Abstract
Background
Specific data are needed regarding the impact of transfusion on operative complications in pancreatectomy. The objectives of this study were to determine risk factors for transfusion and to evaluate the potential association between transfusion and operative complications in elective pancreatectomy procedures.
Study Design
We reviewed our institution’s pancreatectomy and ACS-NSQIP databases. Multivariate analysis was used to determine clinicopathologic risk factors predictive of transfusion, and then a transfusion propensity score was developed to evaluate the impact of transfusion on post-pancreatectomy complications.
Results
Of the 173 patients who were treated from September 2007 to September 2011, 78 patients (45 %) were transfused≥1 unit of blood (median, 3.0 units; range, 1–55). Risk factors for transfusion included increasing Body Mass Index (BMI), smoking, increasing mortality risk score, preoperative anemia, intraoperative blood loss, and benign pathology. After controlling for these risk factors using a transfusion propensity score, transfusion was an independent predictor of increased complications, infectious complications, and hospital costs.
Conclusions
Multiple factors are predictive of transfusion in pancreatectomy, including increasing BMI and smoking. When controlling for transfusion propensity based on these risk factors, RBC transfusion is associated with worse operative outcomes including infectious complications. Development of protocols and strategies to minimize unnecessary transfusion in pancreatectomy are justified.
Keywords: Transfusion, Pancreatectomy, Complications, Pancreatic cancer, Pancreatitis
Introduction
There is increased awareness as to the negative consequences of red blood cell (RBC) transfusion in noncardiac, general surgical procedures.1–4 In addition, several studies have demonstrated a decrease in disease-specific survival in cancer patients who require transfusion when undergoing operations for malignancy.5,6 Several theories have been proposed to account for why RBC transfusion has negative consequences in surgical patients, including factors predictive of a more difficult operation such as blood loss.7 One aspect is the potential for immunosuppression, which may occur despite the standard practice of leukoreduction of donated blood.8,9 One large study demonstrated that as little as 1 unit of blood given in the perioperative period is associated with adverse postoperative events in non-cardiac surgical patients.10
Pancreatectomy is a commonly performed operation, and is now being prospectively audited as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP).11 These operations are performed for both benign and malignant disease and have the potential for significant morbidity and mortality. There is heightened awareness of the importance of collecting and uniformly grading complications following pancreatectomy in an effort to improve operative outcomes.12 Along these lines, a better understanding of the risk factors associated with transfusion and the impact of RBCs on operative outcomes in pancreatectomy are needed.
We have used the ACS-NSQIP database at our institution to comprehensively analyze transfusion in the perioperative period in patients undergoing elective pancreatectomy. Since there are many patient-related and intraoperative factors that may increase the need for transfusion, we devised a transfusion propensity score to allow for a more scrutinized evaluation of the impact of RBCs on post-pancreatectomy complications. The objectives of this study were to determine (1) risk factors associated with transfusion and (2) the association between transfusion and operative complications in elective pancreatectomy procedures.
Patients and Methods
The University of Iowa prospective pancreatectomy and ACS-NSQIP databases were queried for patients who underwent elective pancreatic resection for benign and malignant disease from September 2007 to September of 2011. Patients who underwent resection for traumatic pancreatic injury were excluded from analysis. Following Institutional Review Board approval, the medical records of all patients were reviewed to confirm the prospectively collected data, which included preoperative, intraoperative, and postoperative clinicopathologic and treatment related variables. Preoperative clinical variables obtained included age, sex, race, and body mass index (BMI), history of smoking, previous diagnosis of diabetes mellitus, renal disease, chronic pulmonary disease, cardiac disease, and reported preoperative weight loss. Performance status was determined from the clinical note and designated as independent or partially/totally dependent on others for activities of daily living.
Preoperative severity of illness and mortality risk (measured on a four point scale of minor, moderate, major, or extreme) were based on the patient’s age, gender, and their primary diagnosis and comorbid conditions. The scores were determined using the 3-M All Patient Refined-Diagnostic Related Groups system (Salt Lake City, Utah). This methodology for risk assessment is a highly accurate predictor of outcome and has been validated using the Nationwide Inpatient Sample.13 For this study, severity of illness and mortality risk were grouped into 2 categories: minor and moderate/major/extreme. American Society of Anesthesiologists (ASA) class was determined by the anesthesiology team during the standard preoperative visit. Preoperative laboratory values obtained included serum sodium, blood urea nitrogen, creatinine, albumin, bilirubin, alkaline phosphatase, white blood cell count, aspartate transaminase, hematocrit, and platelet count.
Intraoperative variables obtained included the use of epidural catheter analgesia, type of procedure performed, operative time, and estimated blood loss. Additional procedures beyond the standard resection performed during pancreaticoduodenectomy or distal pancreatectomy were recorded, which included portal vein resection. Intraperitoneal drains were utilized in the majority of cases and the timing of drain removal was at the discretion of the attending surgeon. Postoperative variables collected were any intensive care unit admission, length of hospital stay, and readmission rates within 30 days.
Postoperative complications were prospectively recorded as part of the ACS-NSQIP and included renal failure, infectious complications (wound infection, pneumonia, and urinary tract infection), pulmonary complications (atelectasis, reintubation, and pneumonia), cardiovascular complications (stroke, myocardial infarction, arrhythmia), gastrointestinal complications (ileus and bowel obstruction), deep venous thrombosis and/or pulmonary embolism, wound complications (infection and dehiscence), and delayed gastric emptying (categorized separately from gastrointestinal complications).
Pancreatic fistula was defined in accordance with the International Study Group on Pancreatic Fistula classification.14 This classification defines grade A fistula as a fistula with no clinical impact or deviation from the clinical pathway. For our study, we included patients who had amylase rich drainage initially however had their drains removed less than 3 weeks from the date of operation and required no deviation in the postoperative management strategy. Grade B fistula is defined as a fistula that results in a change in management including the use of TPN, antibiotics, or somatostatin analogues in addition to prolonged hospital stay or readmission. Patients met criteria for grade C fistula if they experienced significant deviation from the clinical pathway and required aggressive interventions—for Grade C fistula, we included patients who required interventional radiology directed percutaneous drainage or reoperation for their fistula.14
Total blood products utilized were recorded including packed RBC, platelets, and/or fresh frozen plasma. Statistical analyses were performed using RBC only—no patient received plasma or platelets independent of RBC in this study. Transfusions included RBCs that were administered intraoperatively and/or within 72 h from the start of the operation.
Direct hospital costs were calculated using the Eclipsys/TSI Financial Decision Support System (Allscripts Healthcare Solutions, Chicago, IL). Direct costs are associated with patient care including operating room costs, nursing, laboratory draws, pharmacy, and transfusion15 and therefore was the endpoint of interest for this study.
Statistical Analysis
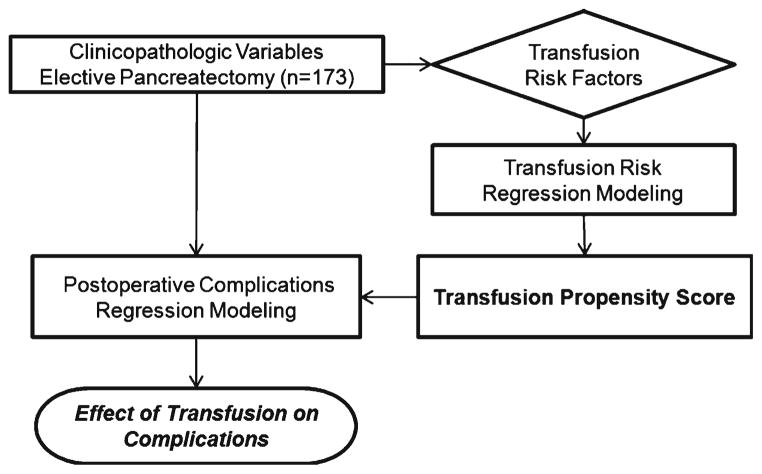
Statistical analysis focused on estimation and testing of the effects of RBC transfusion units on the treatment-related endpoints of overall complications, pancreatic fistula, infectious complications, and direct hospital costs. Complication endpoints were analyzed as dichotomous variables (any/none), and their associations with patient-level predictors modeled with logistic regression. Estimated associations are reported as odds ratios and 95 % confidence intervals. The cost endpoint was modeled with linear regression, and estimated associations reported as mean changes in dollars. Initial regression models were fit to clinicopathologic predictor variables in univariate analysis. Multivariate analysis was then performed to determine whether transfusion was an independent predictor of the endpoints (Fig. 1). A propensity score approach was taken to adjust for individual patient risks of being transfused. To do so, a multivariate negative binomial regression analysis was performed to model RBC transfusion as a function of clinicopathologic variables.
Fig. 1.
Methodology for determination of the transfusion propensity score and impact of transfusions on perioperative complications
The resulting model was used to stratify patients into categories of 0, 1–2, 3–5, 6–9, and 10+ predicted numbers of RBC transfusions (propensity). The transfusion propensity categorization was then adjusted for as a stratification variable in multivariate conditional logistic regression models for complications and as a covariate in linear regression models for hospital costs. Multivariate models were built using stepwise variable selection (with p value cutoffs of 0.15) to identify important predictor variables to include in the models. After the stepwise selection, RBC transfusion was added to the clinical outcomes models to evaluate its incremental effect on the endpoints and to produce the final multivariate models reported in this study. The propensity score therefore attempts to control for volume of transfusion in order to determine if there is an association with the outcome measures independent of the volume transfused. Statistical tests of the effects of transfusion were two-sided and assessed for significance at the 5 % level.
Results
Patient Demographics and Comorbid Conditions
There were 173 patients who underwent elective pancreatectomy from September 2007 to September of 2011. The median age of the cohort was 62 years (range, 20–90 years) (Table 1). The majority of patients were treated for a malignant diagnosis (n=132, 76.3 %), which included pancreatic adenocarcinoma (n=83, 62.9 %), pancreatic neuroendocrine tumor (n=21, 15.9 %), ampullary adenocarcinoma or cholangiocarcinoma (n=15, 11.4 %), duodenal adenocarcinoma (n=12, 9.0 %), and one patient had a renal cell carcinoma invading the pancreas. Indications for pancreatectomy in the 41 patients with benign diagnoses included pancreatitis (n=19, 46.3 %), cystic lesions of the pancreas (n=19, 46.3 %), duodenal adenoma (n=2, 4.9 %), and benign biliary stricture (n=1, 2.4 %).
Table 1.
Patient demographics and comorbid conditions (N=173)
| Variable | N (%) |
|---|---|
| Male | 99 (57 %) |
| Median age (years (range)) | 62 (20–90) |
| Diagnosis | |
| Benign | 41 (23.7) |
| Malignant | 132 (76.3) |
| BMI | |
| ≤20 | 12 (6.9) |
| 21–25 | 50 (28.9) |
| 25–30 | 63 (36.4) |
| 31–35 | 22 (12.7) |
| ≥36 | 26 (15.0) |
| Diabetes mellitus | 50 (28.9) |
| Tobacco use | 55 (31.8) |
| COPD/asthma | 11 (6.4) |
| Renal disease | 5 (2.9) |
| Chronic steroid use | 5 (2.9) |
| Preoperative functional status | |
| Independent | 163 (94.2) |
| Partially/totally dependent | 10 (5.8) |
| Preoperative weight loss | 47 (27.2) |
| Abnormal preoperative laboratory values | |
| Hematocrit<35 % | 33 (19.1) |
| Platelet count<150 K/mm3 | 19 (11) |
| Creatinine>1.2 mg/dL | 15 (8.7) |
| Albumin<3.5 g/dL | 26 (15.0) |
| Bilirubin>1.5 mg/dL | 42 (24.3) |
| ASA class | |
| 1–2 | 72 (41.6) |
| 3–4 | 101 (58.4) |
| Preoperative severity of illness | |
| Minor | 21 (12.1 %) |
| Moderate | 59 (34.1 %) |
| Major/extreme | 93 (53.8 %) |
| Preoperative risk of mortality | |
| Minor | 91 (52.6 %) |
| Moderate/major/extreme | 82 (47.4 %) |
There were 48 patients (27.7 %) who met criteria for obesity, including 22 patients (12.7 %) with a BMI from 31–35 (class I, moderately obese) and 26 patients (15.0 %) with a BMI of ≥36 (class II, severely obese). There were 11 patients (6.4 %) with a BMI of ≥40 (class III, severely obese). As would be expected for selected patients undergoing elective pancreatectomy, the majority of patients had a good performance status (94.2 %). Admission severity of illness was major or extreme in 93 patients (53.8 %).
Operative Procedures and Perioperative Results
Epidural anesthesia was utilized in 136 patients (78.6 %). Operations performed included pancreaticoduodenectomy (n=109, 63.0 %), distal pancreatectomy (n=60, 34.7 %), and total pancreatectomy (n=4, 2.3 %). Among the 113 patients treated with pancreaticoduodenectomy or total pancreatectomy, 71 (62.8 %) had preoperative biliary drainage procedures performed. Additional intraoperative procedures were performed in 18 patients (10.4 %) and included liver-directed therapy for neuroendocrine tumor metastasis (n=6), portal vein resection (n=7), nephrectomy (n=2), gastric resection (n=1), and adrenalectomy (n=1). Mean operative time was 442 min (range, 158–755) and median estimated blood loss was 425 cm3 (range, 50–7,000). Fifty-one patients (29.5 %) required at least one day in the ICU (median ICU days=2; range, 1–40). Median length of stay was 10 days (range, 4–77) and the 30-day readmission rate was 8.7 % (n=15). There were 20 patients (11.6 %) discharged to skilled nursing or rehabilitation facilities and 35 patients (20.2 %) who required home visiting nursing services upon discharge.
Transfusions and Operative Complications
Seventy-eight patients (45.1 %) received at least 1 unit of RBCs and the median number of RBCs administered was 3 units (range, 1–55) (Table 2). There were five patients (6.4 %) who were transfused only 1 unit of blood and the remainder had at least 2 units or more of RBC. In addition to RBC transfusion, 11 patients (6.4 %) received fresh frozen plasma. Postoperative complications are shown in Table 2. The overall complication rate was 56.6 %, and there were six deaths within 90 days (mortality rate=3.5 %). The most common complication was an infectious complication, which included wound infections, urinary tract infections, pneumonia, bacteremia, and/or clostridium difficile colitis. Grade C pancreatic fistula occurred in 20 patients (11.6 %). Compared with patients who did not receive a transfusion, patients who were transfused experienced more total complications, infectious complications, and pancreatic fistulae (Fig. 2).
Table 2.
Operative outcomes associated with elective pancreatectomy
| Variable |
N (%) Median (range) |
|---|---|
| A | |
| Operating time (min) | 442 (158–755) |
| Estimated blood loss (mL) | 425 (50–7,000) |
| Hospital stay (days) | 10 (4–77) |
| Mortality at 90 days | 6 (3.5) |
| Patients requiring transfusion | 78 (45.1) |
| Number of units transfused | 3 (1–55) |
| 1–2 units | 34 (43.6) |
| 3–4 units | 16 (20.5) |
| ≥5 units | 28 (35.9) |
| B | |
| Patients experiencing ≥1complication | 98 (56.6) |
| Complications by system (percentage based on n=173) | |
| Renal | 3 (1.7) |
| Deep venous thrombosis/pulmonary embolism | 6 (3.5) |
| Delayed gastric emptying | 12 (6.9) |
| Cardiovascular | 18 (10.4) |
| Pancreatic fistulaa | 63 (36.4) |
| Grade A/B | 43 (24.9) |
| Grade C | 20 (11.6) |
| Gastrointestinal | 25 (14.5) |
| Wound | 25 (14.5) |
| Pulmonary | 42 (24.3) |
| Infectious | 53 (30.6) |
| C | |
| Direct hospital cost (dollars) | 22,501 (9,953–203,198) |
International Study Group on Pancreatic Fistula definition14
Fig. 2.
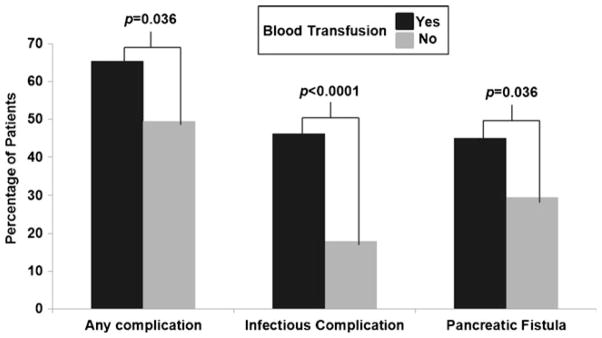
Univariate analysis of operative complications in patients with and without blood transfusion
Evaluation of Risk Factors for Transfusion and Determination of the Transfusion Propensity Score
Univariate analysis revealed BMI, diabetes, severity of illness, mortality risk, and estimated intraoperative blood loss as significant risk factors for transfusion (p<0.05; Table 3). Using a cutoff of p<0.15 in multivariate regression analysis, the following factors were identified as important independent predictors of transfusion risk: BMI, weight loss, smoking status, mortality risk score, preoperative hematocrit, pathology, and estimated blood loss.
Table 3.
Risk factors for red blood cell transfusion—univariate and multivariate analysis
| Variable | Univariate analysis | Multivariate analysisa | p |
|---|---|---|---|
| p | Relative mean change (95 % CI) | ||
| Age | 0.702 | ||
| Gender | 0.417 | ||
| Body Mass Index | 0.005 | 1.57 (1.25–1.97) | <0.001 |
| Weight loss | 0.099 | 0.63 (0.37–1.07) | 0.088 |
| Diabetic | 0.031 | ||
| Smoker | 0.944 | 1.88 (1.18–3.00) | 0.008 |
| Functional status | 0.598 | ||
| ASA class | 0.226 | ||
| Severity of illness score | 0.041 | ||
| Mortality risk score | 0.0003 | 1.98 (1.21–3.23) | 0.007 |
| Procedure type | 0.747 | ||
| Creatinine | 0.194 | ||
| Albumin | 0.260 | ||
| White blood cell count | 0.177 | ||
| Total bilirubin | 0.226 | ||
| Hematocrit | 0.082 | 1.97 (1.47–2.63) | <0.001 |
| Platelet count | 0.111 | ||
| INR | 0.058 | ||
| Epidural | 0.067 | ||
| Operative time | 0.093 | ||
| Estimated blood loss | <0.001 | 3.43 (2.49–4.74) | <0.001 |
| Pathology (malignant vs. benign) | 0.641 | 1.79 (1.00–3.20) | 0.050 |
Variables selected for inclusion (p<0.15) in the multivariate regression model used to calculate the transfusion propensity score
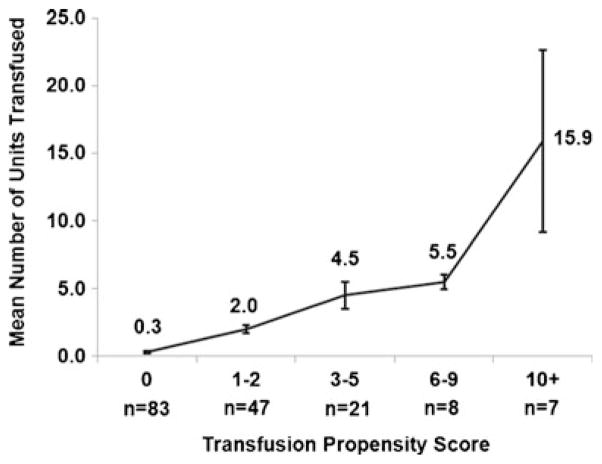
The resulting multivariate regression model provides a predicted number of RBC transfusion units for each patient, and was used to stratify patients into categories of 0, 1–2, 3–5, 6–9, and 10+ predicted transfusions units (transfusion propensity scores). Figure 3 displays the mean observed transfusion units within each of the propensity score categories. As can be seen, there is a positive trend across the categories, with means ranging from 0.3 transfusion units in the lowest category (0) up to a mean of 15.9 in the highest category (10+). The trend suggests agreement between the observed and model-based results. However, the lower-than-expected mean of 5.5 in the second highest category (6–9) indicates imperfect agreement. Such discrepancies are not unexpected and represent unexplained variation in RBC transfusions that can be examined for associations with the clinical outcomes of interest.
Fig. 3.
Mean and standard deviation for observed transfusion units based on transfusion propensity score
Factors Associated with Operative Complications
Univariate analysis was performed using preoperative and intraoperative clinicopathologic variables to determine factors associated with overall complications, infectious complications, and pancreatic fistula. For the multivariate analyses of each of these endpoints, transfusion propensity score was included as an adjustment variable to control for factors known to increase the risk for transfusion.
Overall Complications
Univariate analysis revealed RBC transfusion (p=0.001) and ASA class (p=0.013) to be the only two significant factors associated with overall complications. The multivariate regression analysis identified ASA and diabetes mellitus as important predictors. In the final regression model that controlled for these two factors and the transfusion propensity score, RBC transfusion was an independent predictor of overall complications (p=0.001; Table 4).
Table 4.
Multivariate analysis for complications from elective pancreatectomy
| Endpoint | Variable | Odds ratio (95 % CI) | p value |
|---|---|---|---|
| Overall complications | Red blood cells | 1.54 (1.20–1.97) | 0.001 |
| Diabetes | 2.28 (1.00–5.22) | 0.050 | |
| ASA class | 0.33 (1.16–0.70) | 0.004 | |
| Infectious complications | Red blood cells | 1.73 (1.33–2.62) | <0.001 |
| Platelet count | 1.24 (1.02–1.50) | 0.032 | |
| Operative time | 1.49 (1.20–1.85) | <0.001 | |
| Functional status | 0.09 (0.02–0.49) | 0.005 | |
| Weight loss | 2.60 (0.90–7.51) | 0.078 | |
| Pancreatic fistulaa | Platelet count | 1.24 (1.05–1.46) | 0.012 |
| Operative time | 1.18 (1.00–1.39) | 0.049 | |
| Age | 1.38 (1.05–1.81) | 0.022 | |
| Red blood cells | 1.05 (0.97–1.14) | 0.195 | |
| Diabetes | 2.19 (0.93–5.14) | 0.071 |
The endpoints in both the univariate and multivariate results are based on conditional logistic regression, stratified by the propensity score categories (0, 1–2, 3–5, 6–9, and 10+). The propensity score for risk of RBC transfusion was calculated using a negative binomial model that included estimated blood loss, Body Mass Index, preoperative hematocrit, mortality risk score, weight loss, smoking status, and diagnosis (benign/malignant) as the risk factors
International Study Group on Pancreatic Fistula definition14
Infectious Complications
Factors associated with infectious complications on univariate analysis included preoperative platelet count (p=0.045), operative minutes (p=0.004), RBC transfusion (p<0.001), and operative procedure (p=0.016). As shown in Table 4, multivariate analysis identified platelet count, operative minutes, and poor preoperative functional status as important predictors of infectious complications. Adjusted for these and the transfusion propensity score, RBC remained an independent predictor of infectious complications (p<0.001).
Pancreatic Fistula
On univariate analysis, the only factor associated with pancreatic fistula (grade A, B, and C) was platelet count (p= 0.011), and operative minutes approached significance (OR, 1.69; p=0.079). Multivariate analysis (Table 4), which stratified patients based on their transfusion propensity score, revealed platelet count, operative time, and age as independent risk factors for pancreatic fistula. RBC was not found to be a significant predictor of pancreatic fistula (p=0.195).
Direct Hospital Costs Analysis
The median direct hospital cost was $22,501 (range, $9,953–$203,198). Univariate analysis revealed the following factors as significant predictors of increased direct hospital costs: increased BMI (p=0.032), lower preoperative albumin (p= 0.003), and WBC (p=0.026), elevated preoperative bilirubin (p=0.043), decreased platelet count (p<0.001), longer operative time (p<0.001), increased EBL (p=0.010), RBC transfusion (p<0.001), distal pancreatectomy (p< 0.001), diabetes (p=0.044), ASA class (p=0.020), epidural use (p=0.010), and preoperative mortality risk score (p=0.002). On multivariate analysis (Table 5), RBC transfusion and operative time were the strongest independent predictors of increased direct hospital costs.
Table 5.
Multivariate analysis for direct costs associated with elective pancreatectomy
| Endpoint | Variable | Mean change (dollars (95 % CI)) | p value |
|---|---|---|---|
| Direct observed costs | Red blood cells | 2,477 (1,929 to 3,027) | <0.001 |
| Operative time | 2,960 (1,922 to 3,997) | <0.001 | |
| Platelet count | 1,612 (576 to 2,648) | 0.003 | |
| Epidural | −6,838 (−12,500 to −1,177) | 0.018 | |
| Age | 1,812 (126 to 3,497) | 0.035 |
The multivariate results for the continuous outcome of direct observed cost are based on linear regression adjusted for transfusion propensity score by including it in the model as a categorical predictor
Discussion
There is longstanding evidence that RBC transfusion is a significant contributor to postoperative complications in patients undergoing cardiac surgical procedures.2,4 In several studies, RBC transfusion was demonstrated to be an independent predictor of mortality, renal failure, infectious complications, hospital stay, and cost.1–4 A prospective randomized trial comparing a restrictive RBC transfusion strategy to a liberal strategy in critically ill patients revealed a significant decrease in in-hospital mortality in the restrictive transfusion group.16 Despite these available data, transfusion rates in major medical centers remains high and transfusion practices do not follow clear guidelines.17
Transfusion is a necessary option for patients undergoing complex pancreatic operations. Despite the importance of this treatment for patients with hemorrhage or severe blood loss, there is now increased awareness that RBC transfusion independently has negative consequences in general surgical patients. RBC transfusion has been linked to worse survival following cancer operations 5,18,19 and decreased immunity and susceptibility to infectious complications.20–22 There are multiple variables that contribute to the need for perioperative transfusion, and some of these factors may account for the worse outcomes seen in transfused patients. However, careful analysis reveals that independent of the risk for transfusion, RBC transfusion itself has a negative impact on patient outcomes.
Previous studies have demonstrated an association between RBC transfusions and morbidity and mortality in patients following general surgical operations.10,23,24 Using NSQIP, Bernard and colleagues evaluated the impact of transfusion on operative outcomes in more than 125,000 general surgical patients.10 As in our study, the authors utilized a transfusion risk score to control for ‘transfusion propensity’ in order to assess the impact of RBCs on postoperative complications and mortality. In contrast to our study, the transfusion risk score was calculated using a predetermined set of clinical and pathologic variables. When controlling for transfusion risk, the authors reported that only 1 unit of RBC transfused was associated with a significant increase in operative mortality, morbidity, pneumonia, and sepsis. Two units of blood transfused further increased the odds of the same endpoints and also predicted surgical site infection. A similar study by Ferraris and colleagues evaluated the outcomes of more than 940,000 general surgical patients and reported that transfusion of only 1 unit of blood in the operating room was a significant risk factor for postoperative morbidity and mortality.23 These studies included more than 40 procedure groups, and therefore specific conclusions regarding transfusion in pancreatectomy were not able to be drawn from these data.
Our first objective was to determine risk factors for transfusion in elective pancreatectomy. On multivariate analysis, increasing BMI was a significant predictor of transfusion. This finding is similar to previous studies that have reported increased operative blood loss and complications in obese patients undergoing pancreatic resection.25,26 In addition, smoking history, higher mortality risk score, lower preoperative hematocrit, benign pathology, and increased intraoperative EBL were all independent predictors of transfusion. These clinicopathologic factors can be utilized preoperatively for informed consent regarding risk for transfusion and also for operative planning that may implement blood conservation maneuvers.
Forty-five percent of the patients in our study received at least 1 unit of RBC. Transfusion rates in elective pancreatectomy range from as low as 0 % to as high as 52 % in the currently published literature.27–30 Of note, 48 patients in our series (27.7 %) had a BMI of 30 or greater, which has been shown to correlate with estimated blood loss and complications following pancreatectomy in other reports.26 However, our transfusion rates do not differ significantly from other retrospective series of pancreatectomy patients from major medical centers.25,30 We continue to refine our own protocols to help reduce unnecessary transfusion through collaboration with anesthesia, surgical intensive care, and education of our faculty and house staff.
Our second objective was to determine the impact of RBC transfusion on postoperative complications following elective pancreatectomy. Due to the inherent risk that the previously mentioned clinicopathologic variables would have on the need for transfusion, we utilized these variables to calculate a transfusion propensity score. After demonstrating that the transfusion propensity score fit well with our observed results, we stratified patients based on this score in a multivariate analysis for postoperative complications. Transfusion was independently predictive of both overall complications and infectious complications following pancreatectomy. Despite being associated with pancreatic fistula on univariate analysis, RBC was not an independent risk factor on multivariate analysis. This result strengthens the findings of our study since there is no clear biologic plausibility for a direct link between RBC transfusion and pancreatic fistula beyond factors controlled for by the propensity score (e.g., EBL and BMI).
In addition to RBC transfusion, diabetes mellitus was an independent risk factor for postoperative complications. Diabetes intuitively can contribute to operative complications in numerous ways, including its association with other medical problems and abnormal perioperative glucose control.31,32 In addition to RBC, platelet count, operative time, and preoperative functional status were independent risk factors for increased infectious complications. An abnormal platelet count can be present in numerous disease processes, including splenic vein thrombosis, liver disease, and has previously been shown to correlate with complications following pancreatectomy.33 We did not have any patients in our series with uncompensated cirrhosis, yet splenic vein thrombosis can be present in patients with chronic pancreatitis or with large tumors of the pancreatic body or tail. Abnormal platelet count was also a risk factor for pancreatic fistula, which again may be accounted for by local tumor factors which cannot be accounted for in a multivariate analysis.
The analysis of hospital costs revealed that there is nearly a $2,500 increase in direct hospital costs per unit of RBC transfused. Complications following pancreatic resection are intuitively associated with increased hospital cost.34 Transfusion has been directly linked to increased cost in previous studies; however, this was not independent of factors that would increase risk for transfusion such as operative time, estimated blood loss, and hospital stay.4
This study has limitations. Despite utilizing a prospective ACS-NSQIP database, clinical variables that dictate transfusion in the operating room and postoperatively were not able to be determined from the medical record. There is likely significant variability between surgeons and anesthesia staff with regard to transfusion practices, which may limit the applicability of this dataset to other centers. Selection bias also needs to be taken in to account when evaluating data from a retrospective study. Furthermore, there are other clinical factors that may account for complications that were independent of our clinical variables available in the NSQIP database. For example, there were seven patients in this study who had major vascular resection. Due to the small number of events, we were not able to include this as an additional risk factor in our model. However, the significant impact that this procedure has on the need for transfusion cannot be ignored and likely increases the patient’s risk for complications independent of RBC transfusion.35
Despite these study limitations, the significant impact of RBC transfusion on operative complications and costs needs attention. What can be done with this information? Certainly there are patients who will require transfusion that is beyond the surgeon’s control, and this will likely be dictated by the severity of the case and other tumor- or patient-related factors. However there is clearly a need for protocol-driven transfusion guidelines for surgeons, anesthesiologists, and other physicians and house staff who care for these patients in the perioperative period. Transfusion thresholds need to be determined based on clinical factors as opposed to simply treating numbers. Despite being evaluated in a randomized controlled trial, many clinicians still advocate that their patient’s hemoglobin be equal to or greater than 10 mg/dL when they remain asymptomatic and without significant risk factors for coronary ischemia.16 Or, some physicians typically transfuse 2 units of blood when 1 unit may suffice. Awareness as to the potential negative consequences associated with RBC transfusion is another important step in improving perioperative outcomes following pancreatectomy.
Conclusions
Herein we have analyzed risk factors for transfusion and complications associated with transfusion in pancreatectomy. It is important to note that even though we attempted to control for transfusion risk using a propensity score, the results reported here only reflect an association between transfusion and complications. There are likely many factors that contribute to transfusion during pancreatectomy that cannot be controlled in a retrospective study. At our institution, we have initiated efforts to utilize specific teams in the operating room and are developing perioperative protocols that can be followed by anesthesia and house staff.36 Continued efforts to develop protocols for transfusion and to avoid transfusion when unnecessary in pancreatectomy are justified.
Acknowledgments
The authors wish to thank Mary Belding-Schmidt for excellent construction and maintenance of our department’s ACS-NSQIP database. We also thank Mary Kay Brooks for her dedication and hard work to construct and maintain our institution’s pancreatectomy and transfusion databases. The authors dedicate this work to pancreatic cancer patients and their families.
Abbreviations
- RBC
Red blood cell
- ACS-NSQIP
American College of Surgeons National Surgical Quality Improvement Program
- CT
Computed tomography
- ASA
American Society of Anesthesiology
Footnotes
This paper was presented in part at the Society for Surgery of the Alimentary Tract 53rd annual meeting May 2012, San Diego, California.
Disclosures The authors have no conflict of interest
Contributor Information
Raphael C. Sun, Department of Surgery, University of Iowa Hospitals and Clinics, Iowa City, IA, USA
Anna M. Button, Epidemiology and Biostatistics, University of Iowa Hospitals and Clinics, Iowa City, IA, USA
Brian J. Smith, Epidemiology and Biostatistics, University of Iowa Hospitals and Clinics, Iowa City, IA, USA
Richard F. Leblond, Internal Medicine, University of Iowa Hospitals and Clinics, Iowa City, IA, USA
James R. Howe, Email: james-mezhir@uiowa.edu, Department of Surgery, University of Iowa Hospitals and Clinics, Iowa City, IA, USA
James J. Mezhir, Department of Surgery, University of Iowa Hospitals and Clinics, Iowa City, IA, USA. Division of Surgical Oncology and Endocrine Surgery, University of Iowa Hospitals and Clinics, 200 Hawkins Drive; 4642 JCP, Iowa City, IA 52242, USA
References
- 1.Habib RH, Zacharias A, Schwann TA, et al. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcome. Crit Care Med. 2005;33:1749–56. doi: 10.1097/01.ccm.0000171531.06133.b0. [DOI] [PubMed] [Google Scholar]
- 2.Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–16. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 3.Koch CG, Li L, Duncan AI, et al. Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Ann Thorac Surg. 2006;81:1650–7. doi: 10.1016/j.athoracsur.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 5.Kneuertz PJ, Patel SH, Chu CK, et al. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol. 2011;18:1327–34. doi: 10.1245/s10434-010-1476-3. [DOI] [PubMed] [Google Scholar]
- 6.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256:235–44. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 7.Katz SC, Shia J, Liau KH, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617–23. doi: 10.1097/SLA.0b013e31819ed22f. [DOI] [PubMed] [Google Scholar]
- 8.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: fact or fiction? Blood. 2001;97:1180–95. doi: 10.1182/blood.v97.5.1180. [DOI] [PubMed] [Google Scholar]
- 9.Fergusson D, Khanna MP, Tinmouth A, Hebert PC. Transfusion of leukoreduced red blood cells may decrease postoperative infections: two meta-analyses of randomized controlled trials. Can J Anaesth. 2004;51:417–24. doi: 10.1007/BF03018302. [DOI] [PubMed] [Google Scholar]
- 10.Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208:931–7. 7e1–2. doi: 10.1016/j.jamcollsurg.2008.11.019. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 11.Parikh P, Shiloach M, Cohen ME, et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB (Oxford) 2010;12:488–97. doi: 10.1111/j.1477-2574.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204:356–64. doi: 10.1016/j.jamcollsurg.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Grendar J, Shaheen AA, Myers RP, et al. Predicting in-hospital mortality in patients undergoing complex gastrointestinal surgery: determining the optimal risk adjustment method. Arch Surg. 2012;147:126–35. doi: 10.1001/archsurg.2011.296. [DOI] [PubMed] [Google Scholar]
- 14.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Taheri PA, Butz D, Griffes LC, Morlock DR, Greenfield LJ. Physician impact on the total cost of care. Ann Surg. 2000;231:432–5. doi: 10.1097/00000658-200003000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 17.Carless PA, Henry DA, Carson JL, Hebert PP, McClelland B, Ker K. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2010:CD002042. doi: 10.1002/14651858.CD002042.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328:1372–6. doi: 10.1056/NEJM199305133281902. [DOI] [PubMed] [Google Scholar]
- 19.Heiss MM, Mempel W, Delanoff C, et al. Blood transfusion-modulated tumor recurrence: first results of a randomized study of autologous versus allogeneic blood transfusion in colorectal cancer surgery. J Clin Oncol. 1994;12:1859–67. doi: 10.1200/JCO.1994.12.9.1859. [DOI] [PubMed] [Google Scholar]
- 20.Shorr AF, Duh MS, Kelly KM, Kollef MH. Red blood cell transfusion and ventilator-associated pneumonia: A potential link? Crit Care Med. 2004;32:666–74. doi: 10.1097/01.ccm.0000114810.30477.c3. [DOI] [PubMed] [Google Scholar]
- 21.Jensen LS, Andersen AJ, Christiansen PM, et al. Postoperative infection and natural killer cell function following blood transfusion in patients undergoing elective colorectal surgery. Br J Surg. 1992;79:513–6. doi: 10.1002/bjs.1800790613. [DOI] [PubMed] [Google Scholar]
- 22.Vamvakas EC. Possible mechanisms of allogeneic blood transfusion-associated postoperative infection. Transfus Med Rev. 2002;16:144–60. doi: 10.1053/tmrv.2002.31463. [DOI] [PubMed] [Google Scholar]
- 23.Ferraris VA, Davenport DL, Saha SP, Austin PC, Zwischenberger JB. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg. 2012;147:49–55. doi: 10.1001/archsurg.2011.790. [DOI] [PubMed] [Google Scholar]
- 24.Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114:283–92. doi: 10.1097/ALN.0b013e3182054d06. [DOI] [PubMed] [Google Scholar]
- 25.House MG, Fong Y, Arnaoutakis DJ, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12:270–8. doi: 10.1007/s11605-007-0421-7. [DOI] [PubMed] [Google Scholar]
- 26.Gaujoux S, Torres J, Olson S, et al. Impact of Obesity and Body Fat Distribution on Survival After Pancreaticoduodenectomy for Pancreatic Adenocarcinoma. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2301-y. [DOI] [PubMed] [Google Scholar]
- 27.Fischer M, Matsuo K, Gonen M, et al. Relationship between intraoperative fluid administration and perioperative outcome after pancreaticoduodenectomy: results of a prospective randomized trial of acute normovolemic hemodilution compared with standard intraoperative management. Ann Surg. 2010;252:952–8. doi: 10.1097/SLA.0b013e3181ff36b1. [DOI] [PubMed] [Google Scholar]
- 28.Mezhir JJ, Brennan MF, Baser RE, et al. A matched case–control study of preoperative biliary drainage in patients with pancreatic adenocarcinoma: routine drainage is not justified. J Gastrointest Surg. 2009;13:2163–9. doi: 10.1007/s11605-009-1046-9. [DOI] [PubMed] [Google Scholar]
- 29.Winter JM, Cameron JL, Campbell KA, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2006;10:1280–90. doi: 10.1016/j.gassur.2006.07.020. discussion 90. [DOI] [PubMed] [Google Scholar]
- 30.Goh BK, Tan YM, Chung YF, et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg. 2008;143:956–65. doi: 10.1001/archsurg.143.10.956. [DOI] [PubMed] [Google Scholar]
- 31.Subhedar PD, Patel SH, Kneuertz PJ, et al. Risk factors for pancreatic fistula after stapled gland transection. Am Surg. 2011;77:965–70. [PubMed] [Google Scholar]
- 32.Eshuis WJ, Hermanides J, van Dalen JW, et al. Early postoperative hyperglycemia is associated with postoperative complications after pancreatoduodenectomy. Ann Surg. 2011;253:739–44. doi: 10.1097/SLA.0b013e31820b4bfc. [DOI] [PubMed] [Google Scholar]
- 33.Kelly KJ, Greenblatt DY, Wan Y, et al. Risk stratification for distal pancreatectomy utilizing ACS-NSQIP: preoperative factors predict morbidity and mortality. J Gastrointest Surg. 2011;15:250–9. doi: 10.1007/s11605-010-1390-9. discussion 9–61. [DOI] [PubMed] [Google Scholar]
- 34.Enestvedt CK, Diggs BS, Cassera MA, Hammill C, Hansen PD, Wolf RF. Complications nearly double the cost of care after pancreaticoduodenectomy. Am J Surg. 2012;204:332–8. doi: 10.1016/j.amjsurg.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda S, Oussoultzoglou E, Bachellier P, et al. Significance of the depth of portal vein wall invasion after curative resection for pancreatic adenocarcinoma. Arch Surg. 2007;142:172–9. doi: 10.1001/archsurg.142.2.172. discussion 80. [DOI] [PubMed] [Google Scholar]
- 36.Salvia R, Malleo G, Butturini G, et al. Perioperative management of patients undergoing pancreatic resection: Implementation of a care plan in a tertiary-care center. J Surg Oncol. 2012 doi: 10.1002/jso.23285. [DOI] [PubMed] [Google Scholar]