Abstract
Background
Despite recent advances in intestinal transplantation (ITx), infection (INF) and acute cellular rejection (ACR) remain major causes of patient and graft loss. Studies in other solid-organ transplantations indicate that low levels of serum immunoglobulin G (IgG) negatively impact outcomes. To date, there have been no studies on IgG after ITx.
Methods
A retrospective review of an IgG measurement protocol in primary ITx recipients between 2007 and 2011 was undertaken. IgG levels were measured at the time of evaluation, transplantation, and at weekly intervals for 2 months. Hypogammaglobulinemia (HGG) was defined as IgG levels below the lower limit of the 95% confidence interval for age. Associations between HGG, INF, and ACR were tested, and the incidence and timing of INF and ACR were compared.
Results
Thirty-four patients were transplanted at a mean (SD) age of 12.4 (17.2) years. Most were Latino children with gastroschisis who received multivisceral grafts. Relative to pre-ITx levels, a statistically significant decrease in IgG levels was observed after ITx (PG0.05). Twenty patients (59%) developed HGG during the post-ITx period at a mean (SD) of 9.8 days. No significant associations were identified between HGG and INF or ACR.
Conclusions
This is the first study to describe serum IgG levels after ITx. A marked decrease in serum IgG levels was observed early on, in most patients. The etiology is potentially related to immunotherapy. HGG was not associated with INF or ACR, possibly related to the sample size and our practice of exogenous intravenous immunoglobulin replacement.
Keywords: Intestinal transplantation, Hypogammaglobulinemia, Immunosuppression, Infection, Acute cellular rejection
For the past decade, there has been a progressive improvement in outcomes after intestinal transplantation (ITx), with 5-year survival rates increasing from 40% before 1999 to 70% thereafter (1). The reasons are multifactorial and likely include center experience, improved surgical techniques, and advances in immunosuppressive and antimicrobial agents (2, 3). However, infection (INF) and acute cellular rejection (ACR) remain the major causes of early patient and graft loss (2, 3).
No doubt, the INF risk is complex (4). Proposed predisposing factors after ITx include a history of multiple INFs with antimicrobial resistant organisms, the magnitude of the surgical intervention, the requirement for gastrointestinal anastomoses, and poor preoperative bowel preparation caused by dysmotility and fistulas. Post-ITx predisposing factors include prolonged hospital/intensive care unit stay, open abdominal cavity, the presence of an ostomy, reoperations, strong immunosuppressive regimens, ACR, and multiple central venous catheters.
Hypogammaglobulinemia (HGG) is reported to occur after heart, kidney, liver, and lung transplantations with incidences of 10% to 73% (5–14). The etiology has commonly been attributed to immunosuppressive regimens (15). HGG is a significant risk factor for developing an opportunistic INF after other types of solid-organ transplantation (5–14). Despite these findings, serum immunoglobulin G (IgG) levels have never been studied after ITx. We hypothesized that HGG would be associated with higher rates of post-ITx INF. We aimed to determine the prevalence, timing, risk factors, and impact of HGG in ITx recipients.
Results
Demographics
During the study interval, forty-eight ITx were performed. Twelve retransplantations and two patients with primary immunodeficiency were excluded. Thirty-four patients with a mean (SD) age of 12.4 (17.2) years were included. Most were children (76%), male (62%), and Latino (62%). The most common causes of intestinal failure were gastroschisis (35%), intestinal atresia (15%), mesenteric vascular thrombosis (12%), and necrotizing enterocolitis (9%) (Table 1). The types of transplantation were isolated intestine (29%) and multivisceral (71%). All patients received standard triple-drug maintenance immunosuppression with tacrolimus, mycophenolate mofetil (MMF), and steroids. Induction therapy was interleukin-2 receptor antagonist (IL-2RA; n=22) and antithymocyte globulin (ATG; n=12).
Table 1. Patient characteristics at time of transplantation.
| Variable | Value |
|---|---|
| Age, mean (SD), y | 12.4 (17.3) |
| Age group, % | |
| Children | 76 |
| Adult | 24 |
| Ethnicity, % | |
| Latino | 62 |
| White | 23 |
| Asian | 9 |
| African-American | 3 |
| Others | 3 |
| Cause of intestinal failure, % | |
| Gastroschisis | 35 |
| Jejunoileal atresia | 15 |
| Mesenteric vascular thrombosis | 12 |
| Necrotizing enterocolitis | 9 |
| Chronic intestinal pseudoobstruction | 6 |
| Tufting enteropathy | 6 |
| Others | 17 |
Infectious Episodes
During the 8-week study period, 57 INFs were diagnosed after operation in 29 patients. The mean (SD) time to INF was 18.2 (17.4) days. The primary source of INF was intraabdominal (within the peritoneal cavity; 28%), pulmonary (23%), blood (17%), enteric (within the gastrointestinal tract; 17%), urinary (9%), wound (4%), and central nervous system (2%). The infectious organisms were bacterial (68%), fungal (18%), and viral (14%). Opportunistic INFs accounted for 21% of the INFs, predominantly caused by Candida species.
Rejection Episodes
The mean (SD) number of surveillance endoscopies per patient was 4.0 (2.1). There were 24 episodes of biopsyproven rejection diagnosed in 12 patients. The mean (SD) time to first rejection episode was 22.8 (14.9) days. The grades of rejection were as follows: 1, indeterminate (8%); 2, mild (67%); 3, moderate (0%); and 4, severe (25%). Rejection treatments were none (46%), steroids only (38%), antibody only (8%), antibody and steroids (4%), and antibody and intravenous immunoglobulin (IVIG) (4%).
Survival
The median follow-up time for the group was 56 days. During the 8-week study interval, patient and death-censored graft survival were 91% and 97%, respectively. The causes of death were intracerebral hemorrhage (n=1), cerebral herniation (n=1), and Candida peritonitis (n=1). The cause of graft loss was severe rejection of the intestinal allograft (n=1).
IgG Levels and HGG
The results for the IgG monitoring are shown in Figure 1. The mean (SD) IgG levels at evaluation and before ITx were 1095 (602) and 1287 (629) mg/dL, respectively. After ITx, the mean IgG levels initially drop by more than 50% and gradually increase over the study period but never return to pre-ITx levels. IgG levels at evaluation were statistically significantly higher than post-ITx levels in weeks 1 to 6 (P<0.02), whereas pretransplantation levels were significantly higher than all weeks after ITx (P<0.001). There was no statistical difference between the IgG levels in any week after ITx.
Figure 1.
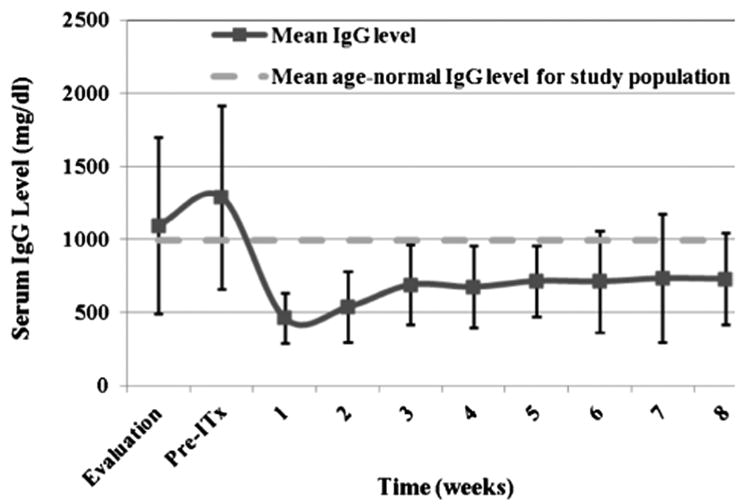
Mean immunoglobulin G (IgG) levels of intestinal transplant recipients during study intervals. For reference only, the mean ageYnormal IgG level for the study population is shown by a dashed line. IgG levels at evaluation were statistically significantly higher than postYintestinal transplantation (ITx) levels in weeks 1 to 6 (P<0.02), whereas pre-ITx levels were statistically significantly higher than those of all weeks after ITx (P<0.001).
IgG was found to be below the lower limit of the 95% confidence interval for age, a total of 57 times in 20 patients (59%), after ITx. The mean (SD) time to first HGG was 8.4 (4.9) days. Fourteen (41%) patients did not have HGG detected during the 8-week study period. Using alternative cutoffs for HGG, 20 patients (59%) showed severe, 5 patients (15%) showed moderate, and 5 patients (15%) showed mild IgG deficiencies.
Immunoglobulin Replacement Therapy
IVIG was administered 42 times in 20 patients after ITx. Thirteen patients received multiple IVIG administrations with a mean (SD) of 2.0 (1.1) administrations. The mean (SD) time to first administration of IVIG was 20.4 (14.5) days. For patients that received multiple IVIG administrations, the mean (SD) time between administrations was 12.1 (6.4) days. The most common reason for IVIG treatment was low IgG levels (83%). Other indications for IVIG administration were the presence of donor-specific antibodies (10%), positive donor-to-recipient crossmatch (2.3%), cytomegalovirus INF (2.3%), and ACR (2.3%). Of note, IVIG was administered in 85% of patients with HGG.
Analysis of Risk Factors for HGG and Association Between INF and ACR
Several demographic factors were analyzed as potential risk factors for developing HGG (Table 2). There was a trend for patients who received isolated intestinal grafts to have a higher likelihood of developing HGG (78%) than those who received multivisceral grafts (52%) (P=0.14). In addition, there was a trend for males (71% vs. 38% of females; P=0.05), adults (88% vs. 50% of children; P=0.20), and patients who received ATG (75% vs. 50% of IL-2RA; P=0.27) to develop HGG more commonly. No statistically significant differences were observed in the number of INF or ACR episodes or in the mean time to these events between HGG and non-HGG patients (Table 2). This held true when comparing severe and moderate IgG levels versus mild and normal IgG levels (data not shown). In addition, no statistical differences were observed in the number of opportunistic INFs observed between HGG and non-HGG patients (data not shown).
Table 2. Risk factors for posttransplantation hypogammaglobulinemia and infection/acute cellular rejection outcomes.
| Variable | HGG | Non-HGG | P |
|---|---|---|---|
| Gender, n | |||
| Female | 5 | 8 | |
| Male | 15 | 6 | 0.05 |
| Induction immunosuppressant, n | |||
| IL-2RA | 11 | 11 | 0.27 |
| ATG | 9 | 3 | |
| Age, n | |||
| Adult | 7 | 1 | 0.2 |
| Child | 13 | 13 | |
| Graft type, n | |||
| Intestine | 8 | 2 | 0.14 |
| Multivisceral | 12 | 12 | |
| INF, mean (SD), n | 1.4 (1.0) | 1.6 (1.0) | 0.56 |
| Time to INF, mean (SD), d | 20.4 (17.1) | 15.4 (18.2) | 0.45 |
| ACR, mean (SD), n | 0.9 (1.3) | 0.4 (0.8) | 0.11 |
| Time to ACR, mean (SD), d | 17.5 (6.3) | 37.5 (15.0) | 0.07 |
| Patients who ever had an INF, n | 16 | 13 | 0.34 |
| Patients who ever had an ACR, n | 8 | 4 | 0.71 |
ACR, acute cellular rejection; ATG, antithymocyte globulin; HGG, hypogammaglobulinemia; IL-2RA, interleukin-2 receptor antagonist; INF, infection.
Discussion
To our knowledge, this is the first report describing serum IgG levels after ITx. The observational portion of this study revealed a marked and significant reduction in IgG levels after ITx in most (59%) patients. This incidence represents one of the highest reported in the literature relative to other solid-organ transplantations—heart (10%), lung (14%–49%), liver (26%), and kidney (45%) (5–14) (Table 3). One of the strengths of this study lies in the protocolized assessment of IgG levels on a weekly basis after transplantation, thus providing a temporal pattern of IgG. There was a nonstatistically significant difference in mean IgG levels between evaluation and before ITx, presumably caused by time elapsed, natural variations in Ig levels, and evolving severity of illness. A profound and sudden decrease in IgG levels was frequently observed within 1 week after ITx. This reduction persisted throughout the 8-week study period. In contrast, the onset of HGG after other solid-organ transplantations seems to have more of a delay (Table 3). The reasons behind these differences are unknown and beyond the scope of this study but do raise interesting questions as to the pathophysiology involved in HGG in ITx recipients.
Table 3. Published studies on hypogammaglobulinemia in solid-organ transplantation.
| Organ Transplanted | Adult Versus Pediatric | No. Patients | Immunosuppression | Incidence of Severe HGG, % | Mean/Median (SD) Time to Severe HGG, d | Mean/Median (SD) Follow-up Period, mo | Reference |
|---|---|---|---|---|---|---|---|
| Heart | Adult | 111 | AZA, CYA, MMF, TAC | 10 | 196 (125) | 13.8 (5.7) | 6 |
| Lung | Adult | 57 | AZA, CYA, IL-2RA, MMF, steroid, TAC | 14 | 291 | V | 7 |
| Lung | Pediatric | 51 | CYA, IL-2RA, MMF, steroid | 48.8 | – | – | 8 |
| Lung | Adult | 40 | AZA, CYA, IL-2RA, MMF, steroid, TAC | 15 | 190 (30–458) | 2.6 (1.5–4.9) | 12 |
| Lung | Adult and pediatric | 67 | AZA, CYA, MMF, steroid, TAC | 37 | 899 (14–2555) | – | 13 |
| Liver | Adult | 112 | AZA, CYA, steroid | 26 | 59 (28–208) | 70 (1.3–94.7) | 10 |
| Renal | Adult | 152 | ATG, CYA, IL-2RA, MMF, OKT3, steroid, TAC | 45 | – | 3 (median) | 14 |
| Intestine | Adult and pediatric | 34 | ATG, IL-2RA, MMF, steroid, TAC | 59 | 8.4 (4.9) | 2 (0.6–2) | This study |
ATG, antithymocyte globulin; AZA, azathioprine; CYA, cyclosporine; HGG, hypogammaglobulinemia; IL-2RA, interleukin-2 receptor antagonist; MMF, mycophenolate mofetil; OKT3, Muromonab-CD3; TAC, tacrolimus.
The contribution of immunosuppression to HGG cannot be understated. Because of historically higher rates of ACR, ITx recipients require overall more in the way of induction immunosuppression than other solid-organ transplant recipients. In this study, patients received induction immunosuppression with either IL-2RA or ATG. Although neither induction agent was associated with an increased risk of HGG, a greater percentage of patients who received ATG went on to develop HGG. Perhaps, this is not surprising because ATG profoundly depletes many immune cell lineages, whereas IL-2RA prevents T-cell activation, which in turn reduces B-cell IgG production. Maintenance immunosuppression used in this study include tacrolimus, MMF, and steroids. There is little data regarding tacrolimus use and IgG deficiency, although its mechanism of action through the blockade of IL-2 production could have similar effects as noted above. The use of MMF, an inhibitor of lymphocyte proliferation, is also another plausible causative agent. In a randomized controlled trial, kidney transplant recipients on MMF-based regimens showed a significant reduction in IgG levels in contrast to those taking azathioprine (16). Adult lung transplant recipients on MMF also have incurred low IgG levels (12). Conversely, heart transplant recipients, similar to our study, did not show a significant difference in the development of HGG among patients using azathioprine, MMF, cyclosporine, or tacrolimus (5). Lastly, steroids, through the inhibition of lymphocyte activation, are another potential cause of HGG. In a study of heart transplant recipients, the use of IV pulse steroid therapy for the treatment of rejection was associated with an increased risk of developing severe HGG (6). Furthermore, one cannot discount the additive effects of all of these agents in combination.
Beyond immunosuppression, additional risk factors that render ITx recipients prone to developing HGG are the perioperative blood loss and subsequent transfusion requirements, protein-losing enteropathy, and renal dysfunction. ITx recipients typically require significant intraoperative blood transfusions after ITx, thus creating a dilutional effect, which may contribute to the immediate HGG observed in this study. Protein-losing enteropathies can be seen commonly after ITx during episodes of mucosal injury such as ACR or infectious enteritis. Protein-losing enteropathy has been described as a common secondary cause of HGG in nontransplant recipients (17, 18), and its incidence after ITx is unknown. Another potential source of protein/IgG loss is nephropathy. Renal injury is prevalent after all forms of transplantation, particularly ITx. Ojo et al. (19), using the Scientific Registry of Transplant Recipients database, reported that ITx recipients showed the highest rate of renal failure after transplantation. Our own single-center analysis also confirmed the high incidence of renal dysfunction after ITx (20). This study did not capture rates of protein-losing enteropathy or proteinuria, both of which would be important to examine in potential future investigations.
The association between HGG and opportunistic INFs is strong. In one of the earliest analysis, Weineke et al. (21) reported that 19% of renal transplant recipients with HGG developed severe INFs. In a study of lung transplant recipients, Goldfarb et al. (13) found that HGG patients showed a significantly higher incidence of posttransplantation INFs including pneumonia, bacteremia, cytomegalovirus (CMV) INF, and aspergillus. Similarly, Yamani et al. (6) assessed 111 heart transplant recipients and found a significantly higher incidence in opportunistic INFs among patients with severe HGG. Doron et al. (10) studied a group of 112 liver transplant recipients and found a strong association between HGG and mortality and opportunistic INF.
With these published reports combined with the fact that sepsis is the leading cause of mortality in ITx recipients, it would have been unethical to withhold IgG replacement therapy in this study group. As a result, IgG replacement was left to the discretion of the clinical team. This therapeutic intervention potentially impacted the assessment of the relationship between HGG and INF. This also impacted analysis of IgG levels between post-ITx weeks. A revealing statistic from this study demonstrating this complex interaction is the fact that the mean times to first INF, ACR, and IVIG infusion were all approximately 3 weeks. Therefore, in contrast with the other studies, we did not find a relationship between HGG and INF but strongly caution the interpretation of this finding.
We did not demonstrate a significant relationship between HGG and ACR; however, Yamani et al. (6) identified that patients with severe HGG after heart transplantation showed an incidence of ACR that was two times higher than those without. In contrast, Doron et al. (10) found no correlation between HGG and ACR after liver transplantation. Admittedly, it is difficult to decipher these conflicting results, and it may relate to whether HGG or ACR is the inciting event. That is, escalation of immunotherapy is associated with HGG, and it may be that patients with ACR have received steroid boluses or antibody therapy, thus resulting in the low IgG levels. Our study does not support or refute either of these assertions because we found no correlation between ACR and HGG. Exogenous IgG administration certainly may confound our results.
Although this is the first study reported on HGG in ITx, there are several weaknesses. The first is the small sample size, which is reflective of the number of ITx performed worldwide. Second, is the follow-up period of 8 weeks, which may be insufficient to determine the impact of HGG in interferon and ACR. Lastly, the use of immunosuppressants and IVIG was not randomized. To determine appropriate indications for monitoring and treating post-ITx HGG, a better understanding is needed of the risk factors contributing to its development and its impact on infectious complications, rejection, and survival.
In summary, HGG occurred in a substantial proportion of ITx recipients. HGG developed quite early after ITx and never recovered back to normal levels. Our experience revealed that the mean times to first INF, ACR, and IVIG administration were approximately the same, perhaps limiting out the ability to demonstrate an association between HGG and INF or ACR. Although this study is the first to describe IgG levels and HGG after ITx, it should serve as a catalyst for the further investigation of this complex field.
Materials and Methods
Study Design and Patient Population
A retrospective review of an institutional review boardYapproved, prospectively maintained electronic database was undertaken. All primary ITx recipients between January 1, 2007, and December 31, 2011, were included. Retransplant recipients and those with known pretransplantation immunodeficiencies were excluded. A battery of demographic data including age, race, gender, type of graft, induction immunosuppression, and primary diagnosis were recorded. For the purpose of this analysis, all liver-intestine and multivisceral transplantations were grouped together and are henceforth referred to as multivisceral. All isolated intestine and modified multivisceral recipients were categorized as intestine only. The details regarding surgical techniques are described in our previous experience (1).
Immunoglobulin Measurement, Classification of HGG, and IVIG Replacement
In 2005, a protocol of IgG measurement was initiated. Serum IgG was measured at the time of transplantation evaluation, immediately before ITx, and weekly thereafter for the first 2 months after transplantation. Whole blood was analyzed through the medical center clinical laboratory department. A normal adult level for IgG is 690 to 1600 mg/dL. For children, agespecific reference ranges were obtained from published norms (Table 4) (22). Clinically, recipients who were found to have a serum IgG level below the lower limit of the 95% confidence interval for age were eligible to receive IVIG (Privigen; CSL Behring, King of Prussia, PA) at the discretion of the clinical team. IVIG replacement was administered at a dose of 500 mg/kg of body weight. All instances of IVIG supplementation were recorded as a study variable. Besides HGG, other clinical indications for IVIG administration included the presence of donor-specific antibody or a positive donor:recipient crossmatch. In these cases, IVIG is prescribed at immunomodulatory doses, that is, 2 g/kg of body weight.
Table 4. Normal age specific values for serum immunoglobulin G levels (21).
| Age | Mean IgG (95% confidence interval), mg/dL |
|---|---|
| 1 mo | 503 (251–906) |
| 2 mo | 365 (206–601) |
| 3 mo | 334 (176–581) |
| 4 mo | 343 (196–558) |
| 5 mo | 403 (172–814) |
| 6 mo | 407 (215–704) |
| 7 to 9 mo | 475 (217–904) |
| 10 to 12 mo | 594 (294–1069) |
| 1 y | 679 (345–1213) |
| 2 y | 685 (424–1051) |
| 3 y | 728 (441–1135) |
| 4 to 5 y | 780 (463–1236) |
| 6 to 8 y | 915 (633–1280) |
| 9 to 10 y | 1007 (608–1572) |
| Adults | 994 (639–1349) |
IgG, immunoglobulin G.
For the analysis, the results of the IgG testing were grouped at the time intervals of evaluation, pretransplantation, and posttransplantation week (days 1Y7 [week 1], days 8Y14 [week 2], days 15Y21 [week 3], days 22Y28 [week 4], days 29Y35 [week 5], days 36Y42 [week 6], days 43Y49 [week 7], and days 50Y56 [week 8]). If more than one level was measured during each time interval, all results were included. HGG was defined as a serum IgG level below the lower limit of the 95% confidence interval for age. Alternative cutoffs that were analyzed included less than the 5th (severe), from the 6th to 25th (moderate), 26th to 50th (mild), and greater than the 50th (normal) percentiles of normal IgG levels for age.
Immunosuppression Protocol
Standard immunosuppression is triple-drug therapy based on tacrolimus (Prograf; Astellas Pharma, Deerfield, IL), MMF (CellCept; Roche Pharma, Nutley, NJ), and steroids (methylprednisolone, hydrocortisone, and prednisone). Target 12-hr trough tacrolimus level for postoperative days (PODs) 1 to 14 is 12 to 18 ng/mL, whereas for PODs 15 to 100, it is 10 to 15 ng/mL. All patients received induction immunotherapy using an IL-2RA: daclizumab (Zenapax; Roche Pharma, Nutley, NJ), basiliximab (Simulect; Novartis Pharma, East Hanover, NJ), or ATG (rabbit ATG Thymoglobulin; Genzyme Pharma, Cambridge, MA). Daclizumab induction was given at a dose of 2 mg/kg IV before ITx (POD 0) and then on PODs 4, 14, 28, 42, and 56. Basiliximab induction therapy is given at a dose of 20 mg for adults and 10 mg for children on PODs 0, 4, 21, 42, and 63. ATG induction therapy is 2 mg/kg, given on PODs 0, 1, 2, 3, and 4. Patients who received isolated intestinal transplants and those who were highly presensitized received ATG induction, whereas those who received liver-inclusive multivisceral grafts received IL-2RAs.
A protocol using prophylactic and preemptive therapy against CMV disease was systematically applied to ITx recipients using the following protocol: from days 0 to 14 after ITx, 10-mg/kg per day ganciclovir was given IV two times per day; from days 15 to 100 after ITx, 6-mg/kg per day ganciclovir was given four times daily; and acyclovir or valganciclovir was given everyday thereafter. A monitoring protocol using a blood polymerase chain reaction for CMV and Epstein-Barr virus was also initiated with samples collected weekly early on after ITx, followed by monthly during the intermediate phase, and quarterly for long-term survivors. Based on the results of these polymerase-chain reaction testing, preemptive therapy with ganciclovir and/or CMV Ig (CMV-IVIG, Cytogam; CSL Behring) was applied as previously described (23).
All patients received pneumocystis jiroveci pneumonia prophylaxis for up to 5 years after transplantation. Prophylaxis is restarted if a patient is treated for rejection. First-line prophylaxis includes cotrimoxazole (Bactrim; Hoffmann-La Roche, Inc., Philadelphia, PA)—pediatric dose: trimethoprim component, 3 to 5 mg/kg per day, Monday to Friday; adult dose: one double strength tab daily, Monday to Friday. Second line therapy for those who are sulfaallergic or develop marrow suppression on Bactrim included atovaquone, (Mepron; GlaxoSmithKline, Research Triangle Park, NC), dapsone (Jacobus Pharmaceutical Co., Inc., Princeton, NJ), or IV pentamidine (Pentam; APP Pharmaceuticals, LLC, Schaumburg, IL).
Classification of INF and ACR
Endoscopic surveillance of ITx recipients is protocolized during the first 8 weeks after operation. The initial endoscopy is done through the ileostomy only with later endoscopies performed through the ileostomy and orally. Standard biopsy practice is to obtain one to three specimens at one to five different distances. Any grossly abnormal endoscopic areas are biopsied. Biopsies are sent rush in formalin to surgical pathology. Processing includes fixation in formalin, paraffin embedding, thin slicing, and then staining with hematoxylin and eosin. Specimens are then graded by a clinical pathologist based on the standard criteria set forth for intestinal rejection (24). These results are then independently classified into the following categories/scores: 0, normal or no abnormalities; 1, indeterminant for ACR based on borderline apoptosis; 2, mild ACR with greater than eight apoptotic bodies per high power field; 3, moderate ACR with apoptosis, crypt damage, and villous blunting; 4, severe ACR with villous injury and crypt damage. Only biopsy-proven episodes of ACR episodes were included in the analysis.
All patients receive pre-ITx bowel preparation with standard enteral antibiotics. Preoperative IV antibiotic therapy includes antifungal drugs (Ambisome; Astellas Pharma US, Northbrook, IL; Caspofungin; Merck & Co., Whitehouse Station, NJ; and Fluconazole; Pfizer, New York, NY) combined with broad-spectrum antibacterial agents (Cefoxitin, Merck & Co., Whitehouse Station, NJ; Ceftazidime; Hospira, Lake Forest, IL; Unasyn; Pfizer, New York, NY; and Gentamycin; Wedgewood Pharmacy, Merck & Co., Whitehouse Station, NJ). Perioperative antibiotics are continued for the first 3 days after operation. Any further antimicrobial therapy is based on culture results or clinical conditions such as fever or leukocytosis. Patients are routinely cultured for temperature elevations higher than 38.0°C. Standard cultures are obtained from the peripheral blood, central venous catheters, sputum (if intubated), and urine. Documented INFs are those associated with clinical findings/suspicion and confirmed with the appropriate culture results. Standard antimicrobial treatment courses are 7 to 14 days. Culture-proven INFs were analyzed as a study variable.
Statistical Methods
Chi-square (Fisher exact test) methods were used to test for associations between HGG and INF or ACR. Student t test was used to compare the mean number of, and time to onset of, INF or ACR between HGG and non-HGG patients. Patient and graft survivals during the study period were determined using the Kaplan-Meier method. Graft survival was death censored. Statistical analysis was performed using SAS (SAS Inc., Cary, NC).
Acknowledgments
D.G.F., O.M.K., and R.S.V. participated in making the research design, performing the research, analyzing the data, and writing the paper. L.J.W. participated in making the research design, performing the research, and analyzing the data. E.M., S.P., and V.H. participated in making the research design and collecting the data. S.V.M. and R.W.B. participated in making the research design and writing the paper.
Footnotes
The authors declare no funding or conflicts of interest.
References
- 1.Farmer DG, Venick RS, Colangelo J, et al. Pretransplant predictors of survival after intestinal transplantation: analysis of a single-center experience of more than 100 transplants. Transplantation. 2010;90:1574. doi: 10.1097/TP.0b013e31820000a1. [DOI] [PubMed] [Google Scholar]
- 2.Mazariegos GV, Superina R, Rudolph J, et al. Current status of pediatric intestinal failure, rehabilitation, and transplantation: summary of a colloquium. Transplantation. 2011;92:1173. doi: 10.1097/TP.0b013e318234c325. [DOI] [PubMed] [Google Scholar]
- 3.Fishbein TM. Intestinal transplantation. N Engl J Med. 2009;361:998. doi: 10.1056/NEJMra0804605. [DOI] [PubMed] [Google Scholar]
- 4.Michaels MG, Green M. Infections in pediatric transplant recipients: not just small adults. Infect Dis Clin North Am. 2010;24:307. doi: 10.1016/j.idc.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Yamani MH, Avery R, Mawhorter S, et al. Hypogammaglobulinemia after heart transplantation: impact of pre-emptive use of immunoglobulin replacement (CytoGam) on infection and rejection outcomes. Transpl Infect Dis. 2001;3:40. doi: 10.1034/j.1399-3062.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamani MH, Avery RK, Mawhorter SD, et al. Hypogammaglobulinemia following cardiac transplantation: a link between rejection and infection. J Heart Lung Transplant. 2001;20:425. doi: 10.1016/s1053-2498(00)00331-4. [DOI] [PubMed] [Google Scholar]
- 7.Kawut SM, Shah L, Wilt JS, et al. Risk factors and outcomes of hypogammaglobulinemia after lung transplantation. Transplantation. 2005;79:1723. doi: 10.1097/01.tp.0000159136.72693.35. [DOI] [PubMed] [Google Scholar]
- 8.Robertson J, Elidemir O, Saz EU, et al. Hypogammaglobulinemia: incidence, risk factors, and outcomes following pediatric lung transplantation. Pediatr Transplant. 2009;13:754. doi: 10.1111/j.1399-3046.2008.01067.x. [DOI] [PubMed] [Google Scholar]
- 9.Ganschow R, Englert C, Grabhorn E, et al. Hypogammaglobulinemia in pediatric liver transplant recipients. Pediatr Transplant. 2005;9:215. doi: 10.1111/j.1399-3046.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 10.Doron S, Ruthazer R, Werner BG, et al. Hypogammaglobulinemia in liver transplant recipients: incidence, timing, risk factors, and outcomes. Transplantation. 2006;81:697. doi: 10.1097/01.tp.0000180531.66518.9e. [DOI] [PubMed] [Google Scholar]
- 11.Van Thiel DH, Finkel R, Friedlander L, et al. The association of IgA deficiency but not IgG or IgM deficiency with a reduced patient and graft survival following liver transplantation. Transplantation. 1992;54:269. doi: 10.1097/00007890-199208000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Yip NH, Lederer DJ, Kawut SM, et al. Immunoglobulin G levels before and after lung transplantation. Am J Respir Crit Care Med. 2006;173:917. doi: 10.1164/rccm.200510-1609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldfarb NS, Avery RK, Goormastic M, et al. Hypogammaglobulinemia in lung transplant recipients. Transplantation. 2001;71:242. doi: 10.1097/00007890-200101270-00013. [DOI] [PubMed] [Google Scholar]
- 14.Broeders EN, Wissing KM, Hazzan M, et al. Evolution of immunoglobulin and mannose binding protein levels after renal transplantation: association with infectious complications. Transpl Int. 2008;21:57. doi: 10.1111/j.1432-2277.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 15.Mawhorter S, Yamani MH. Hypogammaglobulinemia and infection risk in solid organ transplant recipients. Curr Opin Organ Transplant. 2008;13:581. doi: 10.1097/MOT.0b013e3283186bbc. [DOI] [PubMed] [Google Scholar]
- 16.Keven K, Sahin M, Kutlay S, et al. Immunoglobulin deficiency in kidney allograft recipients: comparative effects of mycophenolate mofetil and azathioprine. Transpl Infect Dis. 2003;5:181. doi: 10.1111/j.1399-3062.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 17.Waldmann TA. Protein-losing enteropathy. Gastroenterology. 1966;50:422. [PubMed] [Google Scholar]
- 18.Umar SB, DiBaise JK. Protein-losing enteropathy: case illustrations and clinical review. Am J Gastroenterol. 2010;105:43. doi: 10.1038/ajg.2009.561. [DOI] [PubMed] [Google Scholar]
- 19.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 20.Watson MJ, Venick RS, Kaldas F, et al. Renal function impacts outcomes after intestinal transplantation. Transplantation. 2008;86:117. doi: 10.1097/TP.0b013e31817d55ae. [DOI] [PubMed] [Google Scholar]
- 21.Wieneke H, Otte B, Lang D, et al. Predictive value of IgG subclass levels for infectious complications in renal transplant recipients. Clin Nephrol. 1996;45:22. [PubMed] [Google Scholar]
- 22.Jolliff CR, Cost KM, Stivrins PC, et al. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin Chem. 1982;28:126. [PubMed] [Google Scholar]
- 23.Farmer DG, McDiarmid SV, Winston D, et al. Effectiveness of aggressive prophylatic and preemptive therapies targeted against cytomegaloviral and EpsteinYBarr viral disease after human intestinal transplantation. Transplant Proc. 2002;34:948. doi: 10.1016/s0041-1345(02)02710-0. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz P, Bagni A, Brown R, et al. Histological criteria for the identification of acute cellular rejection in human small bowel allografts: results of the pathology workshop at the VIII International Small Bowel Transplant Symposium. Transplant Proc. 2004;36:335. doi: 10.1016/j.transproceed.2004.01.079. [DOI] [PubMed] [Google Scholar]


