Abstract
The availability of 24 antiretroviral (ARV) drugs within six distinct drug classes has transformed HIV-1 infection (AIDS) into a treatable chronic disease. However, the ability of HIV-1 to develop resistance to multiple classes continues to present challenges to the treatment of many ARV treatment-experienced patients. In this case report, we describe the response to ibalizumab, an investigational CD4-binding monoclonal antibody (mAb), in a patient with advanced immunodeficiency and high-level five-class antiretroviral resistance. After starting an ibalizumab-based salvage regimen, the patient had an approximately 4.0 log10 reduction in viral load. An inadvertently missed infusion at week 32 led to the rapid loss of virologic response and decreased susceptibility to the remainder of the patient’s salvage therapy regimen. Following the reinstitution of ibalizumab, phenotypic and genotypic resistance to ibalizumab was detected. Nonetheless, plasma HIV-1 RNA levels stabilized at ~2.0 log10 copies/ml below pre-ibalizumab levels. Continued ARV drug development may yield additional clinical and public health benefits. This report illustrates the promise of mAbs for HIV-1 therapy in highly treatment-experienced patients. Therapeutic mAbs may also have a role in pre-exposure prophylaxis in high-risk uninfected populations and may facilitate directly observed therapy (DOT) if two or more synergistic long acting agents become available.
Keywords: HIV-1, monoclonal antibody, drug resistance, ibalizumab
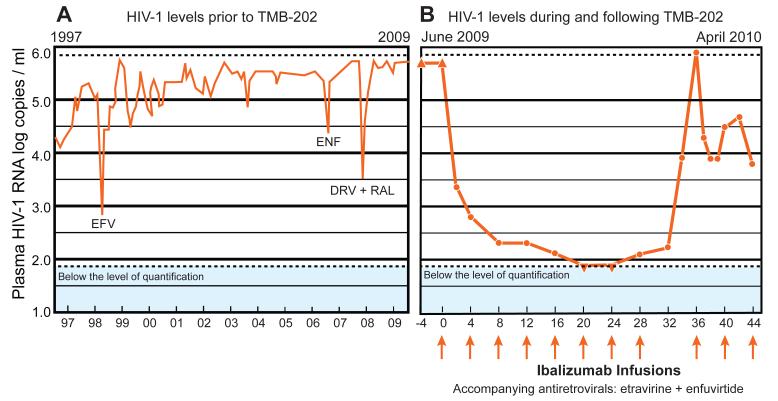
We describe the course of a 56 year-old HIV-1-infected man that underwent treatment with 20 antiretroviral (ARV) drugs between 1991 and 2009 but who nonetheless had persistent HIV-1 viremia. Since 1997, the patient had plasma HIV-1 RNA levels usually exceeding 500,000 copies/ml and advanced immunosuppression, with most CD4 lymphocyte counts below 100 cells/mm3 (Figure 1A). During this time, the patient experienced three transient virologic responses to ARV therapy characterized by reductions in plasma HIV-1 RNA levels of ≥1.0 log10 following the administration of a new ARV. The first response was a 2.5 log10 reduction in plasma HIV-1 RNA levels in 1997 after administration of an efavirenz-containing regimen, the second was a 1.0 log10 reduction in 2006 after administration of an enfuvirtide-containing regimen, and the third was a 1.9 log10 reduction in 2007 after administration of a regimen containing darunavir and raltegravir (Figure 1A). However, by 2009, the patient’s virus had acquired extensive resistance to ARVs belonging to each of the six drug classes based on the results of both genotypic and phenotypic drug resistance assays (Table 1). Supplementary Table 1 summarizes the results of 11 genotypic resistance tests performed between June 1998 and October 2010.
Figure 1.
The patient’s plasma HIV-1 RNA levels (log copies/ml) during the 13 years prior to enrollment intoTMB-202 (A, left panel) and during the 44 weeks following enrollment into TMB-202 (B, right panel).The mAb ibalizumab was administered as part of a placebo-controlled trial for 24 weeks and open-label thereafter. The orange arrows indicate monthly intravenous infusions of 2,000 mg of ibalizumab. In December 2009, it was discovered that the patient had been randomized to receive ibalizumab. However, a drug labeling error led to receipt of placebo rather than ibalizumab at week 32. Between 1997 and 2009,the patient experienced three transient decreases in plasma HIV-1 RNA levels coincident with the administration of new ARV regimens containing efavirenz (EFV, 1998), enfuvirtide (2006), and darunavir plus raltegravir (DRV+RAL, 2007). The ▴ symbol indicates plasma HIV-1 RNA levels above the limit of quantification (500,000 copies/ml). The ▾ symbol indicates plasma HIV-1 RNA levels below the limit of quantification (75 copies/ml). The week 36 plasma level measurement was performed on a diluted sample and determined to be approximately 5.0 million copies/ml. The results of six-class phenotypic susceptibility testing prior to study enrollment (June 2009) and following virologic rebound(March 2010) are shown in Table 1.
Table 1.
PhenoSense Susceptibility Results for Each of the Six Antiretroviral Drug Classes Prior to the Start of Ibalizumab and Following Virologic Rebound after a Missed Ibalizumab Infusion
| Drug Class | June 2009 (fold- resistant) |
June 2009 Interpretation |
March 2010 (fold- resistant) |
March 2010 Interpretation |
Clinical Cutoffs |
|---|---|---|---|---|---|
| Nucleoside RT inhibitors (NRTIs) | |||||
| Abacavir | 20 | R | 12 | R | 4.5-6.5 |
| Didanosine | 4.9 | R | 4.1 | R | 1.3-2.2 |
| Emtricitabine | >300 | R | 8.7 | R | >3.5 |
| Lamivudine | >300 | R | 6.3 | R | >3.5 |
| Stavudine | >300 | R | 13 | R | >1.7 |
| Zidovudine | >300 | R | 11 | R | >1.9 |
| Tenofovir | 8.4 | R | 444 | R | 1.4-4.0 |
| Non-nucleoside RT inhibitors (NNRTIs) | |||||
| Efavirenz | 5.9 | R | >300 | R | >3.0 |
| Etravirine | 0.7 | S | 5.7 | I | 2.9-10 |
| Nevirapine | 72 | R | >300 | R | >4.5 |
| Protease inhibitors (PIs) | |||||
| Atazanavir | 107 | R | 58 | R | >5.2 |
| Darunavir | 151 | R | 155 | R | 10-90 |
| Fosamprenavir | 143 | R | >300 | R | 4-11 |
| Indinavir | 28 | R | 26 | R | >10 |
| Lopinavir | 65 | R | 126 | R | 9-55 |
| Nelfinavir | 45 | R | 43 | R | >3.0 |
| Saquinavir | 35 | R | 31 | R | 2.3-12 |
| Tipranavir | 12 | R | 9.8 | R | 2.0-8.0 |
| Integrase inhibitors | |||||
| Raltegravir | >200 | R | 9.9 | R | 1.5 |
| Fusion inhibitors | |||||
| Enfuvirtide | 2.1 | S | 40 | R | >9.0 |
| CCR5 inhibitors | |||||
| Maraviroc | R5+/X4+ | R | R5+/X4+ | R | R5+/X4+ |
Genotypic susceptibility data for the NRTIs, NNRTIs, and PIs can be found in Table 1 of the Supplementary Materials. Integrase inhibitor susceptibility testing in 2008 and 2009 demonstrated four Raltegravir-resistance mutations: L74M, T97A, Y143C, and S230R. Phenotypic susceptibility testing in 2007 revealed 152-fold decreased Enfuvirtide susceptibility. The presence of CXCR4 tropic virus (X4+) indicates intrinsic nonresponsiveness to Maraviroc and other CCR5 inhibitors
In June 2009, the patient enrolled in a double-blinded phase IIb dose-response clinical trial entitled “Dose-response study of ibalizumab (TaiMed Biologics Inc., Irvine CA) plus optimized background regimen in patients with HIV-1 (TMB-202; NCT00784147).” This blinded investigation compared monthly infusions of ibalizumab (formerly designated as TNX-355) 2000 mg IV every four weeks with 800 mg IV every two weeks. Because baseline drug resistance testing demonstrated enfuvirtide susceptibility (despite a 152-fold reduction in susceptibility observed in 2007) and susceptibility to etravirine, the patient’s background regimen was optimized to comprise these two ARVs. Two weeks following the start of study treatment, the patient’s plasma virus level decreased by >2.5 log10 (Figure 1B). Within 24 weeks, consecutive plasma HIV-1 RNA levels were <75 copies/ml -- representing the approved lower limit of quantification for the Versant HIV RNA 3.0 assay (“bDNA”; Siemans Healthcare Diagnostics, Berkeley, CA). Despite this favorable virologic response, the patient’s CD4 lymphocyte count remained below 20 cells/mm3.
Following completion of the blinded study in December 2009, the patient was identified as a monthly ibalizumab recipient. At week 32, the patient was inadvertently administered an infusion of placebo in place of ibalizumab. By week 36, the patient’s plasma HIV-1 RNA had risen to >500,000 copies/ml by standard testing and was estimated at approximately five million plasma HIV-1 RNA copies/ml based on viral load testing of a diluted plasma sample. A repeat phenotypic susceptibility test revealed the emergence of a variant that displayed six-fold and 40-fold resistance to etravirine and enfuvirtide, respectively (Table 1). Reinstitution of ibalizumab resulted in an approximately 2.7 log10 reduction in plasma HIV-1 RNA levels within two weeks, but further reductions in virus levels were not observed. Despite the re-administration of various additional ARVs in combination with ibalizumab, enfuvirtide, and etravirine, the patient’s plasma HIV-1 RNA levels have persisted at approximately 4.0 log10 copies/ml through February 2011.
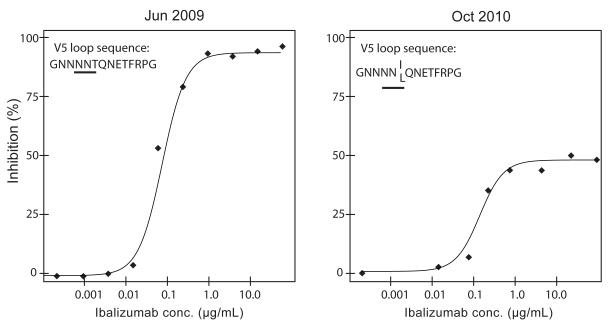
Ibalizumab susceptibility testing was performed prior to the start of TMB-202 and seven months after the reinstitution of ibalizumab following the unintended week 32 interruption (Figure 2). The dose-response curve of the June 2009 virus exhibited a classic sigmoidal shape with a maximum percent inhibition (MPI) approaching 100% and an IC50 value within the normal range for ibalizumab treatment-naïve viruses. In contrast, the dose response curve of the October 2010 virus indicated that only 50% of virus infection was susceptible to ibalizumab inhibition (MPI=50%). No significant differences in the IChalf max values were noted (defined as the concentration of drug required to inhibit 50% of the MPI). Sequencing the HIV-1 envelope gp120 region from the June 2009, July 2010, and October 2010 viruses demonstrated the acquisition of a T (Thr) to I (Ile) mutation in the July 2010 isolate and a T (Thr) to I (Ile) or L (Leu) mutation in the October 2010 isolate which resulted in the disruption of a potential N-linked glycosylation site (N-X-S/T-X (PNGS) in the HIV-1 envelope V5 loop (Supplementary Figure 1). Informed consent was obtained for the clinical trial and human subjects’ approval was obtained for the additional tests performed for this study.
Figure 2.
Ibalizumab susceptibility prior to TMB-202 (June 2009) and seven months following re-institution of ibalizumab following the week 32 unintended interruption (October 2010). The dose-response curve of the June 2009 virus exhibited a classic sigmoidal shape with a maximum percent inhibition (MPI) approaching 100% and an IC50 value within the normal range for ibalizumab treatment-naïve viruses. In contrast, the dose response curve of the October 2010 virus indicated that only 50% of virus infection was susceptible to ibalizumab inhibition (MPI=50%). Sequencing the HIV-1 envelope gp120 region from the June 2009, July 2010, and October 2010 viruses demonstrated the acquisition of a T (Thr) to I (Ile) mutation in the July 2010 isolate and a T (Thr) to I (Ile) or L (Leu) mutation in the October 2010 isolate which resulted in the disruption of a potential N-linked glycosylation site (N-X-S/T-X (PNGS) in the HIV-1 envelope V5 loop.
Two humanized mAbs targeting host receptors are in phase II clinical development: ibalizumab binds domain 2 of the CD4 receptor and PRO140 (Progenics Pharmaceuticals, Tarrytown, NY) attaches to the ligand-binding site of the CCR5 coreceptor (Huber et al., 2008). Ibalizumab binding does not inhibit HIV-1 gp120 attachment to CD4 domain 1, but rather inhibits a post-attachment step required for cell entry (Burkly et al., 1992; Freeman et al., 2010; Moore et al., 1992; Song et al., 2010). In contrast to mAbs that bind CD4 domain 1, ibalizumab does not deplete CD4+ lymphocytes or interfere with MHC Class II immune function (Boon et al., 2002; Jacobson et al., 2009; Kuritzkes et al., 2004). Both PRO140 and ibalizumab are potentially amenable to subcutaneous administration (Jacobson et al., 2009; Jacobson et al., 2010) and contain an IgG4 Fc domain that does not trigger antibody- and complement-dependent cytotoxicity (Burkly et al., 1992; Jacobson et al., 2010; Reimann et al., 1997).
This case report provides insight into the antiretroviral potency of ibalizumab as illustrated by the initial response to therapy (~4.0 log10 reduction in viral load) in combination with etravirine and re-use of enfuvirtide, the rapid loss of that initial response following an inadvertently missed infusion, and the sustained stabilization of plasma HIV-1 RNA levels at ~2.0 log10 copies/ml below pre-ibalizumab levels, despite notable reductions in susceptibility to ibalizumab and the optimized background ARVs. The magnitude of the virologic response reported in this study is greater than responses observed in earlier clinical trials of ibalizumab. This discrepancy may be explained by synergistic effects related to low CD4 counts in this patient coupled with the prevention of new infections of CD4+ lymphocytes by ibalizumab, or synergy between ibalizumab and the fusion inhibitor enfuvirtide, as previously reported (Zhang et al., 2006).
It is possible that the increased number of clinic visits during study period contributed to increased adherence and the resulting virological response. The patient, however, had high-levels of self-reported adherence before the 24 week study period (which was reflected by the history of transient virological responses to earlier salvage therapy regimens) and during the year following the study. Although the inadvertently missed infusion led to a rapid loss of virological response, the possibility that virological failure would have eventually occurred even without the missed infusion cannot be excluded. Indeed despite having plasma HIV-1 RNA levels below the level of detection at weeks 20 and 24, the plasma HIV-1 RNA level at week 28 was approximately 120 copies/ml. Nonetheless, the rapid loss of response to therapy and subsequent development of resistance to all three components of the salvage therapy regimen underscores the potential grave consequences of missing a single dose of an infrequently administered potent ARV.
This case report represents the second description of clinical resistance to ibalizumab. Toma et al. recently elucidated a genotypic and phenotypic mechanism of ibalizumab resistance (Toma et al., 2011). First, they demonstrated that HIV-1 can acquire reductions in ibalizumab susceptibility via mutations that disrupt potential N-linked glycosylation sites (PNGS) in the V5 loop of HIV-1. Second, they demonstrated that reductions in susceptibility to ibalizumab manifest phenotypically as reductions in maximal percent inhibition of virus replication (MPI) at saturating drug concentrations – a characteristic shared with agents that bind to host cell targets and exhibit non-competitive, allosteric inhibition, such as CCR5 inhibitors (Westby et al., 2007) – versus the resistant phenotypes of competitive inhibitors that manifest as increasing IC50 values (rightward shifts of sigmoidal dose response curves that reach 100% inhibition at saturating drug concentrations). The post-ibalizumab treatment gp120 sequences from July 2010 and October 2010 exhibited 8 and 13 amino acid differences, respectively, from the pre-ibalizumab treatment sequences from June 2009 (GenBank Accession numbers JF701703 to JF701706). Notably, one of the differences shared by both of the post treatment sequences resulted in the disruption of one of the previously described potential N-linked glycosylation sites in V5 associated with reductions in ibalizumab susceptibility (Toma et al., 2011).
Given the availability of six drug classes of small molecule medications in most industrialized countries it is not surprising that mAbs have not contributed to our current antiretroviral armamentarium. Manufacturing of biologic therapeutics (e.g. mAb) is expensive compared to chemical agents. Consequently, small molecule inhibitors of virus and host cell targets have been the focus of antiretroviral drug development from the onset. This is best illustrated by the fact that the murine precursor of ibalizumab (5A8) was identified 17 years prior to the use of the humanized mAb for deep salvage therapy in the patient we describe in this report (Burkly et al., 1992). However, mAbs may fill several important niches in HIV-1 treatment and prevention. In addition to an advanced-stage treatment option, therapeutic mAbs may be used for pre-exposure prophylaxis in high-risk uninfected populations. Moreover, if additional synergistic long acting antiretroviral agents are approved, it may become possible to create effective long-acting regimens that could be administered no more than once weekly. Such regimens would make it possible to treat nonadherent patient populations with directly observed therapy (DOT).
Supplementary Material
Supplementary Figure 1 Alignment of gp120 amino acid sequences obtained before (01-2001, 06-2009) and after (07-2010,10-2010) ibalizumab treatment. The complete amino acid sequence of the January 2001 (01-2001) sequence is shown. The amino acids of the remaining sequences are shown only if they differed from the January 2001 sequence. Positions containing electrophoretic mixtures of the 01-2001 amino acid and another amino acid were indicated by a ‘-‘ unless the other amino acid was present in more than one sequence. The variable loop (V1 through V5) regions are shown in bold. The sequences following January 2001 developed a five-amino acid insertion in the V1 sequence. The underlined amino acids in the V5 sequence indicates the site at which a mutation disrupted a potential N-linked glycosylation site. The 5′ and 3′ ends of the 10-2010 sequences were poorly resolved. Each of the sequences was submitted to GenBank (accession numbers JF701703 to JF701706).
Research Highlights.
The monoclonal antibody ibalizumab is active in vivo vs multidrug-resistant HIV-1.
We show this in a subject treated with ibalizumab, enfuvirtide re-use, and etravirine.
A missed ibalizumab infusion markedly affected HIV dynamics and drug resistance.
We confirmed the role of V5 env glycosylation site changes in ibalizumab resistance.
Acknowledgements
The authors thank Karina DeFaria (Monogram BioSciences) for project management. This study was supported by the NIH grant AI068581.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boon L, Holland B, Gordon W, Liu P, Shiau F, Shanahan W, Reimann KA, Fung M. Development of anti-CD4 MAb hu5A8 for treatment of HIV-1 infection: preclinical assessment in non-human primates. Toxicology. 2002;172:191–203. doi: 10.1016/s0300-483x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- Burkly LC, Olson D, Shapiro R, Winkler G, Rosa JJ, Thomas DW, Williams C, Chisholm P. Inhibition of HIV infection by a novel CD4 domain 2-specific monoclonal antibody. Dissecting the basis for its inhibitory effect on HIV-induced cell fusion. J. Immunol. 1992;149:1779–1787. [PubMed] [Google Scholar]
- Freeman MM, Seaman MS, Rits-Volloch S, Hong X, Kao CY, Ho DD, Chen B. Crystal structure of HIV-1 primary receptor CD4 in complex with a potent antiviral antibody. Structure. 2010;18:1632–1641. doi: 10.1016/j.str.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Olson WC, Trkola A. Antibodies for HIV treatment and prevention: window of opportunity? Curr. Top. Microbiol. Immunol. 2008;317:39–66. doi: 10.1007/978-3-540-72146-8_2. [DOI] [PubMed] [Google Scholar]
- Jacobson JM, Kuritzkes DR, Godofsky E, DeJesus E, Larson JA, Weinheimer SP, Lewis ST. Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob. Agents Chemother. 2009;53:450–457. doi: 10.1128/AAC.00942-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JM, Thompson MA, Lalezari JP, Saag MS, Zingman BS, D’Ambrosio P, Stambler N, Rotshteyn Y, Marozsan AJ, Maddon PJ, Morris SA, Olson WC. Anti-HIV-1 activity of weekly or biweekly treatment with subcutaneous PRO 140, a CCR5 monoclonal antibody. J. Infect. Dis. 2010;201:1481–1487. doi: 10.1086/652190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritzkes DR, Jacobson J, Powderly WG, Godofsky E, DeJesus E, Haas F, Reimann KA, Larson JL, Yarbough PO, Curt V, Shanahan WR., Jr. Antiretroviral activity of the anti-CD4 monoclonal antibody TNX-355 in patients infected with HIV type 1. J. Infect. Dis. 2004;189:286–291. doi: 10.1086/380802. [DOI] [PubMed] [Google Scholar]
- Moore JP, Sattentau QJ, Klasse PJ, Burkly LC. A monoclonal antibody to CD4 domain 2 blocks soluble CD4-induced conformational changes in the envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) and HIV-1 infection of CD4+ cells. J. Virol. 1992;66:4784–4793. doi: 10.1128/jvi.66.8.4784-4793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann KA, Lin W, Bixler S, Browning B, Ehrenfels BN, Lucci J, Miatkowski K, Olson D, Parish TH, Rosa MD, Oleson FB, Hsu YM, Padlan EA, Letvin NL, Burkly LC. A humanized form of a CD4-specific monoclonal antibody exhibits decreased antigenicity and prolonged plasma half-life in rhesus monkeys while retaining its unique biological and antiviral properties. AIDS Res. Hum. Retroviruses. 1997;13:933–943. doi: 10.1089/aid.1997.13.933. [DOI] [PubMed] [Google Scholar]
- Song R, Franco D, Kao CY, Yu F, Huang Y, Ho DD. Epitope mapping of ibalizumab, a humanized anti-CD4 monoclonal antibody with anti-HIV-1 activity in infected patients. J. Virol. 2010;84:6935–6942. doi: 10.1128/JVI.00453-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma J, Weinheimer SP, Stawiski E, Whitcomb JM, Lewis ST, Petropoulos CJ, Huang W. Loss of asparagine-linked glycosylation sites in variable region five of human immunodeficiency virus type 1 envelope is associated with resistance to CD4 antibody ibalizumab. J. Virol. 2011;85:3872–3880. doi: 10.1128/JVI.02237-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westby M, Smith-Burchnell C, Mori J, Lewis M, Mosley M, Stockdale M, Dorr P, Ciaramella G, Perros M. Reduced maximal inhibition in phenotypic susceptibility assays indicates that viral strains resistant to the CCR5 antagonist maraviroc utilize inhibitor-bound receptor for entry. J. Virol. 2007;81:2359–2371. doi: 10.1128/JVI.02006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Sorensen M, Fung M, Schooley RT. Synergistic in vitro antiretroviral activity of a humanized monoclonal anti-CD4 antibody (TNX-355) and enfuvirtide (T-20) Antimicrob. Agents Chemother. 2006;50:2231–2233. doi: 10.1128/AAC.00761-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Alignment of gp120 amino acid sequences obtained before (01-2001, 06-2009) and after (07-2010,10-2010) ibalizumab treatment. The complete amino acid sequence of the January 2001 (01-2001) sequence is shown. The amino acids of the remaining sequences are shown only if they differed from the January 2001 sequence. Positions containing electrophoretic mixtures of the 01-2001 amino acid and another amino acid were indicated by a ‘-‘ unless the other amino acid was present in more than one sequence. The variable loop (V1 through V5) regions are shown in bold. The sequences following January 2001 developed a five-amino acid insertion in the V1 sequence. The underlined amino acids in the V5 sequence indicates the site at which a mutation disrupted a potential N-linked glycosylation site. The 5′ and 3′ ends of the 10-2010 sequences were poorly resolved. Each of the sequences was submitted to GenBank (accession numbers JF701703 to JF701706).