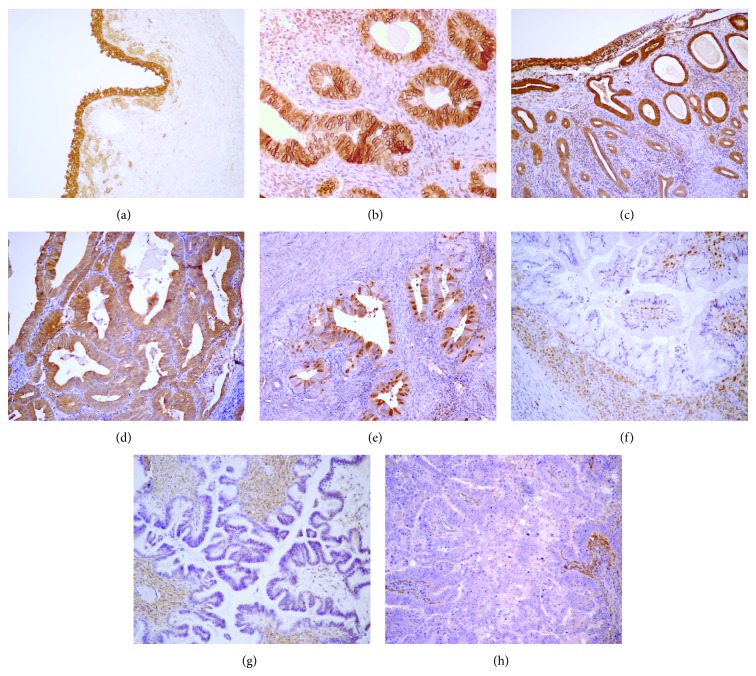
Figure 3.
Immunohistochemical expression of CRBP-1 in normal and neoplastic female reproductive system tissues. (a) CRBP-1 is strongly expressed in normal endocervical epithelial cells, (b) proliferative and (c) atrophic endometrial glandular cells. (d) Well-differentiated (G1) endometrial carcinoma cells show strong and diffuse CRBP-1 positive cells compared to (e) lower and focal CRBP-1 expression in moderately (G2) carcinoma. In the ovary, CRBP-1 is not expressed in (f) mucinous, (g) serous borderline, and (h) well-differentiated (G3) serous carcinoma. Original magnification: (a), (c)–(h) 100x; (b) 200x.
It has been documented that CRBP-1 upregulation exerts a direct antitranscriptional effect through the binding of CRBP-1 promoter to RAR-α [135]. In vitro studies confirmed the downregulation of RAR-α and RAR-γ mRNA levels in CRBP-1-stable-transfected ovarian cancer cells [127]. Abnormal RAR-α upregulation characterizes other malignancies, such as lung cancer and metastatic melanoma [121]. Similarly, RAR-γ upregulation is reported to be prooncogenic and to support the growth/progression of mammary tumours [136]. So, CRBP-1-mediated reactivation of retinol pathways could improve the efficacy of adjuvant retinoid chemotherapy, likely increasing apoptotic susceptibility [127, 137–139] (Figure 4).
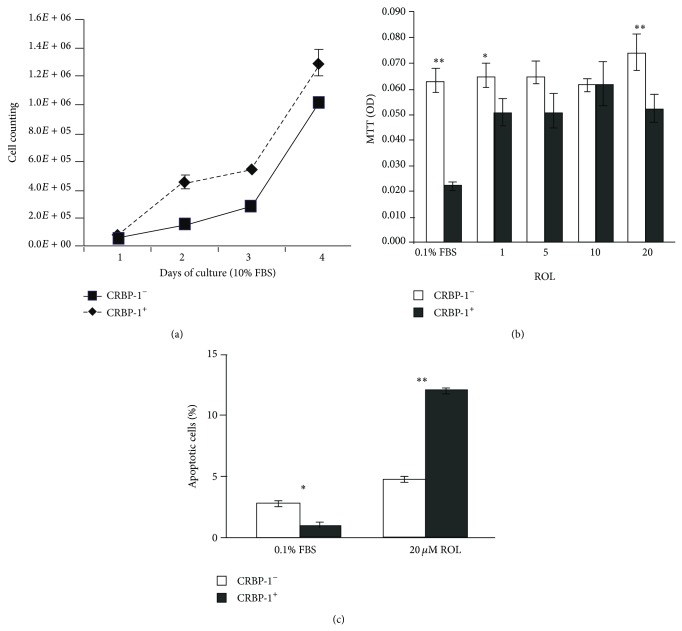
Figure 4.
CRBP-1+ expression influences retinoid-induced A2780 cancer cell viability. (a) Viability of transfected CRBP-1 (CRBP-1+) A2780 cells increased after 4 days in the presence of 10% FBS compared to empty transfected (CRBP-1−) A2780 cells. (b) Retinol induces a reduction of viability in CRBP-1 A2780 cells after 24 h of different ATRA treatments (c) Flow cytometry analysis of Annexin V/PI apoptotic assay shows a higher percentage of dying cells in CRBP-1+ after 2 h of 20 µM retinol treatment compared to CRBP-1− A2780 cells. Values are expressed as means ± SEM of three different experiments; * P < 0.05, ** P < 0.01.
Furthermore, CRBP-1 induces a reduced transcription level and activity of PI3K/Akt pathway, in particular, Akt1 [127]. Although Akt contributes to maintain differentiation of stem cells [140], aberrant pAkt activation sustains cancer progression [127]. CRBP-1 signaling restoration in A2780 ovarian cells induces the downregulation of specific cell proliferation and survival genes STAT1, STAT5, and JUN [127, 141] and downregulation of pErk. The latter is critically involved in the regulation of cell proliferation and survival and its upregulation promotes cancer proliferation, survival, and metastasis [142]. CRBP-1 can have a function independent of its retinol binding ability; in this regard, CRBP-1 also functions in mammary epithelial cell inhibition of the phosphatidylinositol 3-kinase/Akt survival pathway and suppresses anchorage independent growth [121]. Interestingly, somatic CRBP-1 silencing, induced by inoculation of athymic mice with MTSV cells, an SV40-immortalized human mammary epithelial cell line, transfected with empty vector (MTSVvector) or CRBP-1 (MTSVCRBP-1), has shown to prevent tumor cells from taking up, storing, and using retinol in vivo [137]. These in vivo data suggest that somatic CRBP-1 loss results in a local deficit in vitamin A storage and metabolism, with important consequences for the affected tissues.
Reintroduction of CRBP-1 signaling reduced tumorigenicity both in vivo and in vitro, inducing downregulation of survival and proliferative gene pathways and increases retinoid-mediated apoptosis [127, 137, 143]. So, CRBP-1 can represent a potential target for therapeutic strategies aimed at arresting cancer cell growth and tumour progression by increasing intracellular retinol bioavailability [127, 137]. In addition, the presence of detectable CRBP-1 level in a subset of ovarian cancers [127] suggests screening of its expression for a more efficacy and personalized adjuvant retinoid-mediated therapy.
3. Conclusions
Vitamin A and derivatives comprise a group of natural and synthetic molecules, which regulate a variety of essential biological processes during normal development, maintained tissue homeostasis, and also mediate protection from diseases. Retinoids have many important and diverse functions throughout the body including roles in vision, regulation of cell proliferation and differentiation, growth of bone tissue, immune function, and activation of tumour suppressor genes. Genomic functions of the retinoids are mediated via their nuclear DNA-binding receptors, RARs, and RXRs, which regulate gene transcription through recruitment of corepressors and coactivators. Natural and synthetic retinoids have been used as potential chemotherapeutic or chemopreventive agents because of their differentiation, antiproliferative, proapoptotic, and antioxidant effects. The function of the CRBP-1 gene in controlling the availability of retinol to cells suggests that its product has special relevance to inhibition of early steps in transformation. CRBP-1 downregulation occurs in breast and ovarian tumors and compromises RAR activity, leading to loss of cellular differentiation and tumor progression. Furthermore, the CpG island hypermethylation of CRBP-1 is responsible for its inactivation in some cancer cell lines such as cervical, larynx, nasopharyngeal, and gastrointestinal carcinomas and lymphoma [123, 128–130]. So, the loss of CRBP-1 expression is a common event in human cancer that may have important implications for cancer prevention and treatment using retinoids. The possibility to reintroduce CRBP-1-mediated intracellular retinol trafficking in cancer cells can represent a potential tool in strategies aimed at counteracting cancer cell dedifferentiation and minor aggressiveness. Moreover, variability of CRBP-1 expression in some cancers also suggests screening of tumours in order to select patients potentially sensitive to adjuvant retinoid therapy.
Abbreviations
- STRA6:
Stimulated by retinoic acid 6
- RE:
Retinyl ester
- RBP:
Retinol binding protein
- REH:
Retinyl ester hydrolases
- CRBP-1:
Cellular retinol binding protein 1
- RARs:
Retinoic acid receptors
- RXRs:
Retinoid X receptors
- RAREs:
Retinoic acid response elements
- RXREs:
Retinoid X receptor response elements
- RA:
Retinoic acid
- ATRA:
All-trans retinoic acid
- CRABP-1 and CRABP-2:
Cellular retinoic acid binding proteins 1 and 2
- FABPs:
Fatty acid binding proteins
- TNF-α:
Tumour necrosis factor-α
- APL:
Acute promyelocytic leukemia
- PML:
Promyelocytic leukaemia
- ER:
Oestrogen receptor
- RAL:
Retinaldehyde
- SMC:
Smooth muscle cells
- TGF-β1:
Transforming growth factor-β1
- IT:
Intimal thickening
- LEF:
Lymphoid enhancer factor
- TCF:
T-cell factor
- 4-HPR:
Fenretinide.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Gottesman M. E., Quadro L., Blaner W. S. Studies of vitamin A metabolism in mouse model systems. BioEssays. 2001;23(5):409–419. doi: 10.1002/bies.1059. [DOI] [PubMed] [Google Scholar]
- 2.Folli C., Calderone V., Ottonello S., et al. Identification, retinoid binding, and X-ray analysis of a human retinol-binding protein. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):3710–3715. doi: 10.1073/pnas.061455898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawaguchi R., Yu J., Honda J., et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315(5813):820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 4.Matt N., Schmidt C. K., Dupé V., et al. Contribution of cellular retinol-binding protein type 1 to retinol metabolism during mouse development. Developmental Dynamics. 2005;233(1):167–176. doi: 10.1002/dvdy.20313. [DOI] [PubMed] [Google Scholar]
- 5.Donovan M., Olofsson B., Gustafson A.-L., Dencker L., Eriksson U. The cellular retinoic acid binding proteins. The Journal of Steroid Biochemistry and Molecular Biology. 1995;53(1–6):459–465. doi: 10.1016/0960-0760(95)00092-E. [DOI] [PubMed] [Google Scholar]
- 6.Chambon P. A decade of molecular biology of retinoic acid receptors. The FASEB Journal. 1996;10(9):940–954. [PubMed] [Google Scholar]
- 7.Das B. C., Thapa P., Karki R., et al. Retinoic acid signaling pathways in development and diseases. Bioorganic & Medicinal Chemistry. 2014;22(2):673–683. doi: 10.1016/j.bmc.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields A. L., Soprano D. R., Soprano K. J. Retinoids in biological control and cancer. Journal of Cellular Biochemistry. 2007;102(4):886–898. doi: 10.1002/jcb.21530. [DOI] [PubMed] [Google Scholar]
- 9.Beeman C. S., Kronmiller J. E. Temporal distribution of endogenous retinoids in the embryonic mouse mandible. Archives of Oral Biology. 1994;39(9):733–739. doi: 10.1016/0003-9969(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 10.Zile M. H. Function of vitamin A in vertebrate embryonic development. The Journal of Nutrition. 2001;131(3):705–708. doi: 10.1093/jn/131.3.705. [DOI] [PubMed] [Google Scholar]
- 11.Mic F. A., Molotkov A., Benbrook D. M., Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(12):7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molotkov A., Ghyselinck N. B., Chambon P., Duester G. Opposing actions of cellular retinol-binding protein and alcohol dehydrogenase control the balance between retinol storage and degradation. Biochemical Journal. 2004;383(2):295–302. doi: 10.1042/BJ20040621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dirami G., Massaro G. D., Cierch L. B., Ryan U. S., Reczek P. R., Massaro D. Lung retinol storing cells synthesize and secrete retinoic acid, an inducer of alveolus formation. The American Journal of Physiology—Lung Cellular and Molecular Physiology. 2004;286(2):L249–L256. doi: 10.1152/ajplung.00140.2003. [DOI] [PubMed] [Google Scholar]
- 14.Rossant J., Zirngibl R., Cado D., Shago M., Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes & Development. 1991;5(8):1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- 15.Yost R. W., Harrison E. H., Ross A. C. Esterification by rat liver microsomes of retinol bound to cellular retinol-binding protein. Journal of Biological Chemistry. 1988;263(35):18693–18701. [PubMed] [Google Scholar]
- 16.de Carlo Massaro G., Massaro D., Chambon P. Retinoic acid receptor-α regulates pulmonary alveolus formation in mice after, but not during, perinatal period. American Journal of Physiology: Lung Cellular and Molecular Physiology. 2003;284(2):L431–L433. doi: 10.1152/ajplung.00245.2002. [DOI] [PubMed] [Google Scholar]
- 17.Massaro G. D., Massaro D., Chan W. Y., et al. Retinoic acid receptor-beta: an endogenous inhibitor of the perinatal formation of pulmonary alveoli. Physiol Genomics. 2000;4(1):51–57. doi: 10.1152/physiolgenomics.2000.4.1.51. [DOI] [PubMed] [Google Scholar]
- 18.Tannous-Khuri L., Hillemanns P., Rajan N., Wright T. C., Talmage D. A. Expression of cellular retinol- and cellular retinoic acid-binding proteins in the rat cervical epithelium is regulated by endocrine stimuli during normal squamous metaplasia. The American Journal of Pathology. 1994;144(1):148–159. [PMC free article] [PubMed] [Google Scholar]
- 19.Tyson-Capper A. J., Cork D. M. W., Wesley E., Shiells E. A., Loughney A. D. Characterization of cellular retinoid-binding proteins in human myometrium during pregnancy. Molecular Human Reproduction. 2006;12(11):695–701. doi: 10.1093/molehr/gal070. [DOI] [PubMed] [Google Scholar]
- 20.Subbaramaiah K., Cole P. A., Dannenberg A. J. Retinoids and carnosol suppress cyclooxygenase-2 transcription by CREB-binding protein/p300-dependent and -independent mechanisms. Cancer Research. 2002;62(9):2522–2530. [PubMed] [Google Scholar]
- 21.Streb J. W., Miano J. M. Retinoids: pleiotropic agents of therapy for vascular diseases? Current Drug Targets—Cardiovascular and Haematological Disorders. 2003;3(1):31–57. doi: 10.2174/1568006033337393. [DOI] [PubMed] [Google Scholar]
- 22.Orlandi A., Francesconi A., Cocchia D., Corsini A., Spagnoli L. G. Phenotypic heterogeneity influences apoptotic susceptibility to retinoic acid and cis-platinum of rat arterial smooth muscle cells in vitro implications for the evolution of experimental intimal thickening. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(7):1118–1123. doi: 10.1161/hq0701.092144. [DOI] [PubMed] [Google Scholar]
- 23.Orlandi A., Ropraz P., Gabbiani G. Proliferative activity and α-smooth muscle actin expression in cultured rat aortic smooth muscle cells are differently modulated by transforming growth factor-β1 and heparin. Experimental Cell Research. 1994;214(2):528–536. doi: 10.1006/excr.1994.1290. [DOI] [PubMed] [Google Scholar]
- 24.Gidlöf A. C., Ocaya P., Olofsson P. S., Törmä H., Sirsjö A. Differences in retinol metabolism and proliferative response between neointimal and medial smooth muscle cells. Journal of Vascular Research. 2006;43(4):392–398. doi: 10.1159/000094415. [DOI] [PubMed] [Google Scholar]
- 25.Koga M., Ogasawara H. Induction of hepatocyte mitosis in intact adult rat by interleukin-1α and interleukin-6. Life Sciences. 1991;49(17):1263–1270. doi: 10.1016/0024-3205(91)90139-3. [DOI] [PubMed] [Google Scholar]
- 26.Spagnoli L. G., Orlandi A., Mauriello A., et al. Aging and atherosclerosis in the rabbit, 1: distribution, prevalence and mosphology of atherosclerotic lesions. Atherosclerosis. 1991;89(1):11–24. doi: 10.1016/0021-9150(91)90003-L. [DOI] [PubMed] [Google Scholar]
- 27.Orlandi A., Francesconi A., Clément S., Ropraz P., Giusto Spagnoli L., Gabbiani G. High levels of cellular retinol binding protein-1 expression in leiomyosarcoma: possible implications for diagnostic evaluation. Virchows Archiv. 2002;441(1):31–40. doi: 10.1007/s00428-001-0576-7. [DOI] [PubMed] [Google Scholar]
- 28.Orlandi A., Francesconi A., Ferlosio A., et al. Propionyl-L-carnitine prevents age-related myocardial remodeling in the rabbit. Journal of Cardiovascular Pharmacology. 2007;50(2):168–175. doi: 10.1097/FJC.0b013e31805d8ee9. [DOI] [PubMed] [Google Scholar]
- 29.Neuville P., Yan Z.-Q., Gidlöf A., et al. Retinoic acid regulates arterial smooth muscle cell proliferation and phenotypic features in vivo and in vitro through an RARα-dependent signaling pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(6):1430–1436. doi: 10.1161/01.ATV.19.6.1430. [DOI] [PubMed] [Google Scholar]
- 30.Niemann-Jönsson A., Ares M. P. S., Yan Z.-Q., et al. Increased rate of apoptosis in intimal arterial smooth muscle cells through endogenous activation of TNF receptors. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(12):1909–1914. doi: 10.1161/hq1201.100222. [DOI] [PubMed] [Google Scholar]
- 31.Seidel C. L. Cellular heterogeneity of the vascular tunica media: implications for vessel wall repair. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(10):1868–1871. doi: 10.1161/01.ATV.17.10.1868. [DOI] [PubMed] [Google Scholar]
- 32.Niles R. M. Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutation Research. 2004;555(1-2):81–96. doi: 10.1016/j.mrfmmm.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Altucci L., Gronemeyer H. The promise of retinoids to fight against cancer. Nature Reviews Cancer. 2001;1(3):181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 34.Siddikuzzaman, Guruvayoorappan C., Berlin Grace V. M. All trans retinoic acid and cancer. Immunopharmacology and Immunotoxicology. 2011;33(2):241–249. doi: 10.3109/08923973.2010.521507. [DOI] [PubMed] [Google Scholar]
- 35.Niles R. M. Vitamin A and cancer. Nutrition. 2000;16(7-8):573–576. doi: 10.1016/S0899-9007(00)00347-6. [DOI] [PubMed] [Google Scholar]
- 36.Arrieta O., González-De La Rosa C. H., Aréchaga-Ocampo E., et al. Randomized phase II trial of All-trans-retinoic acid with chemotherapy based on paclitaxel and cisplatin as first-line treatment in patients with advanced non-small-cell lung cancer. Journal of Clinical Oncology. 2010;28(21):3463–3471. doi: 10.1200/JCO.2009.26.6452. [DOI] [PubMed] [Google Scholar]
- 37.Bryan M., Pulte E. D., Toomey K. C., et al. A pilot phase II trial of all-trans retinoic acid (Vesanoid) and paclitaxel (Taxol) in patients with recurrent or metastatic breast cancer. Investigational New Drugs. 2011;29(6):1482–1487. doi: 10.1007/s10637-010-9478-3. [DOI] [PubMed] [Google Scholar]
- 38.Hillestad L. K. Acute promyelocytic leukemia. Acta Medica Scandinavica. 1957;159(3):189–194. [PubMed] [Google Scholar]
- 39.Rowley J. D., Golomb H. M., Dougherty C. 15/17 Translocation, a consistent chromosomal change in acute promyelocytic leukaemia. The Lancet. 1977;1(8010):549–550. doi: 10.1016/s0140-6736(77)91415-5. [DOI] [PubMed] [Google Scholar]
- 40.Kakizuka A., Miller W. H., Jr., Umesono K., et al. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor, PML. Cell. 1991;66(4):663–674. doi: 10.1016/0092-8674(91)90112-C. [DOI] [PubMed] [Google Scholar]
- 41.De Thé H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. The PML-RARα fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66(4):675–684. doi: 10.1016/0092-8674(91)90113-D. [DOI] [PubMed] [Google Scholar]
- 42.Borrow J., Goddard A. D., Sheer D., Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science. 1990;249(4976):1577–1580. doi: 10.1126/science.2218500. [DOI] [PubMed] [Google Scholar]
- 43.Dyck J. A., Maul G. G., Miller W. H., Jr., Chen J. D., Kakizuka A., Evans R. M. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994;76(2):333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 44.Bernardi R., Pandolfi P. P. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nature Reviews Molecular Cell Biology. 2007;8(12):1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 45.Dong S., Geng J.-P., Tong J.-H., et al. Breakpoint clusters of the PML gene in acute promyelocytic leukemia: primary structure of the reciprocal products of the PML-RARA gene in a patient with t(15;17) Genes Chromosomes & Cancer. 1993;6(3):133–139. doi: 10.1002/gcc.2870060302. [DOI] [PubMed] [Google Scholar]
- 46.Melnick A., Licht J. D. Deconstructing a disease: RARα, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93(10):3167–3215. [PubMed] [Google Scholar]
- 47.Di Croce L., Raker V. A., Corsaro M., et al. Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science. 2002;295(5557):1079–1082. doi: 10.1126/science.1065173. [DOI] [PubMed] [Google Scholar]
- 48.Nasr R., Guillemin M.-C., Ferhi O., et al. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nature Medicine. 2008;14(12):1333–1342. doi: 10.1038/nm.1891. [DOI] [PubMed] [Google Scholar]
- 49.Kitareewan S., Pitha-Rowe I., Sekula D., et al. UBE1L is a retinoid target that triggers PML/RARα degradation and apoptosis in acute promyelocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):3806–3811. doi: 10.1073/pnas.052011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Y., Qiu J., Chen G., Dong S. Coiled-coil domain of PML is essential for the aberrant dynamics of PML-RARα, resulting in sequestration and decreased mobility of SMRT. Biochemical and Biophysical Research Communications. 2008;365(2):258–265. doi: 10.1016/j.bbrc.2007.10.184. [DOI] [PubMed] [Google Scholar]
- 51.Isakson P., Bjørås M., Bøe S. O., Simonsen A. Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood. 2010;116(13):2324–2331. doi: 10.1182/blood-2010-01-261040. [DOI] [PubMed] [Google Scholar]
- 52.Bushue N., Wan Y.-J. Y. Retinoid pathway and cancer therapeutics. Advanced Drug Delivery Reviews. 2010;62(13):1285–1298. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lo-Coco F., Avvisati G., Vignetti M., et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116(17):3171–3179. doi: 10.1182/blood-2010-03-276196. [DOI] [PubMed] [Google Scholar]
- 54.Lo-Coco F., Avvisati G., Vignetti M., et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. The New England Journal of Medicine. 2013;369(2):111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z.-Y., Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 56.Moon R. C., Grubbs C. J., Sporn M. B. Inhibition of 7,12 dimethylbenz(a)anthracene induced mammary carcinogenesis by retinyl acetate. Cancer Research. 1976;36(7, part 2):2626–2630. [PubMed] [Google Scholar]
- 57.Zhao Z., Zhang Z.-P., Soprano D. R., Soprano K. J. Effect of 9-cis-retinoic acid on growth and RXR expression in human breast cancer cells. Experimental Cell Research. 1995;219(2):555–561. doi: 10.1006/excr.1995.1264. [DOI] [PubMed] [Google Scholar]
- 58.Ribeiro M. P., Santos A. E., Custodio J. B. Interplay between estrogen and retinoid signaling in breast cancer—current and future perspectives. Cancer Letters. 2014;353(1):17–24. doi: 10.1016/j.canlet.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 59.Sheikh M. S., Shao Z.-M., Chen J.-C., Hussain A., Jetten A. M., Fontana J. A. Estrogen receptor-negative breast cancer cells transfected with the estrogen receptor exhibit increased RARα gene expression and sensitivity to growth inhibition by retinoic acid. Journal of Cellular Biochemistry. 1993;53(4):394–404. doi: 10.1002/jcb.240530417. [DOI] [PubMed] [Google Scholar]
- 60.Sheikh M. S., Shao Z.-M., Li X.-S., et al. Retinoid-resistant estrogen receptor-negative human breast carcinoma cells transfected with retinoic acid receptor-α acquire sensitivity to growth inhibition by retinoids. The Journal of Biological Chemistry. 1994;269(34):21440–21447. [PubMed] [Google Scholar]
- 61.Clarke N., Germain P., Altucci L., Gronemeyer H. Retinoids: potential in cancer prevention and therapy. Expert Reviews in Molecular Medicine. 2004;6(25):1–23. doi: 10.1017/S1462399404008488. [DOI] [PubMed] [Google Scholar]
- 62.Wang Q., Lee D., Sysounthone V., et al. 1,25-Dihydroxyvitamin D 3 and retonic acid analogues induce differentiation in breast cancer cells with function- and cell-specific additive effects. Breast Cancer Research and Treatment. 2001;67(2):157–168. doi: 10.1023/A:1010643323268. [DOI] [PubMed] [Google Scholar]
- 63.Mawson A. R. Retinoids in the treatment of glioma: a new perspective. Cancer Management and Research. 2012;4(1):233–241. doi: 10.2147/CMAR.S32449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shibata M.-A., Hirose M., Masuda A., Kato T., Mutai M., Ito N. Modification of BHA forestomach carcinogenesis in rats: inhibition by diethylmaleate or indomethacin and enhancement by a retinoid. Carcinogenesis. 1993;14(7):1265–1269. doi: 10.1093/carcin/14.7.1265. [DOI] [PubMed] [Google Scholar]
- 65.Rogers A. E., Herndon B. J., Newberne P. M. Induction by dimethylhydrazine of intestinal carcinoma in normal rats and rats fed high or low levels of vitamin A. Cancer Research. 1973;33(5):1003–1009. [PubMed] [Google Scholar]
- 66.Han S., Fukazawa T., Yamatsuji T., et al. Anti-tumor effect in human lung cancer by a combination treatment of novel histone deacetylase inhibitors: SL142 or SL325 and Retinoic acids. PLoS ONE. 2010;5(11) doi: 10.1371/journal.pone.0013834.e13834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hail N., Jr., Kim H. J., Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis. 2006;11(10):1677–1694. doi: 10.1007/s10495-006-9289-3. [DOI] [PubMed] [Google Scholar]
- 68.Abou-Issa H., Moeschberger M., Ei-Masry W., Tejwani S., Curley R. W., Jr., Webb T. E. Relative efficacy of glucarate on the initiation and promotion phases of rat mammary carcinogenesis. Anticancer Research. 1995;15(3):805–810. [PubMed] [Google Scholar]
- 69.Kazmi S. M. I., Plante R. K., Visconti V., Lau C. Y. Comparison of N-(4-hydroxyphenyl)retinamide and all-trans-retinoic acid in the regulation of retinoid receptor-mediated gene expression in human breast cancer cell lines. Cancer Research. 1996;56(5):1056–1062. [PubMed] [Google Scholar]
- 70.Moon R. C., Pritchard J. F., Mehta R. G., Nomides C. T., Thomas C. F., Dinger N. M. Suppression of rat mammary cancer development by N-(4-hydroxyphenyl)retinamide (4-HPR) following surgical removal of first palpable tumor. Carcinogenesis. 1989;10(9):1645–1649. doi: 10.1093/carcin/10.9.1645. [DOI] [PubMed] [Google Scholar]
- 71.Pollard M., Luckert P. H., Sporn M. B. Prevention of primary prostate cancer in Lobund-Wistar rats by N-(4-hydroxyphenyl)retinamide. Cancer Research. 1991;51(13):3610–3611. [PubMed] [Google Scholar]
- 72.McCormick D. L., Becci P. J., Moon R. C. Inhibition of mammary and urinary bladder carcinogenesis by a retinoid and a maleic anhydride-divinyl ether copolymer (MVE-2) Carcinogenesis. 1982;3(12):1473–1477. doi: 10.1093/carcin/3.12.1473. [DOI] [PubMed] [Google Scholar]
- 73.McCormick D. L., Moon R. C. Antipromotional activity of dietary N-(4-hydroxyphenyl)retinamide in two-stage skin tumorigenesis in CD-1 and SENCAR mice. Cancer Letters. 1986;31(2):133–138. doi: 10.1016/0304-3835(86)90003-0. [DOI] [PubMed] [Google Scholar]
- 74.Hsieh T.-C., Ng C., Wu J. M. The synthetic retinoid N-(4-hydroxyphenyl) retinamide (4-HPR) exerts antiproliferative and apoptosis-inducing effects in the androgen-independent human prostatic JCA-1 cells. Biochemistry and Molecular Biology International. 1995;37(3):499–506. [PubMed] [Google Scholar]
- 75.Ziv Y., Gupta M. K., Milsom J. W., Vladisavljevic A., Brand M., Fazio V. W. The effect of tamoxifen and fenretinimide on human colorectal cancer cell lines in vitro. Anticancer Research. 1994;14(5A):2005–2009. [PubMed] [Google Scholar]
- 76.Oridate N., Lotan D., Mitchell M. F., Hong W. K., Lotan R. Induction of apoptosis by retinoids in human cervical carcinoma cell lines. International Journal of Oncology. 1995;7(3):433–441. doi: 10.3892/ijo.7.3.433. [DOI] [PubMed] [Google Scholar]
- 77.Supino R., Crosti M., Clerici M. Induction of apoptosis by fenretinide (4HPR) in human ovarian carcinoma cells and its association with retinoic acid receptor expression. International Journal of Cancer. 1996;65(4):491–497. doi: 10.1002/(SICI)1097-0215(19960208)65:4<491::AID-IJC17>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 78.Formelli F., Cleris L. Synthetic retinoid fenretinide is effective against a human ovarian carcinoma xenograft and potentiates cisplatin activity. Cancer Research. 1993;53(22):5374–5376. [PubMed] [Google Scholar]
- 79.Villablanca J. G., London W. B., Naranjo A., et al. Phase II study of oral capsular 4-hydroxyphenylretinamide (4-HPR/fenretinide) in pediatric patients with refractory or recurrent neuroblastoma: a report from the children’s oncology group. Clinical Cancer Research. 2011;17(21):6858–6866. doi: 10.1158/1078-0432.CCR-11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kalemkerian G. P., Slusher R., Ramalingam S., Gadgeel S., Mabry M. Growth inhibition and induction of apoptosis by fenretinide in small-cell lung cancer cell lines. Journal of the National Cancer Institute. 1995;87(22):1674–1680. doi: 10.1093/jnci/87.22.1674. [DOI] [PubMed] [Google Scholar]
- 81.Ohlmann C.-H., Jung C., Jaques G. Is growth inhibition and induction of apoptosis in lung cancer cell lines by fenretinide [N-(4-hydroxyphenyl)retinamide] sufficient for cancer therapy? International Journal of Cancer. 2002;100(5):520–526. doi: 10.1002/ijc.10525. [DOI] [PubMed] [Google Scholar]
- 82.Reynolds C. P., Wang Y., Melton L. J., Einhorn P. A., Slamon D. J., Maurer B. J. Retinoic-acid-resistant neuroblastoma cell lines show altered MYC regulation and high sensitivity to fenretinide. Medical and Pediatric Oncology. 2000;35(6):597–602. doi: 10.1002/1096-911x(20001201)35:6<597::aid-mpo23>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 83.Di Vinci A., Geido E., Infusini E., Giaretti W. Neuroblastoma cell apoptosis induced by the synthetic retinoid N-(4-hydroxyphenyl) retinamide. International Journal of Cancer. 1994;59(3):422–426. doi: 10.1002/ijc.2910590322. [DOI] [PubMed] [Google Scholar]
- 84.Mariotti A., Marcora E., Bunone G., et al. N-(4-hydroxyphenyl)retinamide: a potent inducer of apoptosis in human neuroblastoma cells. Journal of the National Cancer Institute. 1994;86(16):1245–1247. doi: 10.1093/jnci/86.16.1245. [DOI] [PubMed] [Google Scholar]
- 85.Delia D., Aiello A., Lombardi L., et al. N-(4-hydroxyphenyl)retinamide induces apoptosis of malignant hemopoietic cell lines including those unresponsive to retinoic acid. Cancer Research. 1993;53(24):6036–6041. [PubMed] [Google Scholar]
- 86.Ozpolat B., Tari A. M., Mehta K., Lopez-Berestein G. Nuclear retinoid receptors are involved in N-(4-hydroxyphenyl) retinamide (Fenretinide)-induced gene expression and growth inhibition in HL-60 acute myeloid leukemia cells. Leukemia and Lymphoma. 2004;45(5):979–985. doi: 10.1080/1042819031000151833. [DOI] [PubMed] [Google Scholar]
- 87.Maurer B. J., Metelitsa L. S., Seeger R. C., Cabot M. C., Reynolds C. P. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4- hydroxyphenyl)-retinamide in neuroblastoma cell lines. Journal of the National Cancer Institute. 1999;91(13):1138–1146. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 88.Asumendi A., Morales M. C., Alvarez A., Aréchaga J., Pérez-Yarza G. Implication of mitochondria-derived ROS and cardiolipin peroxidation in N-(4-hydroxyphenyl)retinamide-induced apoptosis. British Journal of Cancer. 2002;86(12):1951–1956. doi: 10.1038/sj.bjc.6600356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Erdreich-Epstein A., Tran L. B., Bowman N. N., et al. Ceramide signaling in fenretinide-induced endothelial cell apoptosis. The Journal of Biological Chemistry. 2002;277(51):49531–49537. doi: 10.1074/jbc.M209962200. [DOI] [PubMed] [Google Scholar]
- 90.Wang H., Maurer B. J., Liu Y. Y., et al. N-(4-Hydroxyphenyl)retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Molecular Cancer Therapeutics. 2008;7(9):2967–2976. doi: 10.1158/1535-7163.MCT-08-0549. [DOI] [PubMed] [Google Scholar]
- 91.Maurer B. J., Melton L., Billups C., Cabot M. C., Reynolds C. P. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. Journal of the National Cancer Institute. 2000;92(23):1897–1909. doi: 10.1093/jnci/92.23.1897. [DOI] [PubMed] [Google Scholar]
- 92.Villa M. L., Ferrario E., Trabattoni D., et al. Retinoids, breast cancer and NK cells. British Journal of Cancer. 1993;68(5):845–850. doi: 10.1038/bjc.1993.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Z., Matsuura T., Popoff K., Ross A. C. Effects of N-(4-hydroxyphenyl)-retinamide on the number and cytotoxicity of natural killer cells in vitamin-A-sufficient and -deficient rats. Natural Immunity. 1994;13(5):280–288. [PubMed] [Google Scholar]
- 94.Ribatti D., Alessandri G., Baronio M., et al. Inhibition of neuroblastoma-induced angiogenesis by fenretinide. International Journal of Cancer. 2001;94(3):314–321. doi: 10.1002/ijc.1441. [DOI] [PubMed] [Google Scholar]
- 95.Okusaka T., Ueno H., Ikeda M., Takezako Y., Morizane C. Phase I study of TAC-101, an oral synthetic retinoid, in Japanese patients with advanced hepatocellular carcinoma. Cancer Science. 2012;103(8):1524–1530. doi: 10.1111/j.1349-7006.2012.02334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Minagawa N., Nakayama Y., Inoue Y., et al. 4-[3,5-Bis(trimethylsilyl)benzamido] benzoic acid inhibits angiogenesis in colon cancer through reduced expression of vascular endothelial growth factor. Oncology Research. 2004;14(9):407–414. doi: 10.3727/0965040041791464. [DOI] [PubMed] [Google Scholar]
- 97.Schadt C. R. Topical and oral bexarotene. Dermatologic Therapy. 2013;26(5):400–403. doi: 10.1111/dth.12087. [DOI] [PubMed] [Google Scholar]
- 98.Das B., Yeger H., Tsuchida R., et al. A hypoxia-driven vascular endothelial growth factor/Flt1 autocrine loop interacts with hypoxia-inducible factor-1α through mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 pathway in neuroblastoma. Cancer Research. 2005;65(16):7267–7275. doi: 10.1158/0008-5472.CAN-04-4575. [DOI] [PubMed] [Google Scholar]
- 99.Orlandi A., Bianchi L., Costanzo A., Campione E., Spagnoli L. G., Chimenti S. Evidence of increased apoptosis and reduced proliferation in basal cell carcinomas treated with tazarotene. Journal of Investigative Dermatology. 2004;122(4):1037–1041. doi: 10.1111/j.0022-202X.2004.22414.x. [DOI] [PubMed] [Google Scholar]
- 100.Bianchi L., Orlandi A., Campione E., et al. Topical treatment of basal cell carcinoma with tazarotene: a clinicopathological study on a large series of cases. The British Journal of Dermatology. 2004;151(1):148–156. doi: 10.1111/j.1365-2133.2004.06044.x. [DOI] [PubMed] [Google Scholar]
- 101.Chou A. P., Chowdhury R., Li S., et al. Identification of retinol binding protein 1 promoter hypermethylation in isocitrate dehydrogenase 1 and 2 mutant gliomas. Journal of the National Cancer Institute. 2012;104(19):1458–1469. doi: 10.1093/jnci/djs357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Napoli J. L. Retinoic acid biosynthesis and metabolism. The FASEB Journal. 1996;10(9):993–1001. doi: 10.1096/fasebj.10.9.8801182. [DOI] [PubMed] [Google Scholar]
- 103.Boerman M. H. E. M., Napoli J. L. Cellular retinol-binding protein-supported retinoic acid synthesis: relative roles of microsomes and cytosol. The Journal of Biological Chemistry. 1996;271(10):5610–5616. doi: 10.1074/jbc.271.10.5610. [DOI] [PubMed] [Google Scholar]
- 104.Sundaram M., Sivaprasadarao A., van Aalten D. M. F., Findlay J. B. C. Expression, characterization and engineered specificity of rat epididymal retinoic acid-binding protein. Biochemical Journal. 1998;334(1):155–160. doi: 10.1042/bj3340155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gustafson A.-L., Dencker L., Eriksson U. Non-overlapping expression of CRBP I and CRABP I during pattern formation of limbs and craniofacial structures in the early mouse embryo. Development. 1993;117(2):451–460. doi: 10.1242/dev.117.2.451. [DOI] [PubMed] [Google Scholar]
- 106.Ghyselinck N. B., Båvik C., Sapin V., et al. Cellular retinol-binding protein I is essential for vitamin A homeostasis. The EMBO Journal. 1999;18(18):4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dowling J. E., Wald G. Vitamin A deficiency and night blindness. Proceedings of the National Academy of Sciences of the United States of America. 1958;44(7):648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herr F. M., Ong D. E. Differential interaction of lecithin-retinol acyltransferase with cellular retinol binding proteins. Biochemistry. 1992;31(29):6748–6755. doi: 10.1021/bi00144a014. [DOI] [PubMed] [Google Scholar]
- 109.Mark M., Ghyselinck N. B., Chambon P. Function of retinoic acid receptors during embryonic development. Nuclear Receptor Signaling. 2009;7, article e002 doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tannous-Khuri L., Talmage D. A. Decreased cellular retinol-binding protein expression coincides with the loss of retinol responsiveness in rat cervical epithelial cells. Experimental Cell Research. 1997;230(1):38–44. doi: 10.1006/excr.1996.3399. [DOI] [PubMed] [Google Scholar]
- 111.Hillemanns P., Tannous-Khuri L., Koulos J. P., Talmage D., Wright T. C., Jr. Localization of cellular retinoid-binding proteins in human cervical intraepithelial neoplasia and invasive carcinoma. The American Journal of Pathology. 1992;141(4):973–980. [PMC free article] [PubMed] [Google Scholar]
- 112.Orlandi A., Ferlosio A., Ciucci A., et al. Cellular retinol-binding protein-1 expression in endometrial stromal cells: physiopathological and diagnostic implications. Histopathology. 2004;45(5):511–517. doi: 10.1111/j.1365-2559.2004.01963.x. [DOI] [PubMed] [Google Scholar]
- 113.Xu G., Bochaton-Piallat M.-L., Andreutti D., Low R. B., Gabbiani G., Neuville P. Regulation of α-smooth muscle actin and CRBP-1 expression by retinoic acid and TGF-β in cultured fibroblasts. Journal of Cellular Physiology. 2001;187(3):315–325. doi: 10.1002/jcp.1078. [DOI] [PubMed] [Google Scholar]
- 114.Xu G., Redard M., Gabbiani G., Neuville P. Cellular retinol-binding protein-1 is transiently expressed in granulation tissue fibroblasts and differentially expressed in fibroblasts cultured from different organs. The American Journal of Pathology. 1997;151(6):1741–1749. [PMC free article] [PubMed] [Google Scholar]
- 115.Uchio K., Tuchweber B., Manabe N., Gabbiani G., Rosenbaum J., Desmoulière A. Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Laboratory Investigation. 2002;82(5):619–628. doi: 10.1038/labinvest.3780456. [DOI] [PubMed] [Google Scholar]
- 116.Blomhoff R., Norum K. R., Berg T. Hepatic uptake of [3H]retinol bound to the serum retinol binding protein involves both parenchymal and perisinusoidal stellate cells. The Journal of Biological Chemistry. 1985;260(25):13571–13575. [PubMed] [Google Scholar]
- 117.Blomhoff R., Green M. H., Berg T., Norum K. R. Transport and storage of vitamin A. Science. 1990;250(4979):399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- 118.Neuville P., Geinoz A., Benzonana G., et al. Cellular retinol-binding protein-1 is expressed by distinct subsets of rat arterial smooth muscle cells in vitro and in vivo. The American Journal of Pathology. 1997;150(2):509–521. [PMC free article] [PubMed] [Google Scholar]
- 119.Schwartz M. A., Schaller M. D., Ginsberg M. H. Integrins: emerging paradigms of signal transduction. The Annual Review of Cell and Developmental Biology. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 120.Cvetković D., Williams S. J., Hamilton T. C. Loss of cellular retinol-binding protein 1 gene expression in microdissected human ovarian cancer. Clinical Cancer Research. 2003;9(3):1013–1020. [PubMed] [Google Scholar]
- 121.Kuppumbatti Y. S., Bleiweiss I. J., Mandeli J. P., Waxman S., Mira-Y-Lopez R. Cellular retinol-binding protein expression and breast cancer. Journal of the National Cancer Institute. 2000;92(6):475–480. doi: 10.1093/jnci/92.6.475. [DOI] [PubMed] [Google Scholar]
- 122.Orlandi A., Ferlosio A., Ciucci A., et al. Cellular retinol binding protein-1 expression in endometrial hyperplasia and carcinoma: diagnostic and possible therapeutic implications. Modern Pathology. 2006;19(6):797–803. doi: 10.1038/modpathol.3800586. [DOI] [PubMed] [Google Scholar]
- 123.Esteller M., Guo M., Moreno V., et al. Hypermethylation-associated inactivation of the cellular retinol-binding-protein 1 gene in human cancer. Cancer Research. 2002;62(20):5902–5905. [PubMed] [Google Scholar]
- 124.Lee D. H., Goldberg A. L. Selective inhibitors of the proteasome-dependent and vacuolar pathways of protein degradation in Saccharomyces cerevisiae . The Journal of Biological Chemistry. 1996;271(44):27280–27284. doi: 10.1074/jbc.271.44.27280. [DOI] [PubMed] [Google Scholar]
- 125.Ellenson L. H., Wu T.-C. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5(6):533–538. doi: 10.1016/j.ccr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 126.Jerónimo C., Henrique R., Oliveira J., et al. Aberrant cellular retinol binding protein 1 (CRBP1) gene expression and promoter methylation in prostate cancer. Journal of Clinical Pathology. 2004;57(8):872–876. doi: 10.1136/jcp.2003.014555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Doldo E., Costanza G., Ferlosio A., et al. CRBP-1 expression in ovarian cancer: a potential therapeutic target. Anticancer Research. 2014;34(7):3303–3312. [PubMed] [Google Scholar]
- 128.Mendoza-Rodriguez M., Arreola H., Valdivia A., et al. Cellular retinol binding protein 1 could be a tumor suppressor gene in cervical cancer. International Journal of Clinical and Experimental Pathology. 2013;6(9):1817–1825. [PMC free article] [PubMed] [Google Scholar]
- 129.Peralta R., Baudis M., Vazquez G., et al. Increased expression of cellular retinol-binding protein 1 in laryngeal squamous cell carcinoma. Journal of Cancer Research and Clinical Oncology. 2010;136(6):931–938. doi: 10.1007/s00432-009-0735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kwong J., Lo K.-W., Chow L. S.-N., et al. Epigenetic silencing of cellular retinol-binding proteins in nasopharyngeal carcinoma. Neoplasia. 2005;7(1):67–74. doi: 10.1593/neo.04370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morris E. J., Dreixler J. C., Cheng K.-Y., Wilson P. M., Gin R. M., Geller H. M. Optimization of single-cell gel electrophoresis (SCGE) for quantitative analysis of neuronal DNA damage. BioTechniques. 1999;26(2):282–289. doi: 10.2144/99262st02. [DOI] [PubMed] [Google Scholar]
- 132.Schmitt-Gräff A., Ertelt V., Allgaier H.-P., et al. Cellular retinol-binding protein-1 in hepatocellular carcinoma correlates with β-catenin, Ki-67 index, and patient survival. Hepatology. 2003;38(2):470–480. doi: 10.1053/jhep.2003.50321. [DOI] [PubMed] [Google Scholar]
- 133.Behrens J., Vakaet L., Friis R., et al. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. Journal of Cell Biology. 1993;120(3):757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wong N. A. C. S., Pignatelli M. β-catenin—a linchpin in colorectal carcinogenesis? The American Journal of Pathology. 2002;160(2):389–401. doi: 10.1016/S0002-9440(10)64856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Husmann M., Hoffmann B., Stump D. G., Chytil F., Pfahl M. A retinoic acid response element from the rat CRBPI promoter is activated by an RAR/RXR heterodimer. Biochemical and Biophysical Research Communications. 1992;187(3):1558–1564. doi: 10.1016/0006-291X(92)90480-9. [DOI] [PubMed] [Google Scholar]
- 136.Garattini E., Bolis M., Garattini S. K., et al. Retinoids and breast cancer: from basic studies to the clinic and back again. Cancer Treatment Reviews. 2014;40(6):739–749. doi: 10.1016/j.ctrv.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 137.Farias E. F., Marzan C., Mira-Y-Lopez R. Cellular retinol-bindmg protein-I inhibits PI3K/Akt signaling through a retinoic acid receptor-dependent mechanism that regulates p85-p110 heterodimerization. Oncogene. 2005;24(9):1598–1606. doi: 10.1038/sj.onc.1208347. [DOI] [PubMed] [Google Scholar]
- 138.Campagnolo L., Costanza G., Francesconi A., Arcuri G., Moscatelli I., Orlandi A. Sortilin expression is essential for pro-nerve growth factor-induced apoptosis of rat vascular smooth muscle cells. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0084969.e84969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yasmeen A., Beauchamp M.-C., Piura E., Segal E., Pollak M., Gotlieb W. H. Induction of apoptosis by metformin in epithelial ovarian cancer: involvement of the Bcl-2 family proteins. Gynecologic Oncology. 2011;121(3):492–498. doi: 10.1016/j.ygyno.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 140.Valerio C., Scioli M. G., Gentile P., et al. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Translational Medicine. 2012;1(3):206–220. doi: 10.5966/sctm.2011-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heuser M., Sly L. M., Argiropoulos B., et al. Modeling the functional heterogeneity of leukemia stem cells: role of STAT5 in leukemia stem cell self-renewal. Blood. 2009;114(19):3983–3993. doi: 10.1182/blood-2009-06-227603. [DOI] [PubMed] [Google Scholar]
- 142.Roberts P. J., Der C. J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 143.Farias E. F., Ong D. E., Ghyselinck N. B., Nakajo S., Kuppumbatti Y. S., Lopez R. M. Cellular retinol-binding protein I, a regulator of breast epithelial retinoic acid receptor activity, cell differentiation, and tumorigenicity. Journal of the National Cancer Institute. 2005;97(1):21–29. doi: 10.1093/jnci/dji004. [DOI] [PubMed] [Google Scholar]