Abstract
Background.
Pancreatic ductal adenocarcinoma (PDAC) is a lethal disease generally refractory to standard chemotherapeutic agents; therefore improvements in anticancer therapies are mandatory. A major determinant of therapeutic resistance in PDAC is the poor drug delivery to neoplastic cells, mainly due to an extensive fibrotic reaction. Electroporation can be used in vivo to increase cancer cells’ local uptake of chemotherapeutics (electrochemotherapy, ECT), thus leading to an enhanced tumour response rate. In the present study, we evaluated the in vivo effects of reversible electroporation in normal pancreas in a rabbit experimental model. We also tested the effect of electroporation on pancreatic cancer cell lines in order to evaluate their increased sensitivity to chemotherapeutic agents.
Materials and methods.
The application in vivo of the European Standard Operating Procedure of Electrochemotherapy (ESOPE) pulse protocol (1000 V/cm, 8 pulses, 100 μs, 5 KHz) was tested on the pancreas of normal New Zealand White Rabbits and short and long-term toxicity were assessed. PANC1 and MiaPaCa2 cell lines were tested for in vitro electrochemotherapy experiments with and without electroporation. Levels of cell permeabilization were determined by flow cytometry, whereas cell viability and drug (cisplatin and bleomycin) sensitivity of pulsed cells were measured by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay.
Results.
In healthy rabbits, neither systemic nor local toxic effects due to the electroporation procedure were observed, demonstrating the safety of the optimized electric parameters in the treatment of the pancreas in vivo. In parallel, we established an optimized protocol for ECT in vitro that determined an enhanced anti-cancer effect of bleomycin and cisplatin with respect to treatment without electroporation.
Conclusions.
Our data suggest that electroporation is a safe procedure in the treatment of PDAC because it does not affect normal pancreatic parenchyma, but has a potentiating effect on cytotoxicity of bleomycin in pancreatic tumour cell lines. Therefore, ECT could be considered as a valid alternative for the local control of non-resectable pancreatic cancer.
Keywords: electroporation, bleomycin, cisplatin, electrochemotherapy, preclinical study, safety, pancreatic adenocarcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive disease with a poor 5-year survival, resulting in the fifth leading cause of cancer-related death in Europe.1–3 PDAC is usually diagnosed in advanced stage, with loco-regional invasion and distant metastasis, and usually results largely drug-resistant. Surgical resection, although is currently the only “curative” chance to prolong survival, is suitable only for a minority of patients (< 20%). Standardized protocols of treatment in resectable patients provide a 6-month-course adjuvant chemotherapy following resection4, although the survival is still poor.
The optimal treatment for patients with locally advanced pancreatic cancer (LAPC) with no evidence of metastases remains to be defined. The standard treatment for LAPC is based on Chemotherapy (CT)5–9 and combination of CT and Radiotherapy (RT) with a median overall survival of 10–15 months.10–12
Because of the poor results achieved, some new ablative techniques have been considered for local treatment: radiofrequency ablation (RFA), laser ablation, microwave, ethanol injection and irreversible electroporation (IRE).13 Particularly, RFA has been used in the clinical setting with promising preliminary results.14–17
Patients with metastatic disease undergo systemic palliative chemotherapy. Treatment outcomes are typically poor and resistance to standard chemotherapeutic agents remains the major problem.18–24
Based on recent preclinical data in mouse models, it has been hypothesised that currently available anticancer drugs cannot access tumour cells at an effective concentration due to the presence of an extensive hypo-vascular fibrotic stroma that acts as a barrier shielding tumour cells from the action of systemic therapeutic agents.25 Therefore, the improvement of anticancer drug delivery enhances tumour cells sensitivity and hence would provide a better response and a potential prolongation of survival.
Electroporation is a physical method that employs microsecond length electric pulses to alter temporarily the permeability of cell membranes in order to facilitate chemicals and large molecules delivery to the cells.26–28 Electroporation can be used in all types of isolated cells as well as in tissues. Target cells have to be exposed to an electric field of sufficient strength and for a sufficient time. The magnitude of the electric field depends on cell type, size, orientation and density, pulse duration and number of pulses.29
Electrochemotherapy (ECT) combines the administration of non-permeant or low-permeant cytotoxic drugs (bleomycin or cisplatin) with the application to the tumour of permeabilizing electric pulses, to increase local drug uptake and hence its effectiveness.30, 31
This combination treatment has proven to be very effective in local control of skin metastatic tumour nodules independently of the histotype32–39 and it is now routinely employed for this purpose in a number of European countries, in about 140 cancer treatment centres; recently this technique has been developed for the treatment of deep-seated tumours.40,41
Electrochemotherapy has been used in previous preliminary studies for the treatment of rats and mice bearing subcutaneous implants of melanoma30,41–44, sarcoma30,43–45, and pancreatic tumours46 with encouraging results. Furthermore, liver tumours of rats and rabbits resulted highly responsive to ECT without impairment of the surrounding normal tissue functionality.47–48 The effectiveness of intraoperative ECT was demonstrated in hamster pancreatic adenocarcinoma cell line (PC-1) model.49
We hypothesize that ECT could be used for the treatment of primary non-resectable pancreatic cancer. The aim of the present study was to investigate the feasibility and safety of electroporation in an experimental rabbit model. In vivo local and systemic effects of electric pulses applied to the pancreas were evaluated. Furthermore, we tested ECT in vitro on highly drug resistant pancreatic cancer cell lines in order to prove its efficacy in this subset as well.
Materials and methods
Electroporation apparatus
Electroporation was performed using the Cliniporator™ (IGEA S.p.A., Carpi, Modena, Italy). Different protocols of 100 μs square-wave electric pulses were tested in vitro; one sequences of 8 electrical pulses, 100 μs of duration, at 1000 V/cm were used for in vivo experiments.
Animals and anaesthesia
The experiments were conducted according to the EU Directive 2010/63/EU and to the Guidelines for the welfare and use of animals in cancer research (Br J Cancer 2010; 102:1555–77). The protocol was approved by the Ethical Committee of the Rizzoli Orthopaedic Institute (ELETTRO-RAB 03/25/2009 prot.10499), and submitted to the Italian Ministry of Health (28/04/2009 prot.10771). We used 9 male Hybrid New Zealand rabbits weighing 2.1 ± 0.5 kg, housed under controlled conditions. General anaesthesia was induced with an intramuscular injection of 44 mg/kg ketamine (Imalgene 1000, Merial Italia S.p.a., Milano), 3 mg/kg xylazine (Rompun: Bayer S.p.a. Milano) and maintained by means of facial mask in spontaneous ventilation (O2: 1 L/min, N2O: 0.4 L/min, isoflurane: 2.5% to 3%). Postoperatively, antibiotic and analgesic therapy was administered (Flumequine - Flumexil Fatro, Italy and metamizole Farmolisina Vetem, Italy).
General physiological status of rabbits, including potential onset of anorexia and intestinal blocking, was monitored daily by technician and in charge veterinarian during all the period of the study.
Animal electroporation and sample collection
Animals underwent pancreas and duodenum (gut) electroporation in open surgery according to the standard operating procedure: 8 pulses at 1000 V/cm of amplitude over distance ratio, 100 μs of duration were delivered at 5 KHz using linear N-20-4B electrodes. The electroporated area ranged between 12–15 mm in length. The animals were euthanized 24 hours (n = 2), 72 hours (n = 1), 15 days (n = 3), 30 days (n = 3) after surgery with i.v. injection of 2 ml Tanax (Tanax, Intervet International AN Baxmeer NL) under deep general anaesthesia. The pancreas was removed and treated for the morphological and histological analysis. All the specimens were formalin fixed and paraffin embedded. Four mm tissue sections were stained with Haematoxylin and Eosin (H&E) for histopathological evaluation. Post mortem bowel was visually explored for integrity. No histological evaluation was performed on the bowel.
Biochemical analysis
Peripheral blood was collected before surgery (T0) and at each experimental time: 7 days, 15 days and 30 days following electroporation. After blood collection, serum was separated by centrifugation and parameters of hepatic function such as alanine transaminase (ALT), aspartate transaminase (AST), and pancreatic enzymes, such as amylase, were analysed. The animals did not receive any pharmacological prophylaxis for pancreatitis such as octreotide and Gabesato mesylate. Since the in vivo study is limited to tissue electroporation and no drug was administered to the animals, for ethical reasons, we did not include control groups.
Cell line and drugs
PANC1 and MiaPaCa2 human pancreatic cancer cell lines were obtained from the American Type Culture Collection (ATCC). Various molecular mechanisms have been taken in account to play a role in drug-resistance development in these specific cell lines. One of these mechanisms is mediated by the overexpression of BRG1, a chromatin modulator responsible for gemcitabine resistance also in locally advanced and metastatic pancreatic cancer.50
The two cell lines were cultured in RPMI 1640 medium supplemented with 10% of heat-inactivated foetal calf serum (Gibson, Milan, Italy). Cells were maintained as monolayer in 75 cm2 tissue culture flasks at 37°C in a humidified atmosphere of 5% CO2. The cell lines were Mycoplasma free as assessed by MycoAlert assay (Lonza, Milan, Italy). Cells used for experimental purposes were detached using trypsin-EDTA (0.05% trypsin, Lonza) and collected by centrifugation at 300 rpm. Cell diameter for PANC1 and MiaPaCa2 was 16.8 μm and 12.2 μm respectively, as measured by FACS analysis. The following anticancer drugs were used: bleomycin and cisplatin (Sigma, Milan, Italy). Drugs were dissolved in 0.9% bacteriostatic saline and used at indicated concentrations.
Cell viability and permeabilization
Three aliquots of 1×105 cells (in 100μl of RPMI medium) were placed in separate 2 mm gap electroporation cuvettes (Bio-Rad laboratories). Each cuvette was exposed to electric field strength of 0.5 or 1 kV/cm (8 pulses, 100 μs of duration, 5 KHz). After 30 minutes of incubation, pulsed cells were diluted by a factor 100 in RPMI medium and seeded into 96-well tissue culture plates. Unpulsed cells were subjected to same procedure. The acute effect of electroporation on cell viability was determined using MTS assay (Promega) and the Countess Cell Counter (Invitrogen, Milan, Italy). Cell permeabilization was determined at indicated field strength by uptake of fluorescent dye Lucifer yellow (Sigma, Milan, Italy). Briefly, cells incubated in the presence of Lucifer yellow at a final concentration of 1 mM were exposed to electric pulses and then incubated at 37°C for 5 min. Cells were then chilled on ice, washed in phosphate-buffered saline (PBS) buffer and examined for fluorescence emission in a FACScalibur flow cytometer (Beckton Dickinson). Median fluorescence emission was determined and percent of permeabilized cells was calculated.
In vitro electrochemotherapy (ECT)
Cell sensitivity of PANC1 and MiaPaCa2 to cisplatin and bleomycin was preliminary tested in order to define an appropriate range of drug concentrations to be used for electrochemotherapy experiments (data not shown). For ECT, cells (1×105 suspended in 100μl of RPMI medium) were placed between 2 plate electrodes in 2 mm gap cuvette (Bio-Rad laboratories) in the presence of a range of concentration of drugs or isotonic saline and exposed to a defined electric field strength of 1 kV/cm (8 pulses, 100 μs of duration). Aliquots of cells were left unpulsed in presence of drugs or vehicle. Seven concentrations of cisplatin ranging from 1.6 to 26 μM were used for PANC1, whereas 7 concentrations ranging from 1.6 to 40 μM were tested for MiaPaCa2. For bleomycin, 5 concentrations ranging from 0.1 to 6.6 μM were used for MiaPaCa2, whereas 7 concentrations ranging from 0.3 to 130 μM were tested for PANC1. Pulsed and unpulsed cuvettes were incubated in humidified atmosphere at 37°C for 30 min after electric exposure. Then cells were diluted by a factor of 100 in RPMI medium and three aliquots of 100 μl (1×104 cells) from each cuvette were seeded in 96-well tissue culture plates. Plates were incubated for 72 h and then survival was evaluated by MTS assay (Promega) and the Countess Cell Counter (Invitrogen, Milan, Italy).
Data from the plate reader were analysed using GraphPad Prism version 6. The data were first normalized to the vehicle control and then analysed using the nonlinear regression feature. The curves shown in the figures and the calculated IC50 values were the result of three technical replicates for each cell lines.
Statistical analysis
Comparisons between groups were performed using Student’s T test and p < 0.05 level was considered significant.
Results
In vivo electroporation of non-pathological rabbit pancreata; general toxicity and histological evaluation
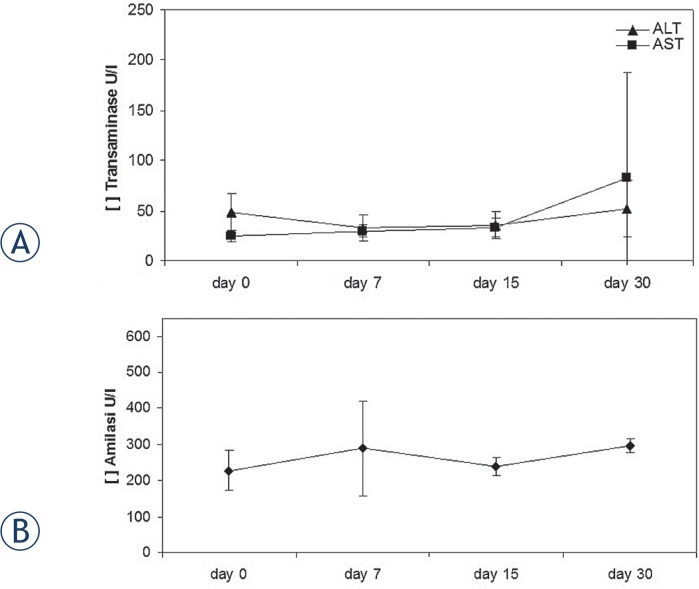
General physiological status of the rabbits was checked daily and no functional deficits as anorexia, intestinal blockage or other clinical status alterations were detected; all the rabbits recovered from general anaesthesia after 1 hour. Transaminase and amylase levels were not statistically modified (p > 0.05) over 30 days of the electroporation procedure (day 0, day 7, day 15, day 30) (Figure 1).
FIGURE 1.
Serum levels of the liver enzymes, AST, ALT (A) and of the pancreatic amylase (B). Data points represent mean ± (standard error) SE, n = 3–6.
Histological evaluation
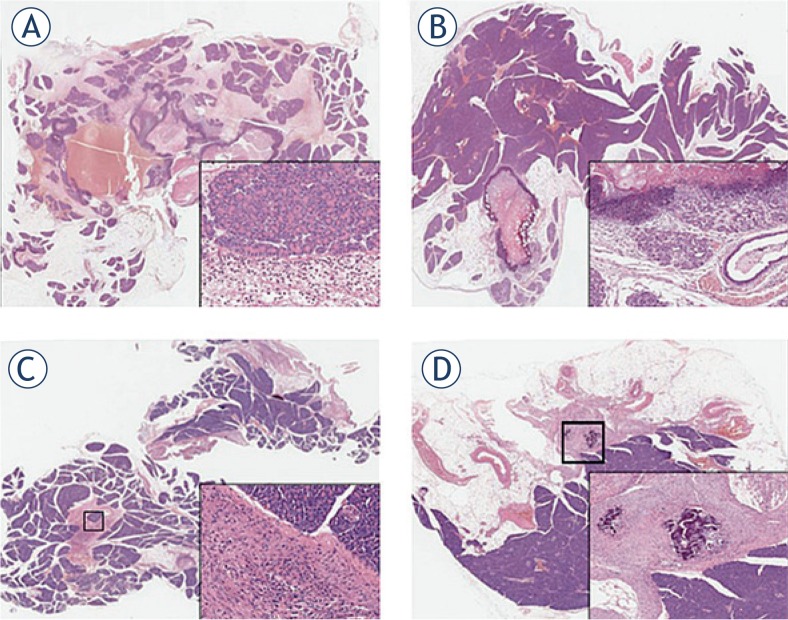
The results of hystopathological analysis are shown in Figure 2A–D. At short term, 24 h post-electroporation, non-pathological rabbit pancreas showed necrosis in the treated areas surrounded by important hyperaemia and oedema in addition to aspects of acute distress of acinar cells (degranulation and vacuolation). An acute inflammatory reaction was also present (Figure 2A). Acinar to ductal metaplasia appeared after 72 h. Elevation of serum amylase is often, but not always, associated with acute pancreatitis. In our study, we found that the ductal metaplasia is not associated with an increase in the serum level of amylase. It is possible that the ductal metaplasia is the consequence of the transient inflammatory response to the insertion of the needle rather than the inflammatory response of the entire organ.
FIGURE 2.
Photomicrographs of rabbit pancreata at different times after electroporation. (A) 24 hours: necrosis in the treated areas surrounded by significant hyperaemia and oedema. Acinar degranulation and vacuolation with granulocytes and lymphocytes infiltration (inset). (B) 72 hours: necrosis is still evident while acinar to ductal metaplasia appears along with intraductal protein plugs (inset). (C) 15 days: fibrotic areas are evident; chronic inflammatory cells are present while pancreatic acini are normal (inset). (D) 30 days: calcium deposition is detectable in fibrotic areas; pancreatic parenchyma is normal (inset).
Moreover, the evidence of intraductal protein plugs, containing degenerating cells, was due to proteins hypersecretion by acinar cells (Figure 2B). After 15 days, fibrotic areas surrounded by normal pancreatic acinar cells were detected. Fat substitution of about 20% of pancreatic parenchyma was also present (Figure 2C). After 30 days, calcium deposition was evident in fibrotic areas (Figure 2D) as a result of mild and transient modification.
When electrical pulses were directly applied to the gut, its integrity was maintained without infection on further clinical complications (data not shown).
Cells survival and permeabilization
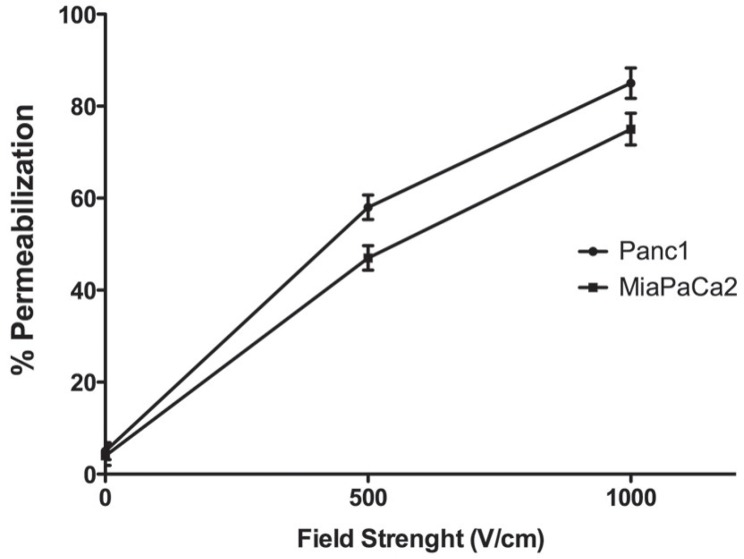
Before testing the toxicity of anticancer drugs on electropermeabilized cells, we defined the optimal parameters for electrical treatment in order to obtain high cell survival and effective permeabilization. Therefore, both PANC1 and MiaPaCa2 cells were subjected to 8 pulses of 100 μs at two different electric field strength of 0.5 or 1 kV/cm. Percent of viable cells was calculated with respect to untreated cells. Field strength of 0.5 kV/cm did not affect cell viability, whereas a slight reduction of about 4% was observed when both cell lines were exposed to field strength of 1 kV/cm (data not shown). Cell permeabilization was assessed by flow cytometry at 1 kV/cm showing that about 90% and about 75% of cells were permeabilized, for PANC1 and MiaPaCa2 respectively (Figure 3). The optimal electric condition was defined as 1 kV/cm, 8 pulses of 100μs duration. In these conditions, the mean value of viability of the pulsed controls was 95% (range 93–97%) of that of the unpulsed controls, with a permeabilization level of at least 75% of pulsed cells.
FIGURE 3.
Cell lines permeabilization as determined by Lucifer yellow uptake and flow cytometry. Mean ± standard deviation (S.D.) of n = 3 independent experiments; circle symbol, Panc1; square symbol, MiaPaCa2
Cell viability following electrochemotherapy
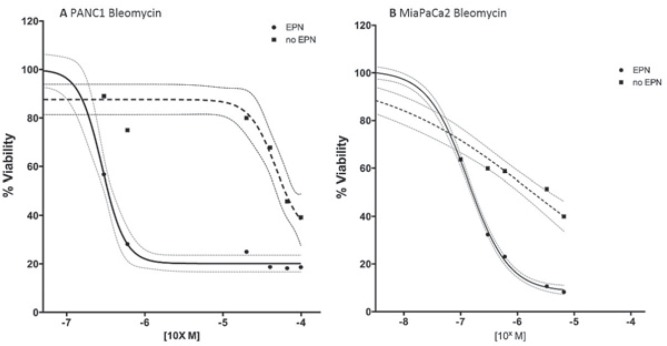
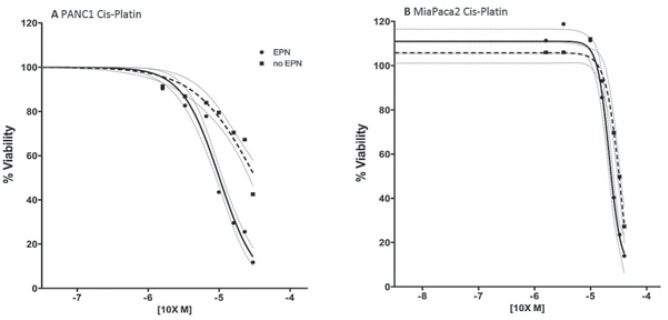
After establishing the optimized parameters for electroporation, 8 pulses of 100μs duration at 1 kV/cm, we investigated the cytotoxicity of two chemotherapeutic agents, bleomycin and cisplatin, against PDAC cell lines. Pulsed PANC1 and MiaPaCa2 cell lines showed enhanced sensitivity to both bleomycin and cisplatin compared to unpulsed cells. Specifically, the IC50 of bleomycin for PANC1 and MiaPaCa2 was reduced by a factor 166 and 18 respectively, after electroporation. Whereas the IC50 of cisplatin was reduced by a factor 2.8 for PANC1 and 1.3 for MiaPaCa2 (Table 1). The cells viability after bleomycin and cisplatin treatment pulsed with electroporation and unpulsed (Figure 4,5).
TABLE 1.
IC50 of bleomycin and cisplatin for PANC1 and MiaPaCa-2 unpulsed (−) or pulsed (+) with electroporation (EP)
| IC 50 of Bleomycin | IC 50 of Cisplatin | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cell | − EP | + EP | P value | − EP | + EP | P value |
| PANC1 | 100 μM | 0.59 μM | <0.0001 | 23 μM | 8 μM | <0.0001 |
| MiaPaCa2 | 3.5 μM | 0.2 μM | <0.0001 | 30 μM | 23 μM | 0.001 |
FIGURE 4.
Dose-response curve of bleomycin treatment. Cell viability was assessed at 72 hours of drug exposure using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega) and the Countess Cell Counter (Invitrogen, Milan, Italy).
Dashed line = no electroporation; solid line = electroporation; grey lines indicate 95 % confident interval [CI]
FIGURE 5.
Dose-response curve of cisplatin treatment. Cell viability was assessed at 72 hours of drug exposure using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega) and the Countess Cell Counter (Invitrogen, Milan, Italy).
Dashed line = no electroporation; solid line = electroporation; grey lines = indicate 95% confident interval [CI]
Discussion
In this study, we demonstrated that a well-defined electroporation protocol does not induce evident signs of local and systemic toxicity when applied to normal pancreas in a pre-clinical model. In addition, the effect of ECT with cisplatin and bleomycin was evaluated in human pancreatic cancer cells lines after. ECT refers to the combined administration of chemotherapy with the local application of electric pulses to the tumour cells in order to increase drug delivery and local cytotoxicity. ECT has been proven effective in the treatment of skin or subcutaneous metastases from solid tumours of different origin. Subsequently to several reports of clinical studies35–38 assessing the use and the efficacy of ECT in the treatment of various primary skin cancers, head and neck cancer, and skin metastasis of different primary tumours, clinicians and researchers are trying to develop novel approaches to extend ECT to the treatment of deep-seated and visceral tumours.39–41 Edhemovic et al., have recently reported for the first time, the feasibility and safety of the procedure highlighting the effectiveness of ECT in the treatment of patients with liver metastases from primary colorectal cancer. The lack of side effect during and after the procedure, demonstrates the safety of the treatment.41 Furthermore, Granata et al. reported a preliminary experience of feasibility and safety of intraoperative electrochemotherapy in locally advanced pancreatic tumour. Twelve patients with tumours of the head or the body of the pancreas underwent ECT. No side effects or major complications have been recorded. No acute intraoperative or postoperative serious adverse effects were related to ECT, showing that electrochemotherapy is a feasible and safe treatment for patients with locally advanced pancreatic adenocarcinoma.51
According to the results reported in further studies, the viability of human tumoural pancreatic cell lines was not modified by electrical pulse alone, while it decreased after exposure to bleomycin and cisplatin. Bleomycin and cisplatin result cytotoxic only at high concentrations. Nevertheless, when the cells were exposed to both the chemotherapeutic agent and the electric pulses, the cytotoxic effect was achieved at lower drug concentration. Specifically, the potentiating effect of electric pulse was more pronounced for bleomycin than cisplatin, confirming previous observations.35,41,49,52–54 Todorovic et al.55 observed a similar sensitization effect in murine colon carcinoma cell-line CMT3 and reported the referral range of IC50 value of different murine and human cell lines treated with bleomycin and cisplatin, with and without electroporation. The pancreatic cell lines PANC1 and MiaPaCa2 tested in the present study, demonstrate IC50 values within the range indicated by Todorovic et al.
The delivery of electric pulses through needles inserted into the pancreas of rabbits elicits a local inflammatory response in the early days after ECT and completely resolves in 30-days. The blood values of pancreatic amylase and transaminases (ALT and AST) were not significantly increased over the whole observational period. Our data agree with previous results reported by Ramirez LH et al., which demonstrate that tissues submitted to electroporation alone, present an immediate inflammatory reaction restricted to electropulsed areas. No diffuse damage of adjacent organs was observed.48 Similarly, Cemazar M et al. analysed in vivo the ECT antitumour efficacy in a number of animal models.56 Our data confirmed that electroporation is a safe procedure in the treatment of pancreatic tumours and ECT could be effective for local control of non-resectable pancreatic cancer. The development of new electrodes and specific software for the assessment of proper preoperative strategies could increase both the safety of ECT and extend its application field.
Footnotes
Disclosure: No potential conflicts of interest were disclosed.
Supported by: FIMP-Italian Ministry of Health (CUP_J33G13000210001)
References
- 1.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Krejs GJ. Pancreatic cancer: epidemiology and risk factors. Dig Dis. 2010;28:355–8. doi: 10.1159/000319414. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos IP, Stocken DD, Friess H, Bassi C, MD, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. NEJM. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 5.Gastrointestinal Tumour Study Group Radiation therapy combined with adriamycin or 5-Fluorouracil for the treatment of locally unresectable pancreatic carcinoma. Cancer. 1985;56:2563–8. doi: 10.1002/1097-0142(19851201)56:11<2563::aid-cncr2820561104>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373–8. doi: 10.1200/JCO.1985.3.3.373. [DOI] [PubMed] [Google Scholar]
- 7.Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–9. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 8.Loehrer PJ, Sr, Feng Y, Cardenes H, Wagner L, Brell JM, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An eastern cooperative oncology group trial. J Clin Oncol. 2011;29:4105–12. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. For the Groupe Tumeurs Digestives of Unicancer and the PRODIGE Intergroup FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 10.Gillen S, Schuster T, Meyer zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR Phase II and III studies. J Clin Oncol. 2007;25:226–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan S, Rana V, Janjan NA, Varadhachary GR, Abbruzzese JL, Das P, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55. doi: 10.1002/cncr.22735. [DOI] [PubMed] [Google Scholar]
- 13.Chu Katrina F, Dupuy Damian E. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nature Rev. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 14.Giardino A, Girelli R, Lusenti A, Auriemma A, Bassi C, Cantore M, et al. Triple approach strategy for patients with locally advanced pancreatic carcinoma. HPB (Oxford) 2013;15:623–7. doi: 10.1111/hpb.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girelli R, Gobbo S, Malleo G, Regi P, Salvia R, Frigerio I, et al. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma. Langenbecks Arch Surg. 2013;398:63–9. doi: 10.1007/s00423-012-1011-z. [DOI] [PubMed] [Google Scholar]
- 16.Cantore M, Frigerio I, Giardino A, Girelli R, Mambrini A, Orlandi M, et al. Combined modality treatment for patients with locally advanced pancreatic adenocarcinoma. Brit J Surg. 2012;99:1083–8. doi: 10.1002/bjs.8789. [DOI] [PubMed] [Google Scholar]
- 17.Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, et al. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Brit J Surg. 2010;97:220–5. doi: 10.1002/bjs.6800. [DOI] [PubMed] [Google Scholar]
- 18.Shi X, Liu S, Kleeff J, Friess H, Büchler MW. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology. 2002;62:354–62. doi: 10.1159/000065068. [DOI] [PubMed] [Google Scholar]
- 19.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–8. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamburrino A, Piro G, Carbone C, Tortora G, Melisi D. Mechanisms of resistance to chemotherapeutic and anti-angiogenic drugs as novel targets for pancreatic cancer therapy. Front Pharmacol. 2013;4:56. doi: 10.3389/fphar.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 23.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Bria E, Milella M, Gelibter A, Cuppone F, Pino MS, Ruggeri EM, et al. Gemcitabine-based combinations for inoperable pancreatic cancer: have we made real progress? A meta-analysis of 20 phase 3 trials. Cancer. 2007;110:525–33. doi: 10.1002/cncr.22809. [DOI] [PubMed] [Google Scholar]
- 25.Olson P, Hanahan D. Cancer. Breaching the cancer fortress. Science. 2009;324:1400–1. doi: 10.1126/science.1175940. [DOI] [PubMed] [Google Scholar]
- 26.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric field. EMBO J. 1982;1:841–5. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann E, Kakorin S, Toesing K. Fundamentals of electroporative delivery of drugs and genes. Bioelectrochem Bioenerg. 1999;48:3–16. doi: 10.1016/s0302-4598(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 28.Orlowski S, Mir LM. Cell electropermeabilization: a new tool for biochemical and pharmacological studies. Biochim Biophys Acta. 1993;1154:51–63. doi: 10.1016/0304-4157(93)90016-h. [DOI] [PubMed] [Google Scholar]
- 29.Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437–47. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 30.Mir LM, Orlowski S, Belehradek M, Paoletti C. Electrochemotherapy: potentiation of antitumor effect of bleomycin by local electric pulses. Eur J Cancer. 1991;1:68–72. doi: 10.1016/0277-5379(91)90064-k. [DOI] [PubMed] [Google Scholar]
- 31.Belehradek M, Domenge C, Luboinski B, Orlowski S, Belehradek J, Jr, Mir LM. Electrochemotherapy, a new antitumor treatment - first clinical phase I–II trial. Cancer. 1993;72:3694–700. doi: 10.1002/1097-0142(19931215)72:12<3694::aid-cncr2820721222>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, et al. Electrochemotherapy – an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases. Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer. 2006;S4:3–13. [Google Scholar]
- 33.Sersa G, Stabuc B, Cemazar M, Jancar B, Miklavcic D, Rudolf Z. Electrochemotherapy with CDDP: potentiation of local CDDP antitumor effectiveness by application of electric pulses in cancer patients. Eur J Cancer. 1998;34:1213–8. doi: 10.1016/s0959-8049(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 34.Sersa G, Stabuc B, Cemazar M, Miklavcic D, Rudolf Z. Electrochemotherapy with cisplatin: clinical experience in malignant melanoma patients. Clin Cancer Res. 2000;6:863–7. [PubMed] [Google Scholar]
- 35.Matthiessen LW, Johannesen HH, Hendel HW, Moss T, Kamby C, Gehl J. Electrochemotherapy for large cutaneous recurrence of breast cancer: A phase II clinical trial. Acta Oncol. 2012;51:713–21. doi: 10.3109/0284186X.2012.685524. [DOI] [PubMed] [Google Scholar]
- 36.Mevio N, Bertino G, Occhini A, Scelsi D, Tagliabue M, Mura F, et al. Electrochemotherapy for the treatment of recurrent head and neck cancers: preliminary results. Tumori. 2012;98:308–13. doi: 10.1177/030089161209800305. [DOI] [PubMed] [Google Scholar]
- 37.Curatolo P, Quaglino P, Marenco F, Mancini M, Nardò T, Mortera C, et al. Electrochemotherapy in the treatment of kaposi sarcoma cutaneous lesions: A two-center prospective phase II trial. Ann Surg Oncol. 2012;1:192–8. doi: 10.1245/s10434-011-1860-7. [DOI] [PubMed] [Google Scholar]
- 38.Campana LG, Valpione S, Mocellin S, Sundararajan R, Granziera E, Sartore L, et al. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg. 2012;99:821–30. doi: 10.1002/bjs.8749. [DOI] [PubMed] [Google Scholar]
- 39.Fini M, Salamanna F, Parrilli A, Martini L, Cadossi M, Maglio M, et al. Electrochemotherapy is effective in the treatment of rat bone metastases. Clin Exp Met. 2013;30:1033–45. doi: 10.1007/s10585-013-9601-x. [DOI] [PubMed] [Google Scholar]
- 40.Edhemovic I, Gadzijev EM, Brecelj E, Miklavcic D, Kos B, Zupanic A, et al. Electrochemotherapy: a new technological approach in treatment of metastases in the liver. Technol Cancer Res Treat. 2011;10:475–85. doi: 10.7785/tcrt.2012.500224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edhemovic I, Brecelj E, Gasljevic G, Marolt Music M, Gorjup V, Mali B, et al. Intraoperative electrochemotherapy of colorectal liver metastases. J Surg Oncol. 2014;110:320–7. doi: 10.1002/jso.23625. [DOI] [PubMed] [Google Scholar]
- 42.Heller R, Jaroszeski M, Perrot R, Messina J, Gilbert R. Effective treatment of B16 melanoma by direct delivery of bleomycin using electrochemotherapy. Melanoma Res. 1997;7:10–8. doi: 10.1097/00008390-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Sersa G, Cemazar M, Miklavcic D, Mir LM. Electrochemotherapy: variable anti-tumor effect on different tumor models. Bioelectrochem Bioenerg. 1994;35:23–7. [Google Scholar]
- 44.Pendez S, Jaroszeski MJ, Gilbert R, Hyacinthe M, Dang V, Hickey J, et al. Direct delivery of chemotherapeutic agents for the treatment of hepatoma and sarcoma in rat models. Radiol Oncol. 1998;21:53–64. [Google Scholar]
- 45.Sersa G, Novakovic S, Miklavcic D. Potentiation of bleomycin antitumor effectiveness by electrochemotherapy. Cancer Lett. 1993;69:81–4. doi: 10.1016/0304-3835(93)90159-7. [DOI] [PubMed] [Google Scholar]
- 46.Nanda GS, Sun FX, Hofmann GA, Hofmann RM, Dev SB. Electroporation enhances therapeutic efficacy of anticancer drugs: treatment of human pancreatic tumor in animal model. Anticancer Res. 1988;18:1361–6. [PubMed] [Google Scholar]
- 47.Jaroszeski MJ, Gilbert RA, Heller R. In vivo antitumor effects of electro-chemotherapy in a hepatoma model. Bioch Biophis Acta. 1997;1334:15–8. doi: 10.1016/s0304-4165(96)00147-x. [DOI] [PubMed] [Google Scholar]
- 48.Ramirez LH, Orlowski S, An D, Gindoula G, Dzodic R, Ardouin P, et al. Electrochemotherapy on liver tumours in rabbit. Br. J Cancer. 1998;77:2104–11. doi: 10.1038/bjc.1998.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaroszeski MJ, Illingworth P, Pottinger C, Hyacinthe M, Miller R. Electrically mediated drug delivery for treating subcutaneous and orthotopic pancreatic adenocarcinoma in a hamster model. Anticancer Res. 1999;19:989–94. [PubMed] [Google Scholar]
- 50.Liu X, Tian X, Wang F, Ma Y, Kornmann M, Yang Y. BRG1 promotes chemoresistance of pancreatic cancer cells through crosstalking with Akt signalling. Eur J Cancer. 2014;50:2251–62. doi: 10.1016/j.ejca.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 51.Granata V, Fusco R, Piccirillo M, Palaia R, Lastoria A, Petrillo A, et al. Feasibility and safety of intraoperative electrochemotherapy in locally pancreatic tumor: A preliminary experience. Eur J Inflamm. 2014;12:467–77. [Google Scholar]
- 52.Allegretti JP, Panje WR. Electroporation therapy for head and neck cancer including carotid artery involvement. Laryngoscope. 2001;111:52–6. doi: 10.1097/00005537-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Bloom DC, Goldfarb PM. The role of intratumour therapy with electroporation and bleomycin in the management of advanced squamous cell carcinoma of the head and neck. Eur J Surg Oncol. 2005;31:1029–35. doi: 10.1016/j.ejso.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Miklavcic D, Sersa G, Brecelj E, Gehl J, Soden D, Bianchi G, Ruggieri P, Rossi CR, Campana LG, Jarm T. Electrochemotherapy: technological advancements for efficient electroporation-based treatment of internal tumors. Med Biol Eng Comput. 2012;50:1213–25. doi: 10.1007/s11517-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Todorovic V, Sersa G, Flisar K, Cemazar M. Enhanced cytotoxicity of bleomycin and cisplatin after electroporation in murine colorectal carcinoma cells. Radiol Oncol. 2009;43:264–73. [Google Scholar]
- 56.Cemazar M, Tamzali Y, Sersa G, Tozon N, Mir LM, Miklavcic D, et al. Electrochemotherapy in veterinary oncology. J Vet Inter Med. 2008;22:826–31. doi: 10.1111/j.1939-1676.2008.0117.x. [DOI] [PubMed] [Google Scholar]