Abstract
Background
The aim of this study was to evaluate the effectiveness of linezolid, teicoplanin, and vancomycin in prevention of prosthetic vascular graft infections in a vascular graft infection model.
Material/Methods
Fifty rats were divided into 5 groups. A polytetrafluoroethylene graft was implanted on the back of each rat. Methicillin-resistant Staphylococcus aureus (MRSA) strain was inoculated into all rats except Group 1. Group 2 was not given any treatment, Group 3 received linezolid, Group 4 received vancomycin, and Group 5 received teicoplanin. The grafts were removed for microbiological and histological examinations on the 7th day. In addition, C-reactive protein and prealbumin levels and leukocyte counts in obtained blood specimens were determined.
Results
Group 1 did not have infection. Group 2 had bacteria 5.7×104 CFU/cm2. Group 3 and Group 4 had less bacterial growth. Group 5 had no bacterial growth. The number of bacteria was significantly higher in Group 2 than in the other experimental groups and the control group (p<0.001). Although there was no bacterial growth in Group 5, it did not significantly differ from Group 3 and Group 4. Group 2 had a significantly higher CRP level and leukocyte count and a significantly lower prealbumin level than the other groups.
Conclusions
Linezolid, teicoplanin, and vancomycin are effective in prevention of prosthetic vascular graft infections.
MeSH Keywords: Anti-Bacterial Agents, Staphylococcal Infections, Vascular Grafting
Background
In spite of recent advances in prevention and treatment of prosthetic vascular graft infections, the mortality (up to 70% for intra-abdominal aortic grafts) and rates of extremity amputation (up to 70% for lower extremity grafts) are still high [1,2]. The mortality has been reported to be 40–75% in aortic grafts and 20–50% in grafts of the distal part of the lower extremities. It is nearly 10% in femoropopliteal grafts [3]. The mechanism for graft infection can be perioperative contamination, postoperative wound infection, or systemic bacteremia [4,5]. The most frequent source of infection is staphylococci found on the skin. Prevention of these infections has an important effect on mortality and hospital costs. Prophylaxis is based on asepsis and perioperative administration of systemic antibiotics [6,7]. First- and second-generation cephalosporins are the most frequently used drugs [8]. However, resistance to these drugs has emerged. The widespread use of many antimicrobial agents in both treatment and prophylactic regimens has resulted in a considerable increase in the rate of many organisms resistant to these agents, including methicillin-resistant staphylococci [9]. In the present study, we attempted to test systemic effectiveness of linezolid, teicoplanin, and vancomycin and to compare their effects in an experimental prosthetic vascular graft infection model caused by MRSA, one of the most significant complications of vascular surgery. The graft infection model was created as described in the literature [10–12].
Material and Methods
Organism
The strain of MRSA used in this study was isolated from a clinical specimen submitted for routine bacteriological investigation to the Department of Microbiology, Faculty of Medicine, Kahramanmaras Sutcu Imam University, Turkey. Commercially available Staphylococcus aureus ATCC 43300 was used as a control strain of methicillin susceptibility test. The organism was incubated overnight on sheep blood agar. The numbers of bacteria were determined by turbidimetry and confirmed by the culture results.
Drugs
Vancomycin (Vancomycin HCL), teicoplanin (Targocid), and linezolid (Zyvoxid) were obtained from Mayne Pharma Plc (Warwickshire UK), Aventis Pharma (Istanbul, Turkey), and Pfizer (Istanbul, Turkey), respectively. Solutions were made fresh on the day of the experiments.
Susceptibility testing
The antimicrobial susceptibilities of the strains were determined by using the microbroth dilution method, according to the procedures outlined by the National Committee for Clinical Laboratory Standards. The minimum inhibition concentration was considered to be the lowest antibiotic concentration at which observable growth was inhibited. Experiments were performed in triplicate.
Rat model
The study was approved by the Ethics Committee of the Faculty of Medicine, Kahramanmaras Sutcu Imam University, Turkey (Reference number: 2012/03-6). The study was performed in accordance with the Declaration of Helsinki. Fifty adult male Wistar rats (weight range: 250 to 300 g) were used. All rats had free access to standard rat chow and tap water. The study included a control group with no graft contamination and no antibiotic treatment (Group 1), 1 contaminated group that did not receive any antibiotic treatment (Group 2), 1 contaminated group that received 10 mg/kg intra-peritoneal linezolid (Group 3), 1 contaminated group that received 10 mg/kg intra-peritoneal vancomycin (Group 4), and 1 contaminated group that received 10 mg/kg intra-peritoneal teicoplanin (Group 5). The intraperitoneal antibiotic doses used in this study were the same as those reported in the literature [11,13]. Each group consisted of 10 animals. The drug administration began at the initiation of surgery and continued once a day in the following 72 h. All operations were carried out under sterile conditions. The rats were anesthetized with intraperitoneal ketamine (10 mg/kg) and xylazine (3 mg/kg), the hair on the backs was shaved, and the skin was cleansed with 10% povidone-iodine solution. One subcutaneous pocket was made on the right side of the median line with a 1.5-cm incision. Aseptically, a 1-cm2 sterile PTFE graft (Gore-Tex; W.L. Gore&Associates Inc, USA) was implanted into the pockets. The pockets were closed with 5/0 polypropylene sutures (Dogsan, Turkey), and a sterile saline solution (1 mL) containing the MRSA strain at a concentration of 2×107 CFU/mL was inoculated onto the graft surface using a tuberculin syringe to create a subcutaneous fluid-filled pocket (Groups 2, 3, 4, and 5). The animals were returned to individual cages and thoroughly examined daily. They were sacrificed by an overdose anesthesia 7 days after implantation. Under sterile conditions, all grafts were explanted for bacteriological study. The perigraft tissue was debrided for histological examinations. Additionally, blood samples were collected via cardiopuncture for proinflammatory markers measurement such as CRP and prealbumin levels and leukocytes count.
Assessment of the infection
The explanted grafts were placed in sterile tubes, washed in sterile saline solution, placed in tubes containing 10 mL of phosphate-buffered saline solution, and ultra-sonicated for 5 min to remove the adherent bacteria from the grafts. Quantification of viable bacteria was performed by preparing serial 10-fold dilutions (0.1 ml) of the bacterial suspensions in 10 mM buffer to minimize the carryover effect and by culturing each dilution on blood agar plates. All plates were incubated at 37°C for 48 h and evaluated for the presence of the MRSA strain. The organisms were quantified by counting the number of CFUs per plate. The limit of detection for this method was approximately 5×101 CFU/cm2 of graft tissue.
Biochemical analyses
Intracardiac blood specimens were obtained for biochemistry and hemogram (3 cc blood specimen for biochemistry and 2 cc blood specimen for hemogram). The biochemical evaluation of the local infection created in the rats was based on leukocyte counts, C-reactive protein (CRP) levels (+ acute phase protein), and prealbumin levels (– acute-phase protein). The blood specimens obtained were analyzed in the biochemistry laboratory to determine CRP and prealbumin levels and leukocyte counts.
Histopathological study
The perigraft tissue was taken. The tissues were fixed in a formalin solution for a maximum of 24–48 h. Samples were washed with water and were soaked in a graded series of ethanol (60, 70, 80, 90, and 100%). Then they were held in a solution of xylene for 90 min and were embedded in paraffin at 60°C. Cross sections (5 mm thick) were cut. Hematoxylin and eosin (H&E) staining was used for the histological examination.
All perigraft tissues were examined for signs of inflammation and infection and classified semiquantitatively as follows: grade 0; no inflammation, grade I; focal interstitial inflammation, grade II; a moderate interstitial inflammation, grade III; an intensive interstitial inflammation, and grade IV; abscess formation with tissue necrosis.
Statistical analysis
Statistical analyses were performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL). Quantitative culture results for all groups are presented as the mean ± standard deviation, and the statistical comparisons between groups were performed using analysis of variance (ANOVA) on the log-transformed data with differences between groups assessed with Tukey significant difference test. Statistical significance was defined as a p value of <0.05.
Results
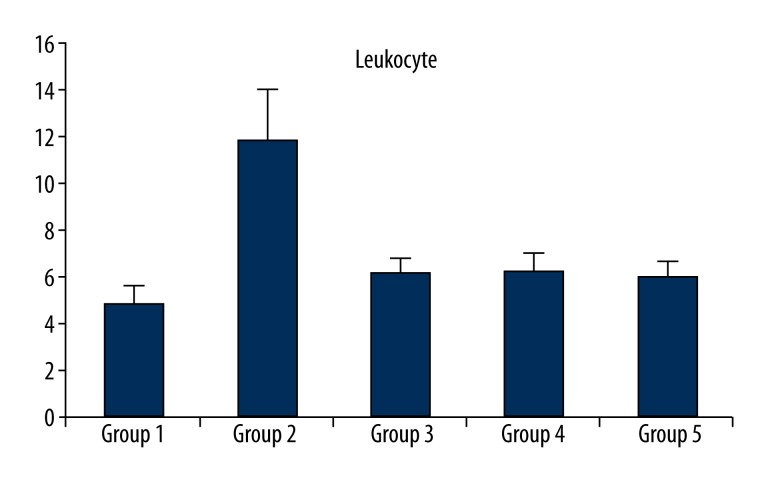
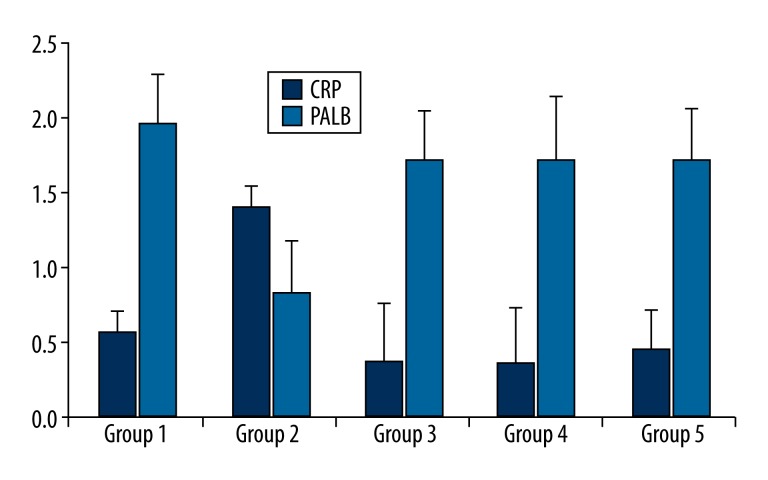
None of the rats died or developed side-effects of the drugs, including anorexia, diarrhea, or behavioral changes. None of the rats in the control group had anatomical or microbiological evidence of graft infection. However, Group 2, which was exposed to inoculation with MRSA but did not receive an antibiotic prophylaxis, had bacterial growth of 5.7×104±1.49×104 CFU/cm2 and the difference between Group 2 and the control group (Group 1) was significant (p<0.001). There was less bacterial growth in Group 3 and Group 4 (4.0×103±9.66×103 and 4.0×103±6.99×103 CFU/cm2, respectively), which were treated with linezolid and vancomycin, respectively. There was not a significant difference in bacterial growth between Group 3 and Group 4 and the control group (p=0.832 and p=0.832, respectively). The quantitative culture results showed no bacterial growth in Group 5, which was treated with teicoplanin. The groups treated with linezolid and vancomycin (Group 3 and Group 4) had far less bacterial growth than Group 2, but there was not a significant difference between Group 3 and Group 4. Although no bacterial growth was observed in the group treated with teicoplanin, the difference between this group and those treated with linezolid and vancomycin was not significant. The difference between Group 2 and Group 3, and Group 4 and Group 5 was significant (p<0.001 for all). The groups, treatment protocols, and results of quantitative results of the microbiological examinations are shown in Table 1. Histopathological examinations demonstrated focal interstitial inflammation in all the groups (Figure 1). An evaluation of the biochemical examinations revealed that CRP levels and leukocyte counts were significantly higher and prealbumin levels were significantly lower in Group 2 than in the control group and the treatment groups (p<0.001) (Table 2, Figures 2, 3).
Table 1.
Study groups and quantitative microbiological results of in vivo experiments.
| Groups | Method of treatments | Quantitative graft culture (cfu/ml) |
|---|---|---|
| Group 1 | No infection, control group | 0.00±0.0 |
| Group 2 | Infection with MRSA, no antibiotic | 5.7×104±1.49×104* |
| Group 3 | Infection with MRSA, linezolid group | 4.0×103±9.66×103** |
| Group 4 | Infection with MRSA, vancomycin group | 4.0×103±6.99×103** |
| Group 5 | Infection with MRSA, teicoplanin group | 0.00±0.0** |
MRSA – Methicillin-resistant Staphylococcus aureus. Each group consisted of 10 animals. Statistical significance was evaluated by the use analysis of variance (ANOVA) on the log-transformed data by the Tukey significant difference test.
Statistically significant versus Group 1;
statistically significant versus Group 2.
Figure 1.
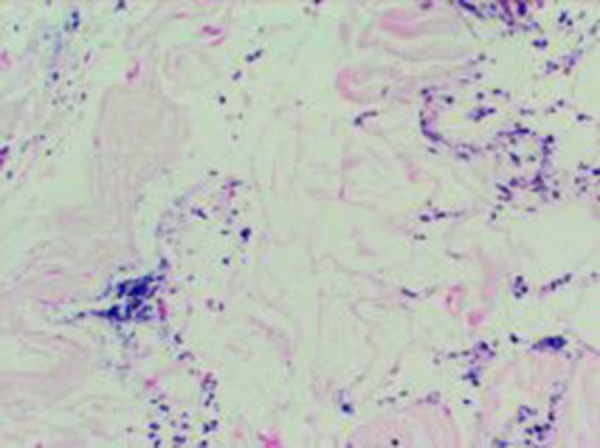
Histopathological findings of the study groups.
Table 2.
Leukocyte, C-reactive protein, and prealbumin values in groups.
| CRP | PALB | LEU | |
|---|---|---|---|
| Group 1 | 0.56±0.15 | 1.98±0.32 | 4.81±.076 |
| Group 2 | 1.42±0.15* | 0.83±0.34* | 11.85±2.23* |
| Group 3 | 0.36±0.39 | 1.72±0.34 | 6.07±0.74 |
| Group 4 | 0.37±0.35 | 1.73±.0.42 | 6.12±0.85 |
| Group 5 | 0.45±0.26 | 1.73±0.34 | 5.92±0.77 |
CRP – C-reactive protein; PALB – Prealbumin; LEU – Leukocyte.
Statistically significant versus Group 1, 3, 4, and 5.
Figure 2.
Leukocyte value in groups.
Figure 3.
C-reactive protein (CRP) and prealbumin (PALB) values in groups.
Discussion
MRSA is one of the most serious and common causes of prosthetic graft infections [14,15]. In an experimental study, S. aureus has been shown to cause bacterial growth in 100% of the animals within 1 month of treatment with graft replacement [16]. In another experimental study, parenteral administration of antibiotics decreased the incidence of bacterial graft infections. The risk of contamination is the highest soon after graft implantation, but decreases as the luminal pseudointimal layer develops [17]. Widespread use of many antimicrobial agents for both prophylaxis and treatment has resulted in a considerable increase in the number of the organisms resistant to MRSA. Mutations causing resistance help bacteria to protect themselves against clinically used antibiotics [7,8]. Emergence of resistant organisms has stimulated investigations of new antimicrobial agents and biological materials. Asepsis and prophylactic administration of systemic antibiotics are necessary to prevent graft infections. However, an increasing number of gram-positive pathogens, especially S. aureus, have started to become resistant to conventional antibiotics such as cefazolin [18]. As a result, penicillinase-producing strains and MRSA have started to emerge. MRSA infections were first treated with glycopeptides (vancomycin and teicoplanin). They have an excellent bactericidal effect on penicillinase-producing bacteria [7]. However, in recent years strains resistant to antibiotics have emerged. Therefore, a need for new antimicrobial agents and new treatment alternatives has arisen. One of these agents, linezolid, is an oxazolidinone, the chemical composition of which is different from that of the agents used now [18]. Linezolid, teicoplanin, and vancomycin seem to be acceptable alternatives for prevention of vascular graft infections. In this context, since antibiotic susceptibility testing is the treatment of choice before testing on humans, we tested susceptibilities of linezolid, teicoplanin, and vancomycin to MRSA on rats. Glycopeptides like vancomycin and teicoplanin are bactericidal agents that can prevent the ability of bacteria to form walls. In the present study, vancomycin effectively reduced bacterial growth (4.0×103±6.99×103 CFU/cm2). In fact, there was a significant difference in bacterial growth between the treated groups and the untreated group (p<0.001). When compared to the control group, the difference was not significant, although a higher number of bacteria were isolated (p=0.832). Antrum et al. suggested that teicoplanin, an agent preferred for use in vascular surgery, better penetrated to the ischemic tissue [19]. Similarly, Turgut et al. concluded that teicoplanin was effective in reduction of prosthetic vascular graft infections [20]. In the present study, teicoplanin effectively reduced bacterial growth. Indeed, the difference between the group receiving teicoplanin and the untreated group was significant (p<0.001). However, there was not a significant difference between the teicoplanin group and the control group (p=1.000). When compared with linezolid and vancomycin, teicoplanin was better in reducing bacterial growth, but the difference was not significant (p=0.832). In the present study, treatment with teicoplanin was given for 3 days because previous studies showed that the best outcome was obtained by a 3-day treatment [21]. In another study we performed previously, single doses of intraperitoneal vancomycin and teicoplanin decreased MRSA growth but did not completely eliminate it [13]. In the present study, a 3-day vancomycin treatment had a similar effect to a single-dose vancomycin treatment, but 3-day teicoplanin treatment had a complete bactericidal effect. In another study conducted in our clinic, effects of vancomycin and teicoplanin administered for differing periods of time on infections due to methicillin sensitive and methicillin resistant S. aureus were evaluated and teicoplanin given for 3 days was found to have a bactericidal effect on both types of infections, although both agents were effective [21]. In another study conducted in our clinic, effects of single doses of vancomycin, teicoplanin, and linezolid on MRSA graft infection were investigated and single doses of teicoplanin and linezolid were found to be more effective than a single dose of vancomycin, although they did not have bactericidal effects [22]. Linezolid is an oxazolidinone, which is a new generation of antibiotics [23]. In an experimental study, Edminston et al. reported that linezolid and daptomycin had a strong antimicrobial effect on staphylococcus strains that stick to medical equipment. It has been reported that effects of linezolid on MRSA can be comparable to those of glycopeptides like vancomycin and teicoplanin and are even stronger. However, in the current study, although linezolid reduced bacterial growth as effectively as vancomycin, its efficiency was not as high as that of teicoplanin [24,25]. Inflammation is a strong and exaggerated physiological response to tissue damage caused by infectious, physical, chemical, and other agents. It is a protective reaction necessary to eliminate the causes of cellular damage (e.g., microorganisms and toxins) and to remove necrotic cells and tissue emerging as a result of cellular damage [26,27]. In the present study, the severity of inflammation, which is a wound-healing parameter, was evaluated histopathologically. Inflammation was expected to be the most severe in Group 2, which did not receive any antibiotics, although inoculated with S. aureus. However, focal interstitial inflammation was observed in all the groups and no significant difference was found between the groups in terms of the severity of inflammation. One of the most important occurrences in inflammation is leukocyte migration to the inflammation region. Leukocytes phagocytize microorganisms, kill bacteria, and eliminate necrotic tissue and foreign antigens. However, they sometimes cause prolongation of inflammation and may cause tissue damage through the enzymes, chemical mediators, and toxic oxygen free radicals they release. So that homeostasis impaired due to bacterial and viral infections, trauma, and tissue damage can be restored, many physiological changes occur in the host. In general, these systemic biochemical changes are known to be acute phase reactions and are a defense response of the organism to restore its integrity. Acute-phase reactions are characterized by fever, changes in vascular permeability, and metabolic and catabolic changes in many organs [28]. During an acute-phase response, leukocyte counts increase because cytokines directly or indirectly stimulate the bone marrow. The most well known acute-phase protein is CRP. CRP levels start to rise 4–6 h after the onset of inflammation and peak 24–48 h later [29]. In the present study, CRP levels and leukocyte counts were significantly higher, but prealbumin levels were significantly lower in the infected and untreated group than in the control group and treatment groups. These results show that inflammation continued in the infected and untreated group, but ceased in the other groups. The significantly increased CRP levels and leukocyte counts and significantly decreased prealbumin levels in the infected and untreated group compared to the control and treatment groups support the results of the microbiological examinations.
Conclusions
It can be concluded that teicoplanin is more effective in preventing vascular graft infection experimentally created in a rat model. When compared to linezolid and vancomycin, teicoplanin was found to suppress bacterial growth completely. However, there are 2 limitations of this study. One limitation is that tissue reaction to the prosthesis rather than the healing process of the prosthetic vascular infection was monitored in the experimental model. The other limitation is that the sample size was small, which might prevent obtaining strong evidence. Nevertheless, in light of the results of the study, it can be suggested that administration of linezolid, teicoplanin, and vancomycin may prevent MRSA infections that occur after prosthetic vascular surgery.
Footnotes
This manuscript was presented as an oral presentation at the 13th Turkish Cardiovascular Surgery National Congress, Antalya, Turkey, 30 October to 2 November 2014
Competing interests
The authors declare that there is no conflict of interest.
Source of support: This research was funded by the Kahramanmaraş Sutcu Imam University Scientific Research Fund
References
- 1.Antonios VS, Noel AA, Steckelberg JM, et al. Prosthetic vascular graft infection: a risk factor analysis using a case-control study. J Infect. 2006;53:49–55. doi: 10.1016/j.jinf.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Seeger JM, Pretus HA, Welborn MB, et al. Long-term outcome after treatment of aortic graft infection with staged extra-anatomic bypass grafting and aortic graft removal. J Vasc Surg. 2000;32:451–59. doi: 10.1067/mva.2000.109471. [DOI] [PubMed] [Google Scholar]
- 3.Goldstone J. Infected prosthetic arterial grafts. 3rd ed. Vascular Surgery: Appleton and Lange; 1989. pp. 564–74. [Google Scholar]
- 4.Lehnhardt FJ, Torsello G, Claeys LG, et al. Systemic and local antibiotic prophylaxis in the prevention of prosthetic vascular graft infection: an experimental study. Eur J Vasc Endovasc Surg. 2002;23:127–33. doi: 10.1053/ejvs.2001.1571. [DOI] [PubMed] [Google Scholar]
- 5.Sago T, Mori Y, Takagi H, et al. Local treatment of Dacron patch graft contaminated with Staphylococcusaureus with antibiotic-releasing porous apatite ceramic: anexperimental study in the rabbit. J Vasc Surg. 2003;37:169–74. doi: 10.1067/mva.2003.105. [DOI] [PubMed] [Google Scholar]
- 6.Ghiselli R, Giacometti A, Goffi L, et al. Efficacy of rifampin-levofloxacin as a prophylactic agent in preventing Staphylococcus epidermidis graft infection. Eur J Vasc Endovasc Surg. 2000;20:508–11. doi: 10.1053/ejvs.2000.1239. [DOI] [PubMed] [Google Scholar]
- 7.Giacometti A, Cirioni O, Ghiselli R, et al. Policationic peptides as prophylactic agents againstmethicillin-susceptible or methicillin-resistant Staphylococcus epidermidis vascular graft infection. Antimicrob Agents Chemother. 2000;44:3306–9. doi: 10.1128/aac.44.12.3306-3309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghiselli R, Giacometti A, Goffi L, et al. Prophylaxis against Staphylococcus aureus vascular graft infection with mupirocin-soaked, collagen-sealed Dacron. J Surg Res. 2001;99:316–20. doi: 10.1006/jsre.2001.6138. [DOI] [PubMed] [Google Scholar]
- 9.Calik S, Turhan T, Yurtseven T, et al. Vancomycin versus linezolid in the treatment of methicillin-resistant Staphylococcus aureus meningitis in an experimental rabbit model. Med Sci Monit. 2012;18(11):SC5–8. doi: 10.12659/MSM.883528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghiselli R, Giacometti A, Cirioni O, et al. Temporin A as a prophylactic agent against methicillin sodium-susceptible and methicillin sodium-resistant Staphylococcus epidermidis vascular graft infection. J Vasc Surg. 2002;36:1027–30. doi: 10.1067/mva.2002.127530. [DOI] [PubMed] [Google Scholar]
- 11.Giacometti A, Ghiselli R, Cirioni O, et al. Therapeutic efficacy of the magainin analogue MSI-78 in different intra-abdominal sepsis rat models. J Antimicrob Chemother. 2004;54:654–60. doi: 10.1093/jac/dkh390. [DOI] [PubMed] [Google Scholar]
- 12.Cirioni O, Giacometti A, Ghiselli R, et al. Prophylactic efficacy of topical temporin A and RNAIII-inhibiting peptide in a subcutaneous rat Pouch model of graft infection attributable to staphylococci with intermediate resistance to glycopeptides. Circulation. 2003;108:767–71. doi: 10.1161/01.CIR.0000083717.85060.16. [DOI] [PubMed] [Google Scholar]
- 13.Yasim A, Gul M, Atahan E, et al. Efficacy of vancomycin, teicoplanin and fusidic acid as prophylactic agents in prevention of vascular graft infection: an experimental study in rat. Eur J Vasc Endovasc Surg. 2006;31:274–79. doi: 10.1016/j.ejvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Nasim A, Thompson MM, Naylor AR, et al. The impact of MRSA on vascular surgery. Eur J Vasc Endovasc Surg. 2001;22:211–14. doi: 10.1053/ejvs.2001.1429. [DOI] [PubMed] [Google Scholar]
- 15.Murphy GJ, Pararajasingam R, Nasim A, et al. Methicillin-resistant Staphylococcus aureus infection in vascular surgicalpatients. Ann R Coll Surg Engl. 2001;83:158–63. [PMC free article] [PubMed] [Google Scholar]
- 16.Roon AJ, Malone JM, Moore WS, et al. Bacteremic infectability: A function of vascular graft material and design. J Surg Res. 1977;22:489–98. doi: 10.1016/0022-4804(77)90031-2. [DOI] [PubMed] [Google Scholar]
- 17.Malone JM, Moore WS, Campagna G, et al. Bacteremic infectability of vascular grafts: The influence of pseudointimal integrity and duration of graft function. Surgery. 1975;78:211–16. [PubMed] [Google Scholar]
- 18.Liekweg WG, Jr, Greenfield LJ. Vascular prosthetic infections: collected experience and results of treatment. Surgery. 1977;81:335–42. [PubMed] [Google Scholar]
- 19.Antrum RM, Bibby SR, Ramsden CH, et al. Teicoplanin: Part I. An evaluation of the concentrations seen in serum and the subcutaneousfat of the relatively ischaemic limb following a single intravenous bolus. Drugs Exp Clin Res. 1989;15:21–23. [PubMed] [Google Scholar]
- 20.Turgut H, Sacar S, Kaleli I, et al. Systemicand local antibiotic prophylaxis in the prevention of Staphylococcus epidermidis graft infection. BMC Infect Dis. 2005;5:91. doi: 10.1186/1471-2334-5-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atahan E, Gul M, Ergun Y, Eroglu E. Vascular graft infection by Staphylococcus aureus: efficacy of cefazolin, teicoplanin and vancomycin prophylaxis protocols in a rat model. Eur J Vasc Endovasc Surg. 2007;34:182–87. doi: 10.1016/j.ejvs.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Atahan E, Katrancioglu N, Oztop Y, et al. Vascular graft infection by Staphylococcus aureus: efficacy of linezolid, teicoplanin and vancomycin systemic prophylaxis protocols in a rat model. Cardiovasc J Afr. 2009;20:122–25. [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens DL, Dotter B, Madaras-Kelly K. A review of linezolid: thefirst oxazolidinone antibiotic. Expert Rev Anti Infect Ther. 2004;2:51–59. doi: 10.1586/14787210.2.1.51. [DOI] [PubMed] [Google Scholar]
- 24.Edmiston CE, Goheen MP, Seabrook GR, et al. Impact of selective antimicrobial agents on staphylococcal adherence to biomedical devices. Am J Surg. 2006;192:344–54. doi: 10.1016/j.amjsurg.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Abb J. In vitro activity of linezolid, quinupristin-dalfopristin, vancomycin, teicoplanin, moxifloxacin and mupirocin against methicillin-resistant Staphylococcus aureus: comparative evaluation by the E test and a broth microdilution method. Diagn Microbiol Infect Dis. 2002;43:319–21. doi: 10.1016/s0732-8893(02)00407-8. [DOI] [PubMed] [Google Scholar]
- 26.Cotran RS, Kumar V, Robbins SL. Robbins, Pathologic Basis of Disease. 8th ed. WB Saunders Company; Philadelphia: 2000. Inflammation and Repair; pp. 25–45. [Google Scholar]
- 27.Kushener I. Regulation of the acute phase response by cytokines. Perspect Biol Med. 1993;36:611–22. doi: 10.1353/pbm.1993.0004. [DOI] [PubMed] [Google Scholar]
- 28.Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunology Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 29.Povoa P. C-reactive protein: A valuable marker of sepsis. Intensive Care Med. 2002;28:235–43. doi: 10.1007/s00134-002-1209-6. [DOI] [PubMed] [Google Scholar]