Abstract
Background
Although aberrant expression of several miRNAs was found during the pathological development of endometriosis to endometriosis-associated ovarian cancer (EAOC), their roles are not fully understood. miR-191 is a miRNA significantly upregulated in endometriosis and EAOC patients. However, its downstream network is still not clear. This study explored its role in malignant transformation of endometriosis to EAOC.
Material/Methods
Tissues from 12 healthy controls, 12 patients with endometriomas, and 12 patients with EAOC were used to verify miR-191 expression by using qRT-PCR. Endometriosis cell line CRL-7566 and ovarian endometrioid carcinoma cell line CRL-11731 were used to explore the downstream regulative function of miR-191.
Results
By using tissue and serum samples from healthy, endometriosis, and EAOC participants, we confirmed that miR-191 expression was significantly higher in endometriosis and EAOC participants. Interestingly, we also observed that TIMP3 expression was negatively correlated with miR-191 expression. Overexpressing miR-191 in CRL-7566 significantly increased cell proliferation and invasion, while miR-191 knockdown in CRL-11731 cells significantly decreased cell proliferation and invasion. These modulating effects of miR-191 are achieved through its regulation of TIMP3.
Conclusions
miR-191 can directly regulate TIMP3 expression, thereby affecting cell proliferation rate and invasion ability. The miR-191-TIMP3 axis might be critical in the malignant transformation of endometriosis to EAOC.
MeSH Keywords: Endometriosis, MicroRNAs, Ovarian Neoplasms
Background
Endometriosis is a benign condition in which endometrial glands and stroma grow outside of the endometrial cavity [1,2]. Previous studies found that endometriosis is associated with significantly increased risk of ovarian cancer [3]. However, the underlying mechanism of malignant transformation of endometriosis is still not clear. There is emerging evidence about aberrant expression of several miRNAs during the pathological development of endometriosis to endometriosis-associated ovarian cancer (EAOC) [4,5]. As a group of conservative and noncoding RNA that regulate expression or degradation of target mRNA, some of them might play critical roles in the malignant transformation of endometriosis [5]. For example, miR-21 and miR-214 are significantly upregulated in EAOC, suppressing the expression of PTEN, a well know tumor suppressor gene [6]. miR-200c is also highly expressed in endometrial tumors and regulates cellular transformation, angiogenesis, inflammation and cell proliferation by directly targeting ZEBs, VEGFA, FLT1, IKKb, KLF9, and FBLN5 at the same time [7]. miR-15b, miR-16, miR-191 and miR-195 are also dysregulated in EAOC [5]. However, their roles in malignant transformation of endometriosis are still not fully understood.
Tissue inhibitor of metalloprotease 3 (TIMP3) is a proapoptotic protein whose expression is negatively correlated with cell growth and invasiveness [8]. Previous studies proved TIMP3 is a direct target of miR-191 in colorectal carcinoma [9]. miR-191 can regulate invasiveness of the cancer cells through directly targeting TIMP3 [9]. Lowered TIMP3 expression was also observed during endometrial transition from normal into cancerous state [10]. However, the exact role of TIMP3 and how it is regulated in endometriosis and EAOC is still not clear. Considering the regulative role of miR-191 over TIMP3 expression, this study hypothesized that the miR-191-TIMP3 axis might play some important roles in malignant transformation of endometriosis to EAOC.
Material and Methods
Human tissues samples
Participants of this study were recruited from the First Hospital affiliated to Hebei Medical University. The study design was approved by the ethics committee of the university. Written informed consent was obtained from each participant before the study. A total of 36 participants were included in this study, including 12 healthy controls, 12 patients with endometriomas, and 12 patients with EAOC (8 endometrioid and 4 clear cell tumor). Endometriomas and EAOC was diagnosed and confirmed by histological examination of laparoscopic biopsy. Patients who were diagnosed with ASRM stage II/III endometriosis were included in this study. Disease tissues from endometriomas and EAOC patients were collected during their laparoscopic surgical examination or resection. Healthy ovarian tissues were collected from participants who received laparoscopic biopsy due to suspicious ovarian cyst but in whom no evidence of ovarian pathology was found. Five-ml blood samples were collected from each participant to isolate serum. Generally, after coagulation, the blood samples were centrifuged at 3,500 rpm for 10 min to isolate serum.
Cell culture
Endometriosis cell line CRL-7566 (which were isolated from both the inner and outer surfaces of the endometriosis cyst), ovarian endometrioid carcinoma cell line CRL-11731 (grade 3, stage IIIc), and HEK293T cells were obtained from ATCC. CRL-7566 and CRL-11731 cells were grown in RPMI-1640 medium (Sigma–Aldrich Co.) supplemented with 10% heat-inactivated FBS, while HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (DEME) (Sigma-Aldrich Co.) supplemented with 10% heat-inactivated FBS. All cells were maintained in an incubator with humidified air and 5% CO2 at 37°C.
Cell transfection
miR-191 mimics, antagomiR-191, TIMP3 siRNA and the corresponding negative controls (NC) were purchased from Ribo Life Science (China). CRL-7566 cells were transfected with 75 nM miR-191 mimics (by using lipofectamine 2000, Invitrogen) or 50 nM si-TIMP3 (by using Oligofectamine, Invitrogen) for overexpression of miR-191 and knockdown TIMP3 mRNA translation, respectively. CRL-11731 cells were transfected with 200 nM antagomiR-191 (by using lipofectamine 2000, Invitrogen) for miR-191 knockdown.
Human TIMP3 lentiviral vector (Lenti-TIMP3) and the empty vector control were purchased from GENECHEM. To generate lentiviral particles for transfection, Lenti-TIMP3 and the corresponding packaging mix were cotransfected to HEK-293T cells. At 48 h after transfection, the viral supernatant was collected for further experiments. CRL-11731 cells were infected with the viral supernatants with the presence of 8 μg/ml Polybrene (Sigma-Aldrich) for overexpressing TIMP3.
qRT-PCR analysis of miR-191 and TIMP3 mRNA expression
To quantify miR-191 in serum and tissue samples, total miRNA from serum were extracted by using miRNeasy Serum/Plasma Kit (QIAGEN), while total miRNA from tissue was extracted by using the mirVana miRNA isolation kit (Ambion), respectively, according to manufacturers’ instructions. Then, the miRNA-specific cDNA was reversely transcribed by using the TaqMan MicroRNA Reverse Transcription Kit. With the cDNA template, mature miR-191 was quantified using Taqman miRNA Assays (Applied Biosystems) with RNU6B as a gene for normalization.
To quantify the expression of TIMP3 mRNA, total RNAs in tissue samples were extracted using Trizol Reagent firstly. Then, the first strand cDNA was reversely transcribed by using RevertAid first strand cDNA synthesis kit (Fermentas). With the cDNA template, TIMP3 mRNA level was quantified by qRT-PCR analysis using Syber Green PCR MasterMix (Applied Biosystems) and TIMP3 specific primers: forward: 5′-TGCAACTTCGTGGAGAGGTG-3′, reverse: 5′-AGGTGATACCGATAGTTCAGCC-3′.GAPDH served as internal control.
Western blot analysis of TIMP3 protein expression
Total proteins from tissue and cell samples were extracted by using RIPA buffer (50 mM TrisHCl, 150 mM NaCl, 2 mM EDTA, 1% NP-40 and 0.1% SDS). Then, the proteins were separated in 10% SDS-PAGE gel and then transferred to a nitrocellulose membrane (Bio-Rad). The membrane was blocked with 5% nonfat milk and then incubated with anti-TIMP3 (1:1000, ab39184, Abcam) and anti-GAPDH (loading control) (1: 2000, ab37168, Abcam) at 4°C overnight. Then, the membrane was included with HRP conjugated anti-rabbit IgG (1:10000, ab97064, Abcam) for 1 h at room temperature. The signals were visualized using ECL reagents (Beyotime, China). The TIMP3-specific signal is a band of approximately 24 kDa.
Colony formation assay
After transfection, 5×102 cells were seeded into 1 well of a 6-well plate and further incubated for 10 days without disturbance. Then, the cells were stained with 0.1% crystal violet and colonies (more than 50 cells) were calculated. Colony formation rate was defined as number of colonies/number of seeded cells ×100%. Each test was performed in triplicate.
In vitro invasion assay
Invasive ability of the cells after transfection were measured by using 24-well transwell cell culture inserts with 8-μm pores (Corning). Generally, cells were first cultured in serum-free RPMI-1640 medium for 24 h and then 5×104 cells were seeded into the top chamber coated with 200 mg/mL of Matrigel (BD Biosciences). The lower chamber was filled with 2.5 mL RPMI-1640 medium containing 20% heat-inactivated FBS, which acts as a chemotactic gradient to stimulate cell penetration. At 24 h after incubation at 37°C, the topside of the membrane was wiped with cotton wool to remove noninvasive cells. The invasive cells on the bottom side were fixed by using 100% methanol for 10 min, then dried at room temperature and stained with 0.1% crystal violet for 20 min. The number of invasive cells was counted under a microscope with 100× magnification. Each test was performed in triplicate.
Statistical analysis
Experimental data are given as mean ±SD based on at least three repeats. Between-group comparison was performed by using unpaired T test. p value of <0.05 was considered significant. *, **, and *** donate significance at 0.05, 0.01 and 0.001 level respectively.
Results
MiR-191 is positively, while TIMP3 is negatively related to endometriosis and EAOC
A total of 36 participants were recruited in this study. Their basic characteristics are summarized in Table 1. qRT-PCR analysis showed that miR-191 expression was lowest in healthy serum and tissue samples, but was significantly increased in endometriosis and further increased in EAOC samples (Figure 1A, 1B). We also observed that the expression of TIMP3, a proven target gene of miR-191 in several types of cancer, showed a reverse trend to miR-191. Its expression was highest in healthy tissues, but dramatically declined in endometriosis and further declined in EAOC samples at both mRNA and protein level (Figure 1C). In endometriosis cell line CRL-7566 and EAOC cell line CRL-11731, we also verified this expression trend. MiR-191 expression was significantly higher in CRL-11731 cells than in CRL-7566 cells (Figure 1D), while TIMP3 expression was significantly lower in CRL-11731 cells than in CRL-7566 cells (Figure 1E). These results suggest that miR-191 is positively, while TIMP3 is negatively, related to endometriosis and EAOC.
Table 1.
The key characteristics of participants.
| Healthy | Endometriosis | EAOC | |
|---|---|---|---|
| No | 12 | 12 | 12 |
| Age (mean ±SD) | 33±4 | 35±5 | 39±3 |
| Stage of disease n (%) | N.A | ASRM stage | FIGO Stage |
| N.A. | Stage II 5 (41.6) | Stage I/II 8 (66.7) | |
| N.A. | Stage III 7 (58.4) | Stage III/IV 4 (33.3) |
EAOC – endometriosis associated ovarian cancer; ASRM – American Society for Reproductive Medicine; FIGO – International Federation of Gynecology and Obstetrics; N.A. – not applicable.
Figure 1.
miR-191 is positively, while TIMP3 is negatively, related to endometriosis and EAOC. (A and B) qRT-PCR analysis of miR-191 expression in serum (A) and tissue (B) samples from 12 healthy controls, 12 patients with endometriomas, and 12 patients with EAOC. (C) qRT-PCR analysis of TIMP3 mRNA and Western blot analysis of TIMP3 protein expression in tissue samples from 12 healthy controls, 12 patients with endometriomas, and 12 patients with EAOC. (D) qRT-PCR analysis of miR-191 expression in endometriosis cell line CRL-7566 and EAOC cell line CRL-11731. (E) qRT-PCR analysis of TIMP3 mRNA and Western blot analysis of TIMP3 protein expression in CRL-7566 and CRL-11731 cells. Values are the average of triple determinations with the S.D. indicated by error bars. *, **, and *** donate significance at 0.05, 0.01 and 0.001 level, respectively.
MiR-191 modulates cell proliferation and invading ability of endometriosis and EAOC cells
To further explore the regulative role of miR-191 in endometriosis and EAOC cell lines, CRL-7566 and CRL-11731 cells were transfected with miR-191 mimics or antagomiR-191, respectively (Figure 2A, 2C). By conducting colony formation assay, we observed that overexpressing miR-191 in CRL-7566 cells significantly increased cell proliferation (Figure 2B), while miR-191 knockdown in CRL-11731 cells significantly decreased cell proliferation (Figure 2D). At the same time, miR-191 overexpression in CRL-7566 also significantly promoted invasive capability of the cells (Figure 2E), while miR-191 knockdown in CRL-11731 cells significantly inhibited the invasive capability (Figure 2F). We also verified the regulative role of miR-191 on TIMP3 expression in these 2 cell lines. MiR-191 overexpression led to TIMP3 downregulation (Figure 2E), while miR-191 knockdown led to increased TIMP expression (Figure 2F). These results suggest that miR-191 can modulate proliferation and invasion ability of endometriosis and EAOC cells.
Figure 2.
MiR-191 modulates cell proliferation and invading ability of endometriosis and EAOC cells. (A and C) Transfection of miR-191 mimics (75 nM) into CRL-7566 cells (A) and antagomiR-191 (200 nM) into CRL-11731 cells (C). (B and D) Colony formation assay of CRL-7566 cells transfected with miR-191 mimics (B) and CRL-11731 cells transfected with antogaomiR-191. (E and F) Cell invasion assay of CRL-7566 cells transfected with miR-191 mimics (75 nM) (E) and CRL-11731 cells transfected with antogaomiR-191 (200 nM) (F). Western blot analysis was also performed to detect TIMP3 expression in these cells after transfection. miR-NC: miR-negative control. Values are the average of triple determinations with the S.D. indicated by error bars. *, **, and *** donate significance at 0.05, 0.01 and 0.001 level, respectively.
MiR-191 modulates cell proliferation and invading ability of endometriosis and EAOC cells through TIMP3
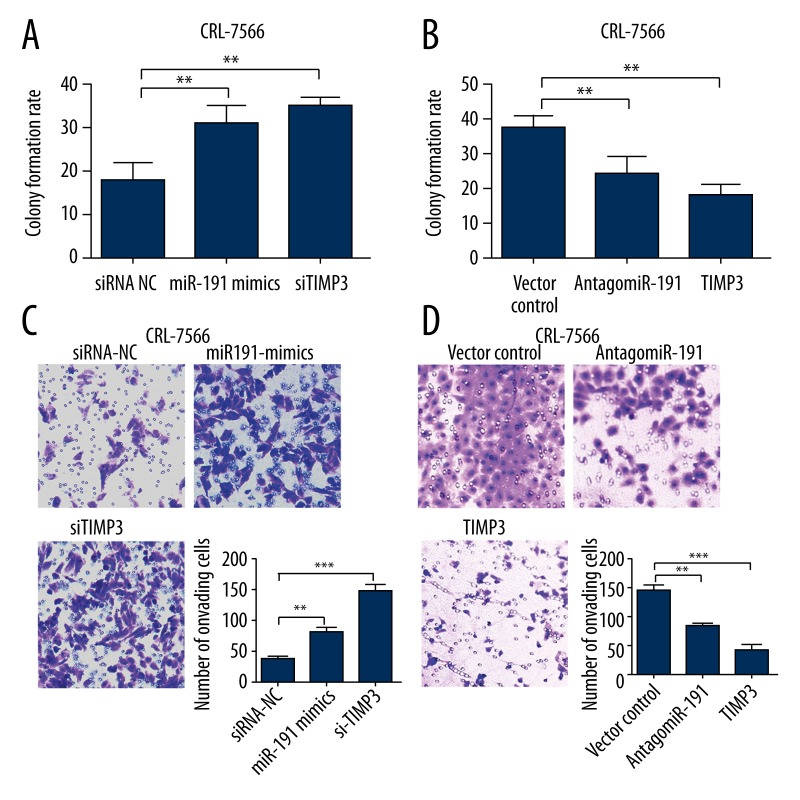
Considering the regulative effect of miR-191 on TIMP3 expression, we further explored the role of TIMP3 in proliferation and invasion of endometriosis and EAOC cells. Knockdown of TIMP3 by siRNA, similar to miR-191 overexpression, could significantly promote cell proliferation (Figure 3A). However, TIMP3 overexpression led to decreased cell proliferation, with an effect similar that of antagomiR-191 (Figure 3B). TIMP3 knockdown significantly increased invasion ability of CRL-7566 cells (Figure 3B). TIMP3 overexpression significantly decreased invasion ability of CRL-11731 cells (Figure 3C). These results suggest that miR-191 can modulate cell proliferation and invasion ability of endometriosis and EAOC cells through TIMP3.
Figure 3.
MiR-191 modulates cell proliferation and invasion ability of endometriosis and EAOC cells through TIMP3. (A and B) Colony formation assay of CRL-7566 cells transfected with miR-191 mimics (75 nM) or TIMP3 siRNA (50 nM) (B) and CRL-11731 cells transfected with antogaomiR-191 (200 nM) or infected with lentiviral TIMP3 vector. (C and D) Cell invasion assay of CRL-7566 cells transfected with miR-191 mimics (75 nM) or TIMP3 siRNA (50 nM) (C) and CRL-11731 cells transfected with antogaomiR-191 (200 nM) or infected with lentiviral TIMP3 vector (D). siRNA-NC: siRNA negative control. Values are the average of triple determinations with the S.D. indicated by error bars. *, **, and *** donate significance at 0.05, 0.01 and 0.001 level, respectively.
Discussion
miRNAs are a group of single-stranded, noncoding, small RNA regulating gene expression through inhibiting translation or promoting degradation of the target mRNAs [11]. Although aberrant expression of several miRNAs was found during the pathological development of endometriosis to EAOC, their roles are not fully understood. MiR-191 is a miRNA significantly upregulated in endometriosis and EAOC patients compared to healthy controls [5]. It is viewed as an OncomiR in several types of cancer. Elevated miR-191 expression and its experimentally validated targets include NDST1 in gastric cancer, TIMP3 in colorectal cancer, and MDM4-C in ovarian cancer [12]. However, although its ectopic high expression was observed in endometriosis and EAOC patients, its downstream network is still not defined. By using tissue and serum samples from healthy, endometriosis, and EAOC participants, we confirmed that miR-191 expression was significantly higher in endometriosis and EAOC participants. Interestingly, we also observed that TIMP3 expression was negatively correlated with miR-191 expression in the samples.
Significantly decreased TIMP3 is observed in several types cancers, including endometrioid carcinomas [13,14]. One recent study analyzed TIMP3 expression in 60 cases of endometrioid carcinomas and found loss of TIMP-3 protein expression in 44 (73%) of the 60 cases [13]. In fact, the metalloprotease family members, including TIMP1, TIMP2, TIMP3, and TIMP4, might be all related to migration and invasion of human immortalized endometriotic epithelial and stromal cells [15]. Inhibiting prostaglandin E2 (PGE2), which is considered as a promoter of the pathogenesis of endometriosis, can suppress migration and invasion of human immortalized endometriotic epithelial and stromal cells by increasing the expression of TIMP1, TIMP2, TIMP3, and TIMP4 proteins [15]. However, the role of TIMP3 in endometriosis and EAOC and its upstream regulative network is still not fully understood. One recent study observed that miR-181a and miR-98 expression were significantly higher in endometrial transition from normal into cancerous state [10]. miR-181a can directly target TIMP3 and suppress its expression at both mRNA and protein levels through binding to the 3′-untranslated regions [10]. Considering the wide regulative network of miRNAs, it is highly possible that other miRNAs are involved in the regulation of TIMP3 in endometriosis and EAOC. In the current study, we found that miR-191 can directly regulate TIMP3 expression in both endometriosis and EAOC cells. TIMP3 overexpression could increase malignant features of CRL-7566 cells, while its downregulation could decrease malignant features of CRL-11731 cells. Therefore, TIMP3 might be a key player modulating cell proliferation and invasion of endometriosis and EAOC cells. Through regulating TIMP3, miR-191 can also promote cell proliferation and invasion of endometriosis cells. Therefore, the miR-191-TIMP3 axis might play an important role in the pathological development of endometriosis to EAOC.
Conclusions
miR-191 can directly regulate TIMP3 expression and thereby affecting cell proliferation rate and invading ability. The miR-191-TIMP3 axis might be critical in the malignant transformation of endometriosis to EAOC.
Footnotes
Source of support: Departmental sources
References
- 1.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Cho HY, Kim K, Jeon YT, et al. CA19-9 elevation in ovarian mature cystic teratoma: discrimination from ovarian cancer – CA19-9 level in teratoma. Med Sci Monit. 2013;19:230–35. doi: 10.12659/MSM.883865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce CL, Templeman C, Rossing MA, et al. Ovarian Cancer Association, Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–94. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braza-Boils A, Mari-Alexandre J, Gilabert J, et al. MicroRNA expression profile in endometriosis: its relation to angiogenesis and fibrinolytic factors. Hum Reprod. 2014;29:978–88. doi: 10.1093/humrep/deu019. [DOI] [PubMed] [Google Scholar]
- 5.Suryawanshi S, Vlad AM, Lin HM, et al. Plasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin Cancer Res. 2013;19:1213–24. doi: 10.1158/1078-0432.CCR-12-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. 2010;16:142–65. doi: 10.1093/humupd/dmp034. [DOI] [PubMed] [Google Scholar]
- 7.Panda H, Pelakh L, Chuang TD, et al. Endometrial miR-200c is altered during transformation into cancerous states and targets the expression of ZEBs, VEGFA, FLT1, IKKbeta, KLF9, and FBLN5. Reprod Sci. 2012;19:786–96. doi: 10.1177/1933719112438448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276:12501–4. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- 9.Qin S, Zhu Y, Ai F, et al. MicroRNA-191 correlates with poor prognosis of colorectal carcinoma and plays multiple roles by targeting tissue inhibitor of metalloprotease 3. Neoplasma. 2014;61:27–34. [PubMed] [Google Scholar]
- 10.Panda H, Chuang TD, Luo X, Chegini N. Endometrial miR-181a and miR-98 expression is altered during transition from normal into cancerous state and target PGR, PGRMC1, CYP19A1, DDX3X, and TIMP3. J Clin Endocrinol Metab. 2012;97:E1316–26. doi: 10.1210/jc.2012-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagpal N, Kulshreshtha R. miR-191: an emerging player in disease biology. Front Genet. 2014;5:99. doi: 10.3389/fgene.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catasus L, Pons C, Munoz J, et al. Promoter hypermethylation contributes to TIMP3 down-regulation in high stage endometrioid endometrial carcinomas. Histopathology. 2013;62:632–41. doi: 10.1111/his.12047. [DOI] [PubMed] [Google Scholar]
- 14.Guan Z, Zhang J, Song S, Dai D. Promoter methylation and expression of TIMP3 gene in gastric cancer. Diagn Pathol. 2013;8:110. doi: 10.1186/1746-1596-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Banu SK, Subbarao T, et al. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits invasion of human immortalized endometriotic epithelial and stromal cells through suppression of metalloproteinases. Mol Cell Endocrinol. 2011;332:306–13. doi: 10.1016/j.mce.2010.11.022. [DOI] [PubMed] [Google Scholar]