Abstract
A recent major cluster randomized trial of screening, active disease treatment, and mass isoniazid preventive therapy for 9 months during 2006–2011 among South African gold miners showed reduced individual-level tuberculosis incidence but no detectable population-level impact. We fitted a dynamic mathematical model to trial data and explored 1) factors contributing to the lack of population-level impact, 2) the best-achievable impact if all implementation characteristics were increased to the highest level achieved during the trial (“optimized intervention”), and 3) how tuberculosis might be better controlled with additional interventions (improving diagnostics, reducing treatment delay, providing isoniazid preventive therapy continuously to human immunodeficiency virus–positive people, or scaling up antiretroviral treatment coverage) individually and in combination. We found the following: 1) The model suggests that a small proportion of latent infections among human immunodeficiency virus–positive people were cured, which could have been a key factor explaining the lack of detectable population-level impact. 2) The optimized implementation increased impact by only 10%. 3) Implementing additional interventions individually and in combination led to up to 30% and 75% reductions, respectively, in tuberculosis incidence after 10 years. Tuberculosis control requires a combination prevention approach, including health systems strengthening to minimize treatment delay, improving diagnostics, increased antiretroviral treatment coverage, and effective preventive treatment regimens.
Keywords: mass community-wide isoniazid preventive therapy, mathematical model, tuberculosis
South Africa experienced the highest tuberculosis incidence globally (800–1,200 per 100,000) in 2011 (1), with extremely high notification rates among gold miners (3,000 per 100,000 in 2008) (2). Following the success of mass community-wide isoniazid preventive therapy (IPT) in Alaska during the 1950s (3), researchers undertook a cluster randomized trial (Thibela TB) of mass tuberculosis screening followed by IPT in South African gold mines (4). The intervention greatly reduced individual-level incidence but had no detectable population-level impact (5): Miners experienced >60% (95% confidence interval (CI): 25, 82) protection while taking IPT, but the relative incidence rate in intervention compared with control clusters was 1.04 (95% CI: 0.73, 1.48) during the year after the intervention stopped.
Several factors, including suboptimal IPT uptake and retention, a high annual risk of infection with Mycobacterium tuberculosis, and population mobility may have helped to reduce the population-level impact (5), but their relative importance is unclear. Because isoniazid targets actively replicating rather than dormant bacilli (6), the lack of impact may be partly attributable to IPT's curing a low proportion of latent infections, a possibility which was not appreciated at the study initiation. Understanding the trial's findings and interventions that might control tuberculosis is important, given the extraordinarily high incidence in gold mines and relevance for other high incidence settings.
Here, we fitted a mathematical model to trial data and explored 1) factors contributing to the lack of population-level impact, including the proportion of infections that were cured by IPT; 2) the best-achievable intervention impact if all implementation characteristics (e.g., IPT uptake, retention) were increased to the highest level achieved during the trial (“optimized intervention”); and 3) how tuberculosis might be better controlled, by estimating the impact of additional interventions (improving sensitivity and screening by using new diagnostics, such as Xpert MTB/RIF (Cepheid, Inc., Sunnyvale, California), reducing treatment delay, providing IPT continuously, and/or scaling up antiretroviral treatment (ART) coverage) both individually and simultaneously.
METHODS
The Thibela TB cluster randomized trial
Thibela TB was a cluster randomized trial in sites belonging to 3 South African gold mining companies (4) comprising 8 intervention and 7 control clusters, including all miners at participating mine shafts and associated hostel residences. Clusters, stratified into 2 groups (low and high tuberculosis notifications) using data from 2004, were randomized to the intervention or standard of care, balancing for company, province, and workforce size. Trial impact calculations accounted for between-site heterogeneity. Consenting miners in intervention clusters were offered tuberculosis screening, active cases were referred for treatment, and those without active tuberculosis or contraindications were offered 9 months of isoniazid (300 mg daily).
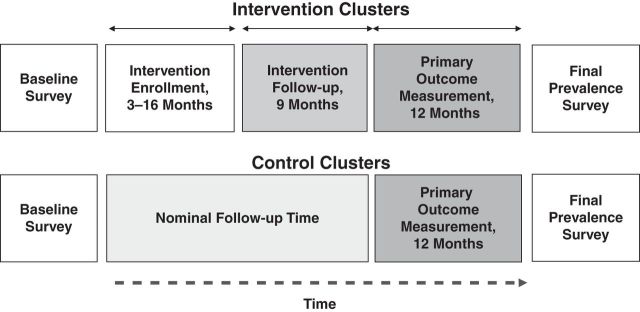
The primary outcome was “tuberculosis incidence” in all clusters, measured during the “primary outcome measurement” period, lasting 12 months after the last person completed IPT in each cluster (Figure 1), with cases mainly ascertained from treatment records. Tuberculosis prevalence was measured by using culture confirmation among systematically sampled employees at the study's end.
Figure 1.
Schematic of the time course of the Thibela TB randomized controlled trial among South African gold miners, 2006–2011. TB, tuberculosis.
Description of the model
Overview
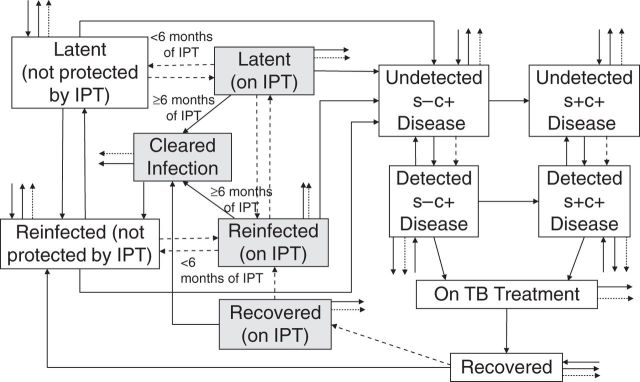
We developed an age-structured deterministic compartmental model (Figure 2) describing M. tuberculosis transmission dynamics for each intervention cluster and its control, matching identically, except for the intervention. The model considers culture-positive tuberculosis (“active disease”), extending previous work (7, 8). Cluster populations comprise 4 age strata (<30, 30–39, 40–49, and ≥50 years) because of age-dependent population mobility and 2 human immunodeficiency virus (HIV) strata (HIV-positive or HIV-negative). Model output is aggregated into 2 age strata (<40 and ≥40 years) or all ages, given insufficient data to further stratify the incidence and prevalence. Clusters are considered independent, because little movement occurred between intervention and control arms.
Figure 2.
General structure of the dynamic transmission model for an intervention cluster in the Thibela TB randomized controlled trial among South African gold miners, 2006–2011. Because of high levels of transmission in the miners, all gold miners were assumed to have been infected at least once in their lifetime, which accounts for the absence of an uninfected compartment. The dashed lines reflect activities relating to case finding or isoniazid preventive therapy. The arrows out of the compartments, which have no destination, reflect out-migration or death. The small arrows into the compartments, which do not start from any destination, reflect in-migration. The shaded boxes reflect people who are taking IPT. IPT, isoniazid preventive therapy; s−c+, smear-negative, culture-positive; s+c+, smear-positive, culture-positive; TB, tuberculosis.
Multiple competing hypotheses exist for why the trial detected no population-level impact, including suboptimal IPT uptake and/or retention or high population mobility (5). To robustly explore this question, we included in the model all known factors (age, HIV, silicosis, ART, in- and out-migration, case detection, initial loss to follow-up after detection, treatment delay, IPT uptake, and retention), at levels supported by detailed data collected during the study or by mine health services (Figure 3; Web Figure 1 (available at http://aje.oxfordjournals.org/)). Parameters (Table 1) were also drawn from publications or estimated by fitting model predictions to trial outcomes. Web Appendixes 1 and 2, Web Tables 1–7, and Web Figures 1–7 provide further details, including the model equations.
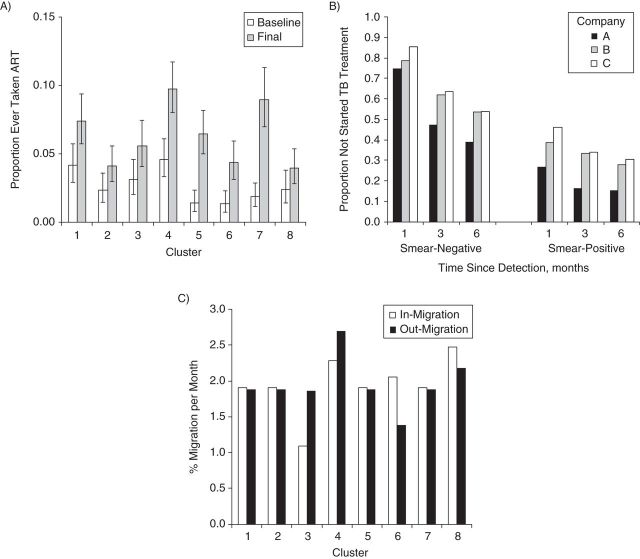
Figure 3.
Some of the key data used to parameterize the model describing the Thibela TB randomized controlled trial among South African gold miners, 2006–2011. A) Proportion of miners in the baseline and final prevalence surveys that reported ever taking ART; B) proportion of smear-positive and smear-negative miners who had not started TB treatment at different times since detection, according to mining company; C) monthly rates of in- and out-migration. Bars (in part A), 95% confidence interval. ART, antiretroviral therapy; TB, tuberculosis.
Table 1.
Summary of the Parameter Values Used in the Base-Case and the Ranges Explored in Sampling Parameter Combinations When Modeling a Cluster Randomized Trial of Mass Tuberculosis Screening Known as “Thibela TB,” 2006–2011
| Definition | Symbola | Base-Case Valueb | Rangeb,c | Commentb |
|---|---|---|---|---|
| Transmission | ||||
| Effective contact rate (average number of individuals effectively contacted by each person per unit of time) | ce | Adjusted to give an annual risk of infection averaged across clusters of 20%/year before the start of the intervention | 5–25/year | No available data |
| Force of infection that is attributable to contact with the outside community | λo | 0.29%/year | Fixed | Based on report by Wood et al. (31)d |
| Infectiousness of sm− TB cases compared with that of sm+ cases | f | 22% | Fixed | Based on report by Behr et al. (32) |
| Disease Onset | ||||
| Rate of onset of reactivation disease for HIV− miners without radiologically confirmed silicosis | dn,z−,a,h− | Estimated | 0.0001–0.014/year | Based on reports by Vynnycky and Fine (7), Sutherland et al. (33), Clark and Vynnycky (34), and Vynnycky et al. (35). The upper limit was set to ensure biological plausibility with the rate of disease following reinfection. |
| Rate of onset of (exogenous) disease in the first year after reinfection | dx,z−,a,h− (0) | Estimated | 0.01–0.11/year | Based on reports by Vynnycky and Fine (7), Sutherland et al. (33), Clark and Vynnycky (34), and Vynnycky et al. (35). |
| Proportion of miners of age a that have radiologically confirmed silicosis | psi,a | Varies by age and between clusters: ≤1% (<40 years of age); 2%–10% (≥40 years of age) | Fixed | Based on baseline prevalence surveyd,e |
| Relative risk of developing TB among miners with radiologically confirmed silicosis, compared with that among miners without radiologically confirmed silicosis | ρsi,h | HIV−: 2.6 | 1–6.5 | Based on report by Corbett et al. (36). For biological plausibility, the selected value for the relative risk for HIV+ was higher than that for HIV−. |
| HIV+: 4.1 | 2.4–7.1 | |||
| Rate at which sm− cases become sm+ | os+,h | HIV−: 0.6%/week | 0.3%–0.9%/week | Based on data from the report by Corbett et al. (37)d,f |
| HIV+: 1.78%/week | 0.89%–2.7%/week | |||
| HIV and ART | ||||
| HIV prevalence in the workforce | h+ | 0.3 | 0.2–0.45 | Based on the report by Lewis et al. (38) |
| ART coverage among miners with a CD4 count of <200 cells/mL at time t | gART,<200(t) | Varies between clusters. Increases from 0 in 2003 to 50%–100% by 2008 | Fixed | Calculated from observed data. Refer to Figure 3Ad,g |
| Protection provided by ART against TB disease among those not on IPT | TTz−,ART+ | 65% | 48%–83% | Based on the report by Suthar et al. (39)d,h |
| Factor by which the rate of disease onset among those with a CD4+ cell count in the range ci − ci+1 cells/µL differs from that among HIV− individuals | CD4 <200: 17 | 8.5–25.5 | Consistent with estimates in the report by Williams et al. (40). The value for those with a CD4 count of <200 cells/mL was constrained to be at least as high as that for miners with a CD4 count of ≥200 cells/mL | |
| CD4 ≥200: 6 | 2.9–8.8 | |||
| Proportion of HIV+ miners who have a CD4+ count in the range ci − ci+1 | CD4 <200: 0.25 | Fixed | Consistent with data in reports by Wlliams et al. (40, 41)d | |
| CD4 ≥200: 0.75 | ||||
| Case Detection | ||||
| Proportion of new employees with TB disease joining the workforce that are detected (“found”) | pin,f,s | sm−: company A, 14%; companies B/C: 1.4% | sm−: 0.7%–20% | Calculated by using the sensitivity of radiographs, proportion of miners followed up, and the sensitivity of the method for subsequent follow-up (culture for company A; smear for company B/C)b,i |
| sm+ (both companies): 25% | sm+: 12.5%–37.5% | |||
| Rate at which cases with smear-status s are detected (found) through the routine medical examination | rf,s,h,y | Company A: sm−, 0.22%/week; sm+, 0.42%/week. Company B/C: sm−, 0.02%/week; sm+, 0.42%/week |
Company A: sm−, 0.1%–0.3%/week; sm+, 0.2%–0.42%/week. Company B: sm−, 0.01%–0.3%/week; sm+, 0.2%–0.42%/week |
Calculated by using the sensitivity of radiographs, proportion of miners followed up, and the sensitivity of the method for subsequent follow-up (culture for company A; smear for company B/C)b,i |
| Rate at which sm+ cases are detected (found) through passive presentation to the health services | rf,s+,h,p | HIV+: 13%/week | 6%–19%/week | Calculated by using data from the report by Corbett et al. (37)b,i |
| HIV−: 1.6%/week | 0.7%–2.3%/week | |||
| Rate at which sm− cases are detected (found) through passive presentation to the health services | rf,s−,h,p | Company A: HIV−, 0.4%/week; HIV+, 1.2%/week. Company B/C: HIV−, 0.6%/week; HIV+, 1.4%/week |
All companies: HIV−, 0.2%–0.9%/week; HIV+, 0.6%–2.2%/week | Calculated by using data from the report by Corbett et al. (37)b,i |
| Treatment | ||||
| Rate at which TB cases with smear status s who have been detected (found) for duration sf start TB treatment | Ts(sf) | Refer to Figure 3B | Fixed | Based on observed data; depends on mining companyd,j |
| Duration of TB (disease) treatment | 6 months | Fixed | Based on observed data. Note that in reality, cases who have previously experienced TB and cases with multiple drug resistance are treated for 8 months and 2 years, respectively. However, such cases make up a small proportion of all TB cases (10% and 2%–2.5%, respectively, based on observed data in the report by van Halsema et al. (2)) | |
| Intervention | ||||
| Rate at which individuals of age a start IPT | iz+,a(t) | Varies between clusters, with the peak proportion on IPT reaching between 10% and 70%k | Fixed | Based on observed data. Differs between clusters and changes over timed,k,l |
| Rate at which individuals of age a stop taking IPT | iz−,a(t) | Varies between clusters with the peak proportion on IPT reaching between 10% and 70%k | Fixed | Based on observed data. Differs between clusters and changes over timed,k,l |
| Rate at which cases are detected through the screening carried out on recruitment into Thibela TB | q(t) | Varies between clusters | Fixed | Based on observed data. Differs between clusters and changes over timed |
| Protection against disease provided by IPT for those not on ART | TTd,z+,ART− | 63% | 25%–81% | Based on the analyses of individual-level data in Thibela TB in the report by Churchyard et al. (5) |
| Protection against disease provided by IPT for those on ART | TTd,z+,ART+ | 82.5% | 41%–83% | Calculated by assuming that IPT provides an additional 50% protection to that provided by ART. Consistent with reports by Samandan et al. (13), Rangaka et al. (42), and Golub et al. (43, 44) |
| Proportion of infections that are cured (i.e., so that reactivation cannot occur) as a result of 6 months of IPT | 100% for IPT assumption 1 | 0%–100% | For biological plausibility, the values for HIV− were constrained to be at least as high as those for HIV+. | |
| Estimated (IPT assumptions 2 and 3) | 0%–100% | |||
| Protection provided against reinfection for individuals while they are on IPT | zr,h | 100% for IPT assumptions 1 and 2 | 0%–100% | No available data |
| Estimated (IPT assumption 3) | 0%–100% | |||
| Minimum duration of IPT required in order to cure infection | 6 months | Fixed | Consistent with the report by Comstock (14) | |
| Maximum duration of IPT | 9 months | Fixed | Determined by Thibela TB | |
| Mortality and Migration | ||||
| Average mortality rate among TB cases before they start TB treatment | μs,h,tr− | HIV−: sm−, 0.2%/month; sm+, 1%/month. HIV+ (both sm+ and sm−): 5%/month |
HIV–: sm−, 0.1%–0.3%/month; sm+: 0.5%–1.5%/month. HIV+ (both sm+ and sm−): 2.5%–7.5%/month |
Consistent with the report by Tiemersma et al. (45)d |
| Average mortality rate among TB cases while they are on TB treatment | μh,tr+ | HIV−: 0.13%/month; HIV+: 1.3%/month | HIV−: 0.06%–0.19%/month; HIV+: 0.6%–1.9%/month | Consistent with the report by Churchyard et al. (46) |
| Average rate at which miners who are not on TB treatment leave the workforce because of out-migration or non-TB-related death | mtr−,a | Varies by age between clusters: 2%–4%/month (<30 years); 1%–2%/month (≥30 years) | Fixed | Based on human resources datad,m |
| Average rate at which miners who are on TB treatment leave the workforce because of out-migration or non-TB-related death | mtr+ | Varies between clusters in the range of 2%–7%/month | Fixed | Based on human resources datad,m |
| Factor by which the prevalence of TB among new mining employees differs from that in the final prevalence survey (after adjustment for the observed impact) |
pin | 1.0 | 0.3–1.5 | No data available |
Abbreviations: ART, antiretroviral therapy; HIV− and HIV+, human immunodeficiency virus-negative and -positive, respectively; IPT, isoniazid preventive therapy; sm− and sm+, smear-negative and smear-positive, respectively; TB, tuberculosis.
a Several of the symbols have subscripts h, z−, z+, or a. h refers to HIV status (which can be positive or negative); z− and z+ refer to those not on IPT and on IPT, respectively; a refers to the age group.
b The 3 mining companies are denoted by “A,” “B,” and “C.”
c The parameters were sampled from the uniform distribution. Unless otherwise stated, the base-case values and ranges are identical for each cluster. The upper and lower limits of the ranges were taken to be 50% and −50% of the base-case value, unless confidence limits were available or the values were constrained for consistency with other parameter values.
e Web Figure 2.
f Web Figure 3.
g Web Figure 4.
h Web Table 2.
i Web Table 3.
k Web Figure 1.
m Web Figure 7.
General structure
All miners are infected in the model, joining the workforce recently reinfected (<2 years previously), with active disease (some of whom are detected on recruitment) or latent infection, consistent with miners transferring between mines or, because of much ongoing transmission (9, 10), being recently reinfected. Miners whose most recent (re)infection occurred >2 years previously are considered to be latently infected. Miners leave the cluster through death or out-migration.
Latently infected miners can develop smear-negative, culture-positive disease through reactivation or following reinfection at estimated rates (see below), depending on the HIV and (radiologically confirmed) silicosis prevalence, CD4 count distributions, and ART coverage, after which they can become smear-positive. Smear status determines their infectiousness. The annual risk of infection, depending on the contact rate among miners and nonminers and the prevalence of infectious individuals, changes with the intervention and is cluster, specific. Cases can die or be detected through self-presenting to mine health services or routine medical examinations. After detection, cases can become lost to follow-up, die, or be treated and recover; disease occurs subsequently only after reinfection.
Once Thibela TB starts, those latently infected, reinfected, or recovered take IPT for ≤9 months at cluster-specific uptake and retention rates, and cases are detected through screening on study recruitment. The assumed protection (63%) during IPT comes from individual-level estimates (5), with increased protection while also taking ART. Scenarios for the proportion of infections that are cleared with 6 months of IPT are described below.
The impact of the intervention
The impact of the intervention on the “measured” tuberculosis incidence (the percentage difference in the measured incidence between intervention and control arms) during the primary outcome measurement period and tuberculosis prevalence at the study end (Figure 1) was calculated for each cluster and averaged across clusters.
Objective 1. Exploring factors contributing to the lack of population-level impact
After inclusion of all known factors and data (age, HIV, silicosis, ART, population mobility, case detection, initial loss to follow-up after detection, treatment delay, and IPT uptake and retention), the model has several unknown parameters: the rates of disease onset through reactivation and following reinfection and the proportion of infections that are cured by IPT.
Because isoniazid targets actively replicating rather than dormant bacilli (6), IPT may not cure all infections. HIV-negative participants in preventive therapy trials typically experienced low tuberculosis incidence many years thereafter (11), suggesting cure of latent infections; those HIV-positive participants in presumed high-transmission settings experienced high disease incidence soon after stopping IPT (12, 13), suggesting that their infections were not cured or that reinfection occurred during IPT, with quick disease progression. When Thibela TB was planned, 6 months of IPT was considered sufficient (14), although 9 months was chosen, compromising between recommendations for HIV-infected people (6 months) or silicotics (12 months) (4). We explored 3 HIV-specific IPT assumptions:
Assumption 1 (100% cure, 100% protection). Six months of IPT cures all infections and reinfection cannot occur during IPT.
Assumption 2 (estimated percentage cured, 100% protection). Six months of IPT can cure <100% of infections, but reinfection cannot occur during IPT. The proportion of infections that are cured by 6 months of IPT is estimated.
Assumption 3 (estimated percentage cured, estimated percentage protection). Six months of IPT can cure <100% of infections, with <100% protection against reinfection during IPT. Both the proportion of infections that are cured by 6 months of IPT and protection against reinfection during IPT are estimated.
For each assumption, we fitted by maximum likelihood cluster-specific model predictions of the measured incidence and prevalence of culture-positive tuberculosis during the primary outcome measurement period and final prevalence survey, respectively (each from 1 time point) for the age groups <40 and ≥40 years to observed data, to estimate the unknown parameters (Web Appendix 3; Web Table 8). These were used to calculate the best-fitting impact, generating a 95% confidence interval using bootstrapping (Web Appendix 3; Web Figures 8 and 9). This fitting used base-case model parameter values (Table 1), which, for IPT assumption 1, facilitate exploring whether factors (IPT coverage, treatment, etc.) explained the lack of detectable population-level impact. By using model predictions of incidence and prevalence, the fitting accounts for correlations between the two quantities.
The sensitivity of the best-fitting impact to alternative values for the annual risk of infection (10% and 30% per year), HIV prevalence (20%, 35%, and 40%), and prevalence of active tuberculosis among newly employed miners was also explored (differing by 0%, 33%, 66%, and 100% from the final prevalence survey estimates), keeping all other parameters at base-case values. Web Appendix 3 provides further details.
The parameters estimated through fitting account for the uncertainty in the intervention's observed impact, assuming that all remaining parameters equaled the values measured in the process data. To account for uncertainty in the latter, we used “Bayesian melding” (15–18) to identify the credible range of key IPT-related parameters. The likelihood of the observed incidence and prevalence was computed for 2.28 million sets of randomly drawn parameter values, comprising 40 parameters each (Table 1). Using fewer (1.5 million) parameter combinations gave similar outcomes. We then resampled 20,000 parameter sets by using the likelihood of the observed incidence as the weight and calculated the median and 95% credible range of the IPT-related parameters and the impact on the incidence and prevalence. Taking additional samples did not affect the outcomes. The resampling was repeated by using the likelihood of the prevalence data as the weight.
In subsequent analyses, the base-case parameters, IPT assumptions, and best-fitting parameters leading to the smallest impact on the measured incidence and prevalence were used.
Objective 2. The best-achievable impact of Thibela TB
The best-achievable impact of Thibela TB was estimated by running the model for base-case and best-fitting parameter values (from objective 1), after changing implementation variables (initial loss to follow-up and treatment delay following diagnosis, the sensitivity of screening on enrollment, and IPT uptake and retention) both individually and concurrently to optimized levels (Table 2; Web Appendix 4; Web Tables 9 and 10).
Table 2.
Summary of Changes Made to Individual Factors When Estimating the Maximum Achievable Impact of Thibela TB (Objective 2) or What Might Control Tuberculosis in the Mines (Objective 3)
| Factor | Value Based on Empirical Data From the Trial | Explored Value |
|
|---|---|---|---|
| Objective 2 (The Best Achievable Impact in Thibela TB) | Objective 3 (What Might Control TB in Gold Mines) |
||
| Initial loss to follow-up | sm+: 25%–40%; sm−: 50%–60% | 5% | As for objective 2 |
| Average treatment delay after detection | sm−: 7–9 weeks (45%–60% within 3 months); sm+: 3–4 weeks (55%–75% within 1 month) | sm−: 3 weeks (90% within 3 months); | Without Xpert MTB/RIF: as for objective 2; with Xpert MTB/RIF: 2 weeks for both sm+ and sm−a,b |
| sm+: 2 weeks (90% within 1 month)a,b | |||
| Sensitivity of case screening on enrollment into Thibela TB | 50% on average | ∼100% (potentially achievable if culture had been used instead of smear for suspected TB at the initial screen) | Not applicable |
| Preventive treatment | 9 months of IPT is provided for all individuals at observed levels of coverage and retention (refer to Web Figure 1 for the proportion on IPT achieved during the trial). | 9 months of IPT is provided for all individuals, but 1) uptake and/or 2) retention equals that in the best-performing cluster in Thibela TB. With both optimized, the proportion on IPT equals that for cluster 7 (70%–80% of the cluster on IPT at the peak)c | 1) Mass community-wide IPT: 9 months of IPT is provided for all individuals, with coverage and retention equaling those in the best-performing cluster (cluster 7) in Thibela TB. The proportion of infections that are cured and the protection provided against reinfection equal those associated with the greatest impact in objective 1. |
| 2) Continuous IPT for 50% of the population: 9 months of IPT is provided as directly above, with those still taking IPT 9 months after starting it (∼50% of the population) continuing to do so, along with 50% of new mining employeesa | |||
| 3) 3-month curing regimen: isoniazid and rifapentine, similar to that described in the report by Sterling et al. (47) provided as directly above. Infections are considered to be cured after completing the regimen, and recipients experience 100% protection against reinfection during the regimen.a | |||
| ART coverage | 0% in 2003, increasing to ∼70% among those with a CD4 count of <200 cells/mL by 2013d,e | No change | Increased from the levels in 2008 to reach 80% by 2009 among those eligible, defined for 3 criteria: those with a CD4 count of <350 cells/mL, <500 cells/mL, or all HIV-positivesa |
| TB disease detection and diagnosis | All miners at their routine medical examination (approximately every 1.5 years) and new mining employees are screened using radiographs. Those with suspected TB are investigated by using either culture (company A) or with culture for those with previous TB and smear otherwise (companies B/C).Cases presenting passively are investigated with culture (company A) or with smear and/or radiological/clinical signs (companies B/C). | No change | Sensitivity of Xpert MTB/RIF is assumed to be 55% and 97% for sm− and sm+ TB disease, respectively; refer to the report by Dorman et al. (48). 1) Screen with radiographs and diagnose suspects with Xpert MTB/RIF. Radiographs are used to screen miners presenting at the routine medical examination or when joining the workforce, in the same way that they are used in current practice (and therefore with the same sensitivity), but Xpert MTB/RIF is used to diagnose suspected TB both in the routine medical examination and on passive presentation.a,f 2) Screen and diagnose with Xpert MTB/RIF. Xpert MTB/RIF is used to screen and diagnose cases in the routine medical examination, when joining the workforce, and for miners who self-present.a,f |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IPT, isoniazid preventive therapy; sm− and sm+, smear-negative and smear-positive, respectively; TB, tuberculosis.
b Web Table 9.
c Web Figure 1.
e Web Figure 4.
f Web Table 10.
Objective 3. What might control tuberculosis in gold mines?
Interventions implemented individually
Using the model calibrated to trial results in objective 1, we simulated the impact of additional interventions, besides reduced initial loss to follow-up and treatment delay (Table 2). These were as follows: 1) preventive treatment with isoniazid or a curative regimen; 2) ART scale-up; and 3) improved diagnosis by using Xpert MTB/RIF introduced into the intervention clusters in 2008 (first year of the primary measurement period) in the model, after removing the Thibela TB intervention (Web Appendix 4; Web Table 10). The annual true culture-positive tuberculosis incidence (measurable by detecting all cases immediately after disease onset), averaged across the intervention clusters, was calculated until 2017 and compared with that in the control condition.
The population attributable fraction for silicosis
The population attributable fraction for silicosis was calculated to determine potential reductions in true tuberculosis incidence achievable by dust control (19) (Web Appendix 4; Web Table 11).
The combined impact of 4 multiple interventions
The combined impact of the following 4 multiple interventions was estimated by using the model (Table 2):
Reduced initial loss to follow-up and treatment delay.
Xpert MTB/RIF is the first test used at routine medical examinations for new mining employees and diagnosis.
Increased ART coverage, reaching 80% of all HIV-positive workers by 2009.
ART recipients receive IPT continuously.
RESULTS
Objective 1. Exploring factors contributing to the lack of population-level impact
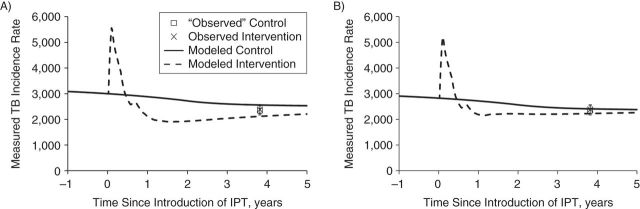
After incorporating all known data and factors (migration, ART uptake, silicosis, IPT uptake and retention, treatment delay, and initial loss to follow-up) and fitting to the observed outcomes, we found that the model based on IPT assumptions 1 (100% cure, 100% protection) fitted the data poorly. The best-fitting impact of 24.5% (95% CI: 24.2, 25.0) and 17.8% (95% CI: 15.0, 21.0) on the measured incidence for IPT assumptions 1 and 2, respectively, exceeded that observed but was inside the latter's 95% confidence interval (−48, 27) (Figure 4A). Findings for the best-fitting impact on prevalence were similar. Web Appendix 5, Web Table 12, and Web Figures 10–14 include further details.
Figure 4.
Summary of the best-fitting impact on the weekly measured tuberculosis disease incidence rate (per 100,000 person-years) during the Thibela TB randomized controlled trial among South African gold miners, 2006–2011. The incidence rate is defined as the incidence that would be observed if it were measured weekly. A) Model predictions obtained by assuming that IPT fully cures all infections and protects against reinfection (IPT assumption 1: 100% cure, 100% protection); B) model is permitted to estimate that 6 months of IPT does not cure all infections and also does not give 100% protection against reinfection during IPT (IPT assumption 3: estimated percentage cured, estimated percentage protection). Note that, for all IPT models, the best-fitting values for the disease rates differed slightly (Web Figure 10), leading to differences in the predicted measured incidence before the introduction of IPT. For each plot, the predicted measured incidence increases in the intervention clusters after the start of the trial because of increased case detection, resulting from screening miners on recruitment into the trial. The cross shows the observed incidence in the intervention arm, aggregated for all intervention clusters; the empty square shows the “observed” incidence in the control arm, taken to equal the incidence in the intervention clusters, divided by 0.98 (the point estimate of the trial impact on incidence). Bars, 95% confidence intervals. IPT, isoniazid preventive therapy; TB, tuberculosis.
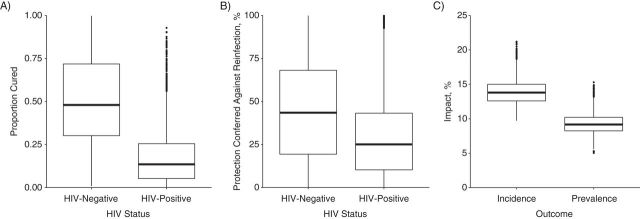
IPT assumption 3 (estimated percentage cured, estimated percentage protected) led to the lowest overall impact of 11.2% (95% CI: 10.7, 15.6) and 7.3% (95% CI: 6.7, 13.9) on the measured incidence and prevalence, respectively (Figure 4). For HIV-positive individuals, IPT cured a small percentage of infections, that is, 0.01% (95% CI: 0.00, 0.53) and provided little protection against reinfection, that is, 0.12% (95% CI: 0.00, 0.55). These estimates are implicitly averaged across CD4 strata. Insufficient power existed to estimate these values for HIV-negative individuals (Web Appendix 5; Web Table 12).
The best-fitting impact for each model remained similar for each assumed value for the annual risk of infection, HIV prevalence, and tuberculosis prevalence among newly employed miners (Web Appendix 5; Web Figure 15).
These findings were consistent across parameter values that were resampled using the likelihood of the incidence as the weight (Figure 5). The percentage of infections that were cured by 6 months of IPT was low for HIV-positive miners (median, 13.5%; 95% credible range, 0.7−66) but in a wide range for those who were HIV-negative (median, 55%; 95% credible range, 10–96), as was the IPT-derived protection against reinfection (median, 25%; 95% credible range, 0.4–75 for HIV-positive miners). The impact on the measured incidence and prevalence associated with these values was 14% (95% credible range, 11–17) and 9% (95% credible range, 6–12), respectively. Using the likelihood of the prevalence data as the weight gave similar findings (Web Figure 16).
Figure 5.
Results of the Bayesian melding (resampling 20,000 parameter combinations from 2.28 million parameter combinations using the likelihood of the measured incidence as the weight). Box plot of estimates of the proportion of infections that were cured by 6 months of IPT (A), the protection provided by IPT against reinfection (B), and the impact of the intervention (C). The boxes reflect the interquartile range (IR), the “whiskers” extend to 1.5 times the IR, and the points outside this range are represented with filled circles. The resampling process resulted in 2,028 unique parameter combinations. HIV, human immunodeficiency virus; IPT, isoniazid preventive therapy.
Objective 2. The best-achievable impact of Thibela TB
The additional impact on tuberculosis incidence measured during the primary measurement period achievable by changing individual factors (treatment delay, sensitivity of case screening, or IPT uptake or retention) was 1%–8% for each IPT model (Web Figures 17 and 18). Additional 21% and 13% reductions were predicted for IPT assumptions 1 (100% cure, 100% protection) and 3 (estimated percentage cure, estimated percentage protection), respectively, with all factors at their optimal achievable level. These reductions were temporary. The predicted measured incidence 5 years after introducing IPT differed by 5%–15% from the base-case value (Web Figure 19). Additional reductions were predicted only after reducing population mobility to unattainable levels (Web Figure 19). These reductions were relatively insensitive to the assumed annual risk of infection, HIV prevalence, and tuberculosis prevalence among new employees (Web Figure 20).
Objective 3. What might control tuberculosis in gold mines?
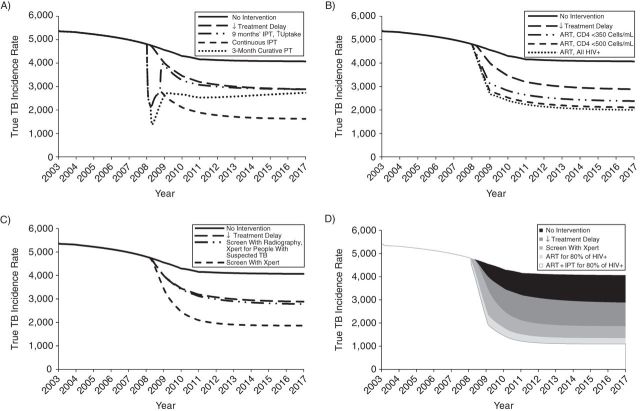
Without additional interventions, the average predicted true tuberculosis incidence declined from about 5,000 to 4,000/100,000/year during 2008–2017 (Figure 6), reflecting increasing ART uptake in some clusters. Reducing initial loss to follow-up to 5% and the average treatment delay to 3 and 2 weeks for all smear-negative and smear-positive cases, respectively, detected by the health services (Table 2) reduced this further to 3,000/100,000/year by 2017 (Figure 6A). Implementing interventions individually resulted in <30% reductions in the predicted long-term true incidence compared with that without interventions (Figure 6). The greatest reductions resulted from scaling-up ART to levels in Table 2, providing continuous IPT or tuberculosis screening (routine medical examination, new employees, and self-presenting miners) with Xpert MTB/RIF.
Figure 6.
Impact of different interventions implemented individually (A–C) or in combination (D) predicted for the Thibela TB randomized controlled trial among South African gold miners, 2006–2011. Summary of the predicted impact of different interventions on the number of cases/100,000/year (the true TB incidence rate), after the treatment delay has been reduced. Each panel shows the impact of reduced treatment delay plus in A) preventive treatment, with 1) IPT provided community-wide in an initial round for 9 months of IPT, with coverage at the highest levels seen in Thibela, and 2) IPT provided community-wide in an initial round for 9 months, with coverage at the highest levels seen in Thibela, followed by continuous community-wide IPT with 50% coverage. This is achieved through keeping those who are still on IPT at the end of the initial round on IPT thereafter and providing IPT to 50% of new mining employees, and 3) a single round with a 3-month fully curing regimen provided community-wide (without 9 months of IPT), with coverage at the highest levels seen in Thibela. B) Scale-up of ART, with ART coverage increasing to reach 80% in 2009 in the HIV-positive groups specified in the figure legend; C) improved diagnosis using Xpert MTB/RIF, with 1) radiographs being used to screen at routine medical examinations and for newly employed miners and Xpert MTB/RIF being used to diagnose people with suspected TB, and 2) Xpert MTB/RIF being used to screen and diagnose at routine medical examinations for newly employed miners and on passive presentation; D) combined interventions. Combined impact of introducing reduced treatment delay, screening with Xpert MTB/RIF, ART for 80% of HIV-positive people, and IPT for those on ART. The shaded areas show the incremental impact of adding each intervention, so that the white area reflects the impact of having all interventions in place simultaneously. For the scenario involving Xpert MTB/RIF, Xpert MTB/RIF is used in routine medical examinations, for newly employed miners, and on passive presentation. For both the ART and ART/IPT scenarios, the coverage is increased to reach 80% by 2009. ART, antiretroviral therapy; HIV+, human immunodeficiency virus–positive; IPT, isoniazid preventive therapy; PT, preventive therapy; TB, tuberculosis.
Introducing 9 months of IPT or a 3-month curative regimen at the highest achievable coverage, with reduced treatment delay and initial loss to follow-up, led to initial reductions in the predicted true incidence, which reversed, eventually matching that predicted without preventive treatment (Figure 6A). The reduction persisted if 50% of miners took IPT continuously, with the true incidence reaching about 40% of that predicted by 2017 by reducing treatment delay and initial loss to follow-up.
Scaling up ART provision to 80% of HIV-positives by 2009 also led to large (30%), sustained reductions in the predicted true incidence (Figure 6B), resembling the impact of providing ART to 80% of those with CD4 counts of <500 cells/mL.
As regards interventions relating to detection and diagnosis (Table 2), the use of radiographs to screen new employees and those attending routine medical examinations (current practice) and Xpert MTB/RIF to confirm suspected tuberculosis (option 1) was predicted to scarcely affect true tuberculosis incidence, after reducing initial loss to follow-up and treatment delay (Figure 6C). Replacing radiographs with Xpert MTB/RIF as the first test for new employees, routine medical examinations and investigation of self-reported symptoms (option 2) led to a 30% reduction in the true incidence predicted by 2017, compared with that without using Xpert MTB/RIF.
For all assumptions about HIV prevalence, <10% of true tuberculosis incidence was attributed to silicosis (Web Figure 21), much less than that attributed to HIV (60%–70%).
The greatest reductions in true incidence were predicted for combined multiple interventions (Figure 6D). Increasing ART coverage to 80% of HIV-positives by 2009, reducing treatment delay, and screening with Xpert MTB/RIF led to a 60% reduction predicted by 2017, compared with that without additional interventions. If IPT were also to be provided to ART recipients, the reduction reached 70% and 80% in the overall population and those HIV-positive, respectively (Web Figure 22).
DISCUSSION
Our analysis, supported by findings from fitting the base-case model to trial data and sampling >2 million parameter combinations, suggests that a possible explanation for the lack of a detectable population-level impact in Thibela TB is that IPT cured a small proportion of latent infections, at least among HIV-positive miners. Other contributing factors include suboptimal IPT uptake and retention. The optimized intervention increased impact by only 10%. Tuberculosis control in gold mines requires implementing interventions simultaneously, including health systems improvements reducing treatment delay and initial loss to follow-up, improved diagnostics, scaling-up ART, and providing IPT continuously to HIV-positive workers.
The model used was complex, which is justifiable, given multiple competing hypotheses for why no population-level impact was detected, including suboptimal IPT uptake and retention, as well as high population mobility. To robustly address why this occurred, we found that the model needed to include all known factors at levels supported by the trial's process data. These factors did not fully explain the lack of detectable population-level impact because, after incorporation of both the known data and the assumption that IPT cured all infections, the model fitted the data poorly. The population-level impact might have changed little even with optimized implementation. A simpler model than that used here would have excluded some factors and could not determine their contribution to the trial's lack of detectable population-level impact.
Our model did not include drug resistance, which probably contributed little to the study's population-level impact, given the low (<15%) prevalence of isoniazid resistance (2) and no evidence that IPT's effectiveness is reduced at such levels (20, 21). We note that our Bayesian melding analyses suggest that 14% of infections were cured, which would have increased to just 16% (0.14/0.85) by including drug resistance in the model.
Our finding that isoniazid may not cure latent infections is consistent with observations that it targets actively replicating rather than dormant bacilli (6). We could not reliably estimate its outcome for HIV-negative workers, given their small contribution to disease incidence and the lack of HIV-specific tuberculosis incidence data. Differences by HIV status probably exist, given the different incidence trends reported between HIV-positive and HIV-negative individuals after stopping IPT (3, 11–14, 22). The lack of detectable impact of preventive therapy elsewhere (23, 24) suggests that ubiquitous silica dust exposure in gold mines, impairing macrophage function (25), may have affected the ability to sterilize latent infections or prevent reinfections, even in HIV-negative miners.
Although improved implementation, including increased IPT uptake and retention, might have helped to increase the impact on tuberculosis incidence, this probably would have been short lived (Web Figure 19), even with a 3-month curative regimen (Figure 6A), largely because of employees with latent infection or tuberculosis disease joining the workforce and, potentially, developing disease and/or transmitting infection. These factors, the HIV prevalence and IPT coverage/retention, partly explain the different impact in the Alaskan IPT trial and Thibela TB. Providing IPT to new gold mining employees could potentially increase the durability.
The durable impact predicted with providing continuous IPT (Figure 4) supports recent South African guidelines recommending ≥36 months of IPT for HIV-positive, tuberculin-positive people (26). We accounted for the minimal IPT-derived benefit for tuberculin-negative individuals (13): The assumed protection was based on all trial participants, irrespective of tuberculin status (5). However, we have not included immune function improvements during prolonged ART, which may help to restore the ability to sterilize latent infections and increase the benefit of providing IPT with ART.
We may have underestimated the impact of improved dust control because of underascertainment of silicosis. Radiology has low (∼30%) sensitivity for detecting nodules (27), and increasing cumulative silica dust exposure increases the risk of tuberculosis even in miners with normal chest radiographs. In South Africa, 20%–30% of gold miners who die from any cause may have histopathological silicosis (28, 29), with 50% and 30% of HIV-positive and HIV-negative miners, respectively, having active tuberculosis (28).
Several factors, including the sensitivity of existing diagnostics and delays in care-seeking, influence the impact of improved diagnostics (30). This may be small if Xpert MTB/RIF is used to diagnose self-reported tuberculosis symptoms and confirm suspected radiological tuberculosis at routine medical examinations (Figure 6). It increases substantially if it replaces radiology at routine medical examination, reflecting the relatively high and unusually low sensitivity of Xpert MTB/RIF and radiological screening, respectively, in gold miners.
Our analyses suggest that tuberculosis incidence in gold mines may decrease substantially by introducing several interventions simultaneously. Tuberculosis morbidity declined rapidly during the Alaskan IPT trials, which were accompanied by greatly intensified case-finding and treatment (11). The reductions attributable to any given intervention will be setting specific. The predicted impact of reducing treatment delay could be large in gold mines that have high losses to treatment following tuberculosis diagnosis and high transmission rates, but minimal elsewhere.
The lack of a detectable impact in Thibela TB strongly suggests that IPT cures a small proportion of latent infections in gold miners. With optimized IPT uptake and retention, as well as case-screening and treatment delays, an increased (albeit nondurable) impact might have been seen. Effective tuberculosis control in gold mines and other high incidence settings requires a combination prevention approach, including health systems improvements to minimize treatment delay, improved diagnostics, increased and improved ART coverage, and effective durable preventive treatment regimens.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Statistics, Modelling, and Economics Department, Public Health England, London, United Kingdom (Emilia Vynnycky); Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom (Emilia Vynnycky, Tom Sumner, Katherine L. Fielding, James J. Lewis, Andrew P. Cox, Richard J. Hayes, Gavin J. Churchyard, Richard G. White); Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom (Elizabeth L. Corbett, Alison D. Grant); Malawi-Liverpool-Wellcome Research Programme, Blantyre, Malawi (Elizabeth L. Corbett); School of Public Health, University of the Witwatersrand, Johannesburg, South Africa (Gavin J. Churchyard); and the Aurum Institute, Johannesburg, South Africa (Gavin J. Churchyard).
Funding was provided by the following: The Consortium to Respond Effectively to the AIDS/TB Epidemic (19790.01) (R.G.W., E.V., T.S., A.P.C., K.L.F., R.J.H., J.J.L.); Bill and Melinda Gates Foundation (grants 21675 and OPP1084276) (R.G.W.); Medical Research Council (United Kingdom) (grant G0802414 and Methodology Research Fellowship G0802414) (R.G.W.); Centers for Disease Control and Prevention/President's Emergency Plan for AIDS Relief via the Aurum Institute (grant 5U2GPS0008111); Wellcome Trust Research Fellowship (grant WT091769) (E.L.C.); and the Medical Research Council (United Kingdom) (grant G0700837) (K.L.F.). A.D.G. was supported by a Public Health Career Scientist award from the United Kingdom Department of Health.
We thank the thousands of mine employees who consented to take part in this study. We also thank the many stakeholders for their support for the study to be implemented, particularly the National Union of Mineworkers, Solidarity, and United Unions of South Africa; Anglogold Ashanti, Gold Fields, and Harmony mining companies; the South African Chamber of Mines; the Mine Health and Safety Council; and the South African government Departments of Mineral Resources, Health, and Labour. We thank the large study team for their commitment and persistent efforts to ensure that the study was successfully implemented.
Conflict of interest: none declared.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report, 2012. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 2.van Halsema CL, Fielding KL, Chihota VN, et al. Trends in drug-resistant tuberculosis in a gold-mining workforce in South Africa, 2002–2008. Int J Tuberc Lung Dis. 2012;167:967–973. [DOI] [PubMed] [Google Scholar]
- 3.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 4.Fielding KL, Grant AD, Hayes RJ, et al. Thibela TB: design and methods of a cluster randomised trial of the effect of community-wide isoniazid preventive therapy on tuberculosis amongst gold miners in South Africa. Contemp Clin Trials. 2011;323:382–392. [DOI] [PubMed] [Google Scholar]
- 5.Churchyard GJ, Fielding KL, Lewis JJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;3704:301–310. [DOI] [PubMed] [Google Scholar]
- 6.Esmail H, Barry CE, 3rd, Wilkinson RJ. Understanding latent tuberculosis: the key to improved diagnostic and novel treatment strategies. Drug Discov Today. 2012;17(9-10):514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;1192:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dye C, Garnett GP, Sleeman K, et al. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet. 1998;3529144:1886–1891. [DOI] [PubMed] [Google Scholar]
- 9.Godfrey-Faussett P, Sonnenberg P, Shearer SC, et al. Tuberculosis control and molecular epidemiology in a South African gold-mining community. Lancet. 2000;3569235:1066–1071. [DOI] [PubMed] [Google Scholar]
- 10.Corbett EL, Mallory KF, Grant AD, et al. HIV-1 infection and risk of tuberculosis after rifampicin treatment. Lancet. 2001;3579260:957–958. [DOI] [PubMed] [Google Scholar]
- 11.Comstock GW, Baum C, Snider DE., Jr Isoniazid prophylaxis among Alaskan Eskimos: a final report of the Bethel isoniazid studies. Am Rev Respir Dis. 1979;1195:827–830. [DOI] [PubMed] [Google Scholar]
- 12.Quigley MA, Mwinga A, Hosp M, et al. Long-term effect of preventive therapy for tuberculosis in a cohort of HIV-infected Zambian adults. AIDS. 2001;152:215–222. [DOI] [PubMed] [Google Scholar]
- 13.Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;3779777:1588–1598. [DOI] [PubMed] [Google Scholar]
- 14.Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis. 1999;310:847–850. [PubMed] [Google Scholar]
- 15.Hallett TB, Gregson S, Mugurungi O, et al. Assessing evidence for behaviour change affecting the course of HIV epidemics: a new mathematical modelling approach and application to data from Zimbabwe. Epidemics. 2009;12:108–117. [DOI] [PubMed] [Google Scholar]
- 16.Alkema L, Raftery AE, Clark SJ. Probabilistic projections of HIV prevalence using Bayesian melding. Ann Appl Stat. 2007;11:229–248. [Google Scholar]
- 17.Brown T, Salomon JA, Alkema L, et al. Progress and challenges in modelling country-level HIV/AIDS epidemics: the UNAIDS Estimation and Projection Package 2007. Sex Transm Infect. 2008;84(suppl 1):i5–i10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alkema L, Raftery AE, Brown T. Bayesian melding for estimating uncertainty in national HIV prevalence estimates. Sex Transm Infect. 2008;84(suppl 1):i11–i16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orroth KK, White RG, Korenromp EL, et al. Empirical observations underestimate the proportion of human immunodeficiency virus infections attributable to sexually transmitted diseases in the Mwanza and Rakai sexually transmitted disease treatment trials: simulation results. Sex Transm Dis. 2006;339:536–544. [DOI] [PubMed] [Google Scholar]
- 20.Chaisson RE, Clermont HC, Holt EA, et al. Six-month supervised intermittent tuberculosis therapy in Haitian patients with and without HIV infection. Am J Respir Crit Care Med. 1996;154(4 Pt 1):1034–1038. [DOI] [PubMed] [Google Scholar]
- 21.Halsey NA, Coberly JS, Desormeaux J, et al. Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet. 1998;3519105:786–792. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JL, Okwera A, Hom DL, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. AIDS. 2001;1516:2137–2147. [DOI] [PubMed] [Google Scholar]
- 23.Cowie RL. Short course chemoprophylaxis with rifampicin, isoniazid and pyrazinamide for tuberculosis evaluated in gold miners with chronic silicosis: a double-blind placebo controlled trial. Tuber Lung Dis. 1996;773:239–243. [DOI] [PubMed] [Google Scholar]
- 24.Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis. 1992;1451:36–41. [DOI] [PubMed] [Google Scholar]
- 25.Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med. 2005;112:169–173. [DOI] [PubMed] [Google Scholar]
- 26.Department of Health. The South African Antiretroviral Treatment Guidelines. Pretoria, South Africa: Department of Health; 2013. [Google Scholar]
- 27.Hnizdo E, Murray J, Sluis-Cremer GK, et al. Correlation between radiological and pathological diagnosis of silicosis: an autopsy population based study. Am J Ind Med. 1993;244:427–445. [DOI] [PubMed] [Google Scholar]
- 28.Murray J, Sonnenberg P, Nelson G, et al. Cause of death and presence of respiratory disease at autopsy in an HIV-1 seroconversion cohort of southern African gold miners. AIDS. 2007;21(suppl 6):S97–S104. [DOI] [PubMed] [Google Scholar]
- 29.Corbett EL, Murray J, Churchyard GJ, et al. Use of miniradiographs to detect silicosis. Comparison of radiological with autopsy findings. Am J Respir Crit Care Med. 1999;1606:2012–2017. [DOI] [PubMed] [Google Scholar]
- 30.Lin HH, Dowdy D, Dye C, et al. The impact of new tuberculosis diagnostics on transmission: why context matters. Bull World Health Organ. 2012;9010:739–747A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood R, Liang H, Wu H, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;144:406–412. [PMC free article] [PubMed] [Google Scholar]
- 32.Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;3539151:444–449. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland I, Svandová E, Radhakrishna S. The development of clinical tuberculosis following infection with tubercle bacilli. 1. A theoretical model for the development of clinical tuberculosis following infection, linking from data on the risk of tuberculous infection and the incidence of clinical tuberculosis in the Netherlands. Tubercle. 1982;634:255–268. [DOI] [PubMed] [Google Scholar]
- 34.Clark M, Vynnycky E. The use of maximum likelihood methods to estimate the risk of tuberculous infection and disease in a Canadian First Nations population. Int J Epidemiol. 2004;333:477–484. [DOI] [PubMed] [Google Scholar]
- 35.Vynnycky E, Borgdorff MW, Leung CC, et al. Limited impact of tuberculosis control in Hong Kong: attributable to high risks of reactivation disease. Epidemiol Infect. 2008;1367:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbett EL, Churchyard GJ, Clayton TC, et al. HIV infection and silicosis: the impact of two potent risk factors on the incidence of mycobacterial disease in South African miners. AIDS. 2000;1417:2759–2768. [DOI] [PubMed] [Google Scholar]
- 37.Corbett EL, Charalambous S, Moloi VM, et al. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med. 2004;1706:673–679. [DOI] [PubMed] [Google Scholar]
- 38.Lewis JJ, Charalambous S, Day JH, et al. HIV infection does not affect active case finding of tuberculosis in South African gold miners. Am J Respir Crit Care Med. 2009;18012:1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suthar AB, Lawn SD, del Amo J, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med. 2012;97:e1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams BG, Granich R, De Cock KM, et al. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci U S A. 2010;10745:19485–19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams BG, Korenromp EL, Gouws E, et al. HIV infection, antiretroviral therapy, and CD4+ cell count distributions in African populations. J Infect Dis. 2006;19410:1450–1458. [DOI] [PubMed] [Google Scholar]
- 42.Rangaka MX, Boulle A, Wilkinson RJ, et al. Randomized controlled trial of isoniazid preventive therapy in HIV-infected persons on antiretroviral therapy. Presented at the XIX International AIDS Conference, Washington, DC, July 22–27, 2012. [Google Scholar]
- 43.Golub JE, Pronyk P, Mohapi L, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;235:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;2111:1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiemersma EW, van der Werf MJ, Borgdorff MW, et al. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One. 2011;64:e17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Churchyard GJ, Kleinschmidt I, Corbett EL, et al. Factors associated with an increased case-fatality rate in HIV-infected and non-infected South African gold miners with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;48:705–712. [PubMed] [Google Scholar]
- 47.Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;36523:2155–2166. [DOI] [PubMed] [Google Scholar]
- 48.Dorman SE, Chihota VN, Lewis JJ, et al. Performance characteristics of the Cepheid Xpert MTB/RIF test in a tuberculosis prevalence survey. PLoS One. 2012;78:e43307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.