Abstract
Importance
Telomeres protect chromosome ends and are markers of cellular aging and replicative capacity.
Objective
To evaluate the association between recipient and donor pre-transplant leukocyte telomere length with outcomes after unrelated donor allogeneic hematopoietic cell transplantation (HCT) for patients with severe aplastic anemia
Design, Participants, and Setting
The study included 330 patients (235 acquired, 85 Fanconi anemia, and 10 Diamond Blackfan anemia) and their unrelated donors who had pre-HCT blood samples, and clinical and outcome data available at the Center for International Blood and Marrow Transplant Research. Patients underwent HCT between 1989 and 2007 in 84 centers, and were followed-up until March 2013
Exposures
Recipient and donor pre-HCT leukocyte telomere length classified into long (3rd tertile) and short (1st and 2nd tertiles combined) based on donor telomere length distribution.
Main Outcomes
Overall survival, neutrophil recovery, and acute and chronic graft-versus-host disease (GvHD), as ascertained by transplant centers through regular patient follow-up.
Results
Longer donor leukocyte telomere length was associated with higher survival probability (5-year overall survival=56% (number at risk=57, cumulative deaths=50 vs. 40% (number at risk =71; cumulative deaths=128), in the long vs. short, respectively; p=0.009). The association remained statistically significant after adjusting for donor age, disease subtype, Karnofsky performance score, graft type, HLA matching, prior aplastic anemia therapy, race, and calendar year of transplant (hazard ratio (HR)= 0.61, 95% confidence intervals=0.44–0.86). Similar results were noted in analyses stratified on severe aplastic anemia subtype, recipient age, HLA matching, calendar year of transplant, and conditioning regimen. There was no association between donor telomere length and neutrophil engraftment at 28 days (cumulative incidence= 86% vs. 85%; HR=0.94, 95% CI= 0.73–1.22), acute GvHD grades III–IV at 100 days (cumulative incidence =22% vs. 28%; HR=0.77, 95% CI= 0.48–1.23), or chronic GvHD at 1-year (cumulative incidence =28% vs. 30%; HR=0.81, 95% CI= 0.53–1.24) for long versus short respectively. Pre-transplant leukocyte telomere length in the recipients was not associated with post-transplant survival (HR= 0.91, 95% CI= 0.64–1.30).
Conclusions and Relevance
Longer donor leukocyte telomere length was associated with increased 5-year survival in patients who received HCT for severe aplastic anemia. Patient leukocyte telomere length was not associated with survival. The results of this observational study suggest that donor leukocyte telomere length may have a role in long-term post-transplant survival.
Keywords: telomeres, aplastic anemia, marrow failure, hematopoietic cell transplant, outcome
Introduction
Telomeres are long (TTAGGG)n tandem nucleotide repeats and an associated protein complex located at chromosome ends that are essential for maintaining chromosomal stability. Telomeres shorten with each cell division due to the inability of DNA polymerases to completely replicate the chromosome ends. Consequently, telomeres are markers of cellular replicative capacity, cellular senescence, and aging.1
Aplastic anemia is a heterogeneous bone marrow failure disorder of multi-factorial etiology. Inherited bone marrow failure is caused by germline defects in telomere biology (e.g., dyskeratosis congenita), ribosomal function (e.g., Diamond Blackfan anemia or Shwachman Diamond syndrome), or DNA repair (e.g., Fanconi anemia).2 The acquired form of aplastic anemia is typically immune-mediated,3 and may also be related to environmental exposures.4 Allogeneic hematopoietic cell transplantation (HCT) is recommended as initial therapy for young patients with acquired severe aplastic anemia when a matched sibling donor is available. Older severe aplastic anemia patients or those who do not have a sibling donor typically receive at least one course of immunosuppressive therapy before considering unrelated donor HCT.5 HCT requires extensive replication and rapid expansion of transplanted donor cells to achieve engraftment.6,7 Several studies have demonstrated accelerated post-transplant telomere shortening in the transplanted hematopoietic cells when compared with age-matched controls or matched donors.7–10 Older donors,8 female gender, and chronic graft-versus-host disease (GvHD) were associated with shorter telomeres in transplanted hematopoietic cells after allogeneic HCT.7 Short telomeres in patients with acquired severe aplastic are associated with relapse, clonal evolution, and poor survival after immunosuppressive therapy.11
In this study, we evaluated the association between pre-HCT recipient and donor relative leukocyte telomere length and outcomes after HCT in patients with severe aplastic anemia.
Methods
Data Source
We identified patients who received an allogeneic HCT for severe aplastic anemia facilitated by the National Marrow Donor Program (NMDP) and who met study eligibility listed below. The NMDP is a non-profit organization that manages the “Be The Match Registry®” to facilitate unrelated HCT for those who lack a matched related donor. Clinical and outcome data from study participants were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR®) database. The CIBMTR is a research collaboration between the NMDP/Be The Match Registry and the Medical College of Wisconsin and collects data from a voluntary working group of more than 450 transplant centers around the world. Participating centers contribute comprehensive baseline and longitudinal follow-up data including patient and donor demographics, patient diagnosis, pre-transplant therapy, and clinical parameters, other transplant-related information, and patient outcomes.
Study Participants and Patient Eligibility Criteria
Patients were eligible for the study if they received a first HCT for severe aplastic anemia (1) prior to age 40 years, (2) between 1989 and 2007, (3) from an unrelated donor, and (4) had an available pre-transplant blood sample from both the patient and the donor in the NMDP/CIBMTR Research Sample Repository.
Of the 518 patients who underwent unrelated donor HCT with a high-resolution HLA typing for severe aplastic anemia during the study time period at 97 centers, 342 met study eligibility criteria (59 patients were excluded because they were older than 40 years of age, and 117 patients because of the unavailability of pre-HCT blood sample for either the recipient or the donor.) We excluded 12 patients from the final analysis because of failed DNA extraction or telomere length assay. The final analyses included 330 patients from 84 centers.
All patients and donors provided informed consent, and the study was approved by the NMDP Institutional Review Board. Cord blood transplants were excluded from this study.
Definitions of Study End Points and Selected Clinical and Demographic Variables
The study outcomes included overall survival, neutrophil and platelet recovery, and acute and chronic GvHD. Neutrophil recovery was defined as an absolute neutrophil count ≥ 0.5×109/L for three consecutive days. Platelet recovery was defined as a platelet count ≥ 20 × 109/L independent of transfusions for 7 consecutive days. Acute and chronic GvHD were defined according to standard criteria.12,13
Conditioning regimens were classified as myeloablative, reduced intensity or non-myeloablative according to standard CIBMTR definitions.14
Patient race was reported by the transplant center using standardized CIBMTR forms, while donor race was self identified at the time of registration to NMDP registry. Recipient and donor race for this study were classified as White, African American, or other if Asian, Pacific Islander, Hispanic, Native American, or other/unknown race.
Telomere Length Measurement
We used samples of peripheral blood mononuclear cells or whole blood collected, processed, and stored in liquid nitrogen or at −80C at the NMDP/CIBMTR repository using standard operating procedures. Samples were transferred to the DNA extraction facility on dry ice where QIAamp Maxi Kit procedure (QIAGEN Inc., Valencia, CA) was used to extract DNA from all samples. While at the DNA extraction facility, all samples went through a tightly regulated, automated DNA staging procedure; DNA volume was quantified using PicoGreen, normalized to 50ng/ul, plated, stored in a robotic automated cold storage unit until transferred (shipped overnight on dry ice) to the telomere assay laboratory. We measured relative leukocyte telomere length in extracted DNA using monoplex quantitative real-time PCR (qPCR), as previously described.15 Briefly, the qPCR assay calculates the ratio between telomeric repeat copy number (T) and that of a single reference gene (beta-globin gene; 36B4) (S). Relative T/S is calculated in relation to a reference curve and final measurements are exponentiated to assure normality. Details are available elsewhere.16 For quality control, all telomeric and 36B4 reactions were measured in triplicate, and the average was used for final calculations. The mean coefficient of variation (CV) among all samples was 0.6% for the telomere assay, 0.34% for the 36B4 assay. The mean CV for the exponentiated T/S measure from quality control samples was 13.2%. Laboratory personnel were blinded to the recipient/donor status, participant age, and recipient outcome.
Statistical Analysis
We used the Kaplan-Meier estimator to calculate the probability of overall survival (OS) and 95% confidence intervals (CI) at 1, 3 and 5 years post-HCT. Follow-up time started at the date of HCT, and ended at death or censored at date of last follow-up (only 3 patients were lost to follow-up), or end of study in March 22, 2013. The log-rank test was used to compare the survival distribution across categories of leukocyte telomere length. The cumulative incidence estimator was used to calculate the probabilities of neutrophil recovery, platelet recovery, and acute and chronic GvHD; death was treated as a competing event for those analyses.
For multivariable analyses, we calculated hazard ratios (HRs) and the 95% CI of the outcome of interest, comparing leukocyte telomere length categories using Cox regression models. The proportional hazard assumption was tested for all variables included in the model, and stratification was used if the proportionality assumption was not met. The associations between recipient or donor leukocyte telomere length and outcomes of interest have been tested in separate models. Variables included in the model were selected by a stepwise forward-backward procedure with a p-threshold of 0.05 for both entry and retention in the model. All variables presented in Table 1 were tested for their eligibility to stay in the final model. Final survival models for both recipient and donor leukocyte telomere length analyses for survival outcome included: corresponding leukocyte telomere length and age, severe aplastic anemia subtype, Karnofsky performance score (KPS), HLA matching, prior therapy for aplastic anemia, race, and calendar year of transplant. Final models for neutrophil recovery was adjusted for patient age, disease subtype, donor age, HLA matching, graft type, and GvHD prophylaxis. Final models for acute GvHD grade III-IV were adjusted for patient age, HLA matching, T-cell depletion, and calendar year of transplant. Final models for chronic GvHD were adjusted for patient age, donor-recipient cytomegalovirus matching status, cyclophosphamide and total body irradiation given.
Table 1.
Characteristics of hematopoietic cell transplant recipients for severe aplastic anemia by donor leukocyte telomere length categories
| 1st Tertile of Donor LTL (T/S <0.62) | 2nd Tertile of Donor LTL (T/S 0.62–<0.81) | 3rd Tertile of Donor LTL (T/S≥ 0.81) | P-value | |
|---|---|---|---|---|
| N=106 | N=111 | N=113 | ||
| N (%) | ||||
| Age at HCT (years) median (range) | 14 (<1–39) | 17 (<1–39) | 11 (<1–39) | 0.02 |
| Age at HCT in decades | 0.09 | |||
| 0 – 9 y | 29 (27) | 23 (21) | 42 (37) | |
| 10 – 19 y | 40 (38) | 47 (42) | 42 (37) | |
| 20 – 29 y | 26 (25) | 22 (20) | 16 (14) | |
| 30 – <40 y | 11 (10) | 19 (17) | 13 (12) | |
| Race | 0.09 | |||
| White | 77 (73) | 83 (75) | 75 (66) | |
| African American | 6 (6) | 11 (10) | 13 (12) | |
| Others | 23 (21) | 17 (15) | 25 (22) | |
| Male sex | 52 (49) | 65 (59) | 59 (52) | 0.36 |
| Pre-HCT Karnofsky ≥ 90 | 82 (80) | 79 (75) | 92 (86) | 0.11 |
| HLA matching | 0.23 | |||
| 8/8 | 51 (48) | 53 (48) | 48 (42) | |
| 7/8 | 26 (25) | 33 (30) | 32 (28) | |
| 6/8 | 19 (18) | 15 (14) | 22 (19) | |
| ≤ 5/8 | 10 (9) | 10 ( 8) | 11 (10) | |
| Graft type | 0.27 | |||
| Bone marrow | 98 (92) | 95 (86) | 99 (88) | |
| Peripheral blood (PBSC) | 8 ( 8) | 16 (14) | 14 (12) | |
| Disease Subtype | 0.09 | |||
| Acquired aplastic anemia | 80 (75) | 85 (77) | 70 (62) | |
| Fanconi Anemia | 22 (21) | 24 (22) | 39 (34) | |
| Diamond Blackfan Anemia | 4 (4) | 2 (2) | 4 (4) | |
| Conditioning regimen | 0.24 | |||
| Myeloablative | 48 (45) | 37 (33) | 36 (32) | |
| Reduced intensity | 29 (27) | 37 (33) | 42 (37) | |
| Non-myeloablative | 18 (17) | 29 (26) | 27 (24) | |
| Other | 11 (10) | 8 (7) | 8 (7) | |
| Donor/Recipient sex match | 0.67 | |||
| Male/Male | 35 (33) | 39 (35) | 40 (35) | |
| Male/Female | 29 (27) | 29 (26) | 30 (27) | |
| Female/Male | 17 (16) | 26 (23) | 19 (17) | |
| Female/Female | 25 (24) | 17 (15) | 24 (21) | |
| Prior severe aplastic anemia therapy | 0.76 | |||
| None | 9 (8) | 11 (10) | 17 (15) | |
| Androgens but no corticosteroids | 10 (9) | 6 (5) | 11 (10) | |
| Corticosteroids but no androgens | 53 (50) | 52 (47) | 47 (42) | |
| Both androgens and corticosteroids | 11 (10) | 11 (10) | 13 (12) | |
| Others | 16 (15) | 21 (19) | 15 (13) | |
| Missing/Unknown | 7 (7) | 10 (9) | 10 (9) | |
| Recipient age at severe aplastic anemia diagnosis | 0.31 | |||
| < 20 years | 80 (75) | 75 (68) | 86 (76) | |
| ≥ 20 years | 25 (24) | 30 (27) | 21 (19) | |
| Missing | 1 (1) | 6 (5) | 6 (5) | |
|
Months from aplastic anemia diagnosis to HCT Median (range) |
20 (<1–318) | 24 (1–340) | 19 (2–186) | 0.66 |
| GvHD prophylaxis | 0.21 | |||
| Tacrolimus + (methotrexate or mycophenolate mofetil or steroids) ± other | 17 (16) | 30 (27) | 15 (13) | |
| Tacrolimus or mycophenolate mofetil ± other | 2 (2) | 2 (2) | 2 (2) | |
| Cyclosporine + methotrexate ± other | 46 (43) | 41 (37) | 41 (36) | |
| Cyclosporine ± other (No methotrexate) | 13 (12) | 11 (10) | 17 (15) | |
| T-cell depletion | 28 (26) | 27 (24) | 36 (32) | |
| Others^ | 0 | 0 | 2 (2) | |
| Donor/Recipient cytomegalovirus match | 0.66 | |||
| Negative/Negative | 32 (30) | 39 (35) | 36 (32) | |
| Negative/Positive | 28 (26) | 32 (29) | 36 (32) | |
| Positive/Negative | 19 (18) | 11 (10) | 14 (12) | |
| Positive/Positive | 27 (25) | 27 (24) | 25 (22) | |
| Unknown | 0 | 2 (2) | 2 (2) | |
| Donor age, median years (range) | 39 (21–57) | 36 (19–57) | 33 (19–53) | 0.003 |
| 18–29 | 20 (19) | 35 (32) | 44 (39) | 0.04 |
| 30–39 | 36 (34) | 37 (33) | 33 (29) | |
| 40–49 | 43 (41) | 32 (29) | 33 (29) | |
| 50 and older | 7 ( 7) | 7 ( 6) | 3 ( 3) | |
| Years of transplant | 0.27 | |||
| 1988 – 1995 | 27 (25) | 25 (23) | 21 (19) | |
| 1996 – 1999 | 33 (31) | 27 (24) | 26 (23) | |
| 2000 – 2007 | 46 (43) | 59 (53) | 66 (58) | |
| Follow-up of survivors, Median Months (range) | 84 (17–248) | 74 (5–221) | 75 (11–217) | 0.62 |
Abbreviations: HCT, hematopoietic cell transplantation; N, number; LTL, leukocyte telomere length; HLA, Human leukocyte antigens; GvHD, graft versus host disease
Others include: tacrolimus± other, mycophenolate mofetil± other, and methotrexate ± other (no cyclosporine)
Interactions between leukocyte telomere length and clinical covariates were tested, and none were statistically significant. Wald test was used to compare stratum-specific hazard ratios. We also tested for the association between patient survival and leukocyte telomere length in the recipient and donor simultaneously by including them in one model.
Pearson’s correlation coefficient was used to evaluate the strength of association between leukocyte telomere length and age. Leukocyte telomere length for both recipient and donor analyses was categorized into 3 categories based on the leukocyte telomere length tertiles in the donors (T/S <0.62, 0.62–<0.81, or ≥0.81). In final analyses, leukocyte telomere length was categorized into short if <0.81, or long if ≥0.81. Combining the two shortest telomere length categories was a post-hoc decision based on the similarity of the calculated hazard ratios between the shortest and intermediate categories in the final model.
For all statistical tests, 2-sided P <0.05 was considered statistically significant. We used SAS Versions [9.2], and [9.3] (SAS Institute Inc., Cary, NC, USA) for all analyses.
Results
Characteristics of Study Participants
Patients in this study were predominantly white (71%) with a nearly equal gender distribution (53% males). They were transplanted at a median age of 15 years (range= 0.5–39 years). Acquired severe aplastic anemia was the most common disease subtype (n=235, 71%), followed by Fanconi anemia (n=85, 26%) and Diamond Blackfan anemia (n=10, 3%). Donors were older than recipients (donor median age=36 years, range=19–57 years), and 55% were sex-matched to the recipients. Most of the patients received bone marrow grafts (88%), 45% received a myeloablative-conditioning regimen, and 46% had an 8/8 HLA matched donor graft. Cyclophosphamide (Cy) and total body irradiation (TBI) >500 cGy single-dose or TBI >800 cGy fractionated ± VP16 was the most commonly used myeloablative regimens (n=95/121; 78.5%), 500≥TBI>200 cGy single or 800≥TBI>200 cGy fractionated was the most prevalent reduced intensity regimen (n=95/108; 88%), and 200 cGy TBI was the most commonly used non-myeloablative regimen (n=34/74; 45.9%) in this population. Post-HCT follow-up for survivors ranged from 5 months to 21 years (median 6 years). Table 1 summarizes patient demographics, clinical, and HCT characteristics by leukocyte telomere length categories.
Leukocyte telomere length was inversely correlated with age in both donors (r= −0.20, p<0.001) and recipients (r= −0.31, p<0.001). Despite the fact that donors were older, patients with severe aplastic anemia had significantly shorter leukocyte telomere lengths (mean T/S =0.66+/−0.27), than their donors (mean T/S=0.77+/−0.22), even after adjusting for age (adjusted mean leukocyte telomere length difference comparing recipients with donors = −0.18, p<0.001).
Recipient pre-transplant leukocyte telomere length and post-HCT outcome
Longer pre-transplant leukocyte telomere length in the recipients was not associated with better survival after HCT. The OS probability of recipients with long versus short pre-HCT leukocyte telomere length were: 52% vs. 55% at 1-year (p=0.65), 50% vs. 50% at 3-years (p =0.99), and 47% vs. 46% at 5-years (p=0.87). Multivariable models adjusting for age, disease subtype, Karnofsky performance score, HLA matching, prior therapy for aplastic anemia, graft type, race, and calendar year of transplant showed similar results (number of events in the long and short telomere length=51, and 113, respectively; HR=0.91, 95% CI= 0.64–1.30, p=0.59) (Table 2).
Table 2.
Adjusted hazard ratio of HCT outcomes in patients with severe aplastic anemia comparing long to short leukocyte telomere length
| Recipient LTL | p-value | Donor LTL | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Number of events/sample size in long LTL | Number of events/sample size in short LTL | Adjusted^ HR (95% CI)* | Number of events/sample size in long LTL | Number of events/sample size in short LTL | Adjusted^ HR (95% CI)* | |||
| Overall survival | 51/92 | 113/193 | 0.91 (0.64–1.30) | 0.59 | 53/113 | 134/215 | 0.61 (0.44–0.86) | 0.006 |
| Neutrophil recovery | 86/92 | 174/192 | 1.03 (0.78–1.37) | 0.83 | 102/111 | 195/215 | 0.94 (0.73–1.22) | 0.64 |
| Acute GvHD grade III–IV | 24/92 | 48/195 | 1.21 (0.74–2.00) | 0.45 | 25/113 | 61/217 | 0.77 (0.48–1.23) | 0.27 |
| Chronic GvHD | 28/89 | 59/191 | 1.00 (0.62–1.60) | 0.98 | 33/111 | 66/212 | 0.81 (0.53–1.24) | 0.34 |
All models compared long (T/S≥0.81) to short LTL (T/S<0.81); overall survival model is adjusted for patient age in recipient LTL model or donor age in donor LTL model, disease subtype, Karnofsky performance score, HLA matching, prior therapy for aplastic anemia, race, graft type, and calendar year of transplant; Neutrophil recovery model is adjusted for patient age, disease subtype, donor age, HLA matching, graft type, and GvHD prophylaxis; Acute GvHD grade III–IV model is adjusted for patient age, HLA matching, T-cell depletion, and calendar year of transplant; Chronic GvHD model is adjusted for patient age, CMV matching status, Cyclophosphamide and TBI given.
Hazard ratio and 95% confidence interval
Additionally, there was no association between recipient pre-HCT leukocyte telomere length and the cumulative incidence of: neutrophil recovery at 28 days post-HCT (86% vs. 86%, p=0.82; number of events in the long and short telomere length=86, and 174, respectively; HR=1.03, 95% CI=0.78–1.37, p=0.83), acute GvHD grades III–IV at 100 days (25% vs. 24%, p=0.88; number of events in the long and short telomere length=24, and 48, respectively; HR=1.21, 95% CI=0.74–2.0, p=0.45) or chronic GvHD at 1-year (29% vs. 30%, p=0.88; number of events in the long and short telomere length=28, and 59, respectively; HR=1.0, 95% CI=0.62–1.60, p=0.98) in the long vs. short pre-HCT recipient leukocyte telomere length, respectively. Adjusted hazard ratios are summarized in Table 2.
Donor leukocyte telomere length and recipient post-HCT outcomes
Longer donor leukocyte telomere length was associated with a significantly higher probability of post-HCT overall survival in severe aplastic anemia patients. Patient OS probabilities for the shortest, intermediate, and longest tertiles at 1 year were: 46% (95% CI=37–56%), 53% (95% CI=43–62%), and 61% (95% CI=52–70%), respectively (p=0.09); at 2 years they were: 41% (95% CI=32–51%), 50% (95% CI=41–59%), and 59% (95% CI=50–68%), respectively (p=0.03); and at 3 years were: 38% (95% CI=29–47%), 49% (95% CI=40–58%), and 59% (95% CI=50–68%), respectively (p=0.006). Multivariable analysis comparing the 2nd or 3rd tertile of donor telomere length with the 1st tertile showed statistical significant results only in patients who received transplant from donors with telomere length in the longest tertile (HR comparing 2nd to 1st tertile=0.92, 95% CI=0.65–1.29, p=0.61; HR comparing 3rd to 1st tertile=0.59, 95% CI=0.41–0.86, p=0.006).
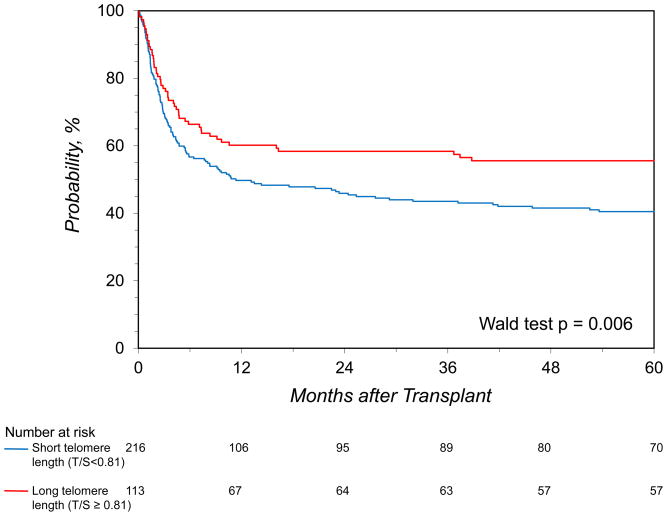
The survival probabilities for patients who received HCT from donors with long (longest tertile) versus short telomeres (1st and 2nd tertiles combined) were 60% vs. 50% at 1 year (p=0.07), 58% vs. 44% at 3 years (p=0.01), and 56% vs. 40% at 5 years (p=0.009) (Figure 1). Multivariable analysis showed a lower probability of post-HCT death for recipients who received transplant from a donor with long leukocyte telomere length (HR=0.61, 95% CI=0.44–0.85, p=0.006). This finding was independent of donor age (Table 2), and not altered by severe aplastic anemia subtype (acquired versus inherited, p-interaction=0.7). When including recipient leukocyte telomere length in the model, neither it nor its interaction with donor measures were statistically significant (p=0.73, and 0.48, respectively).
Figure 1.
The probability of overall survival after hematopoietic cell transplant for patients with severe aplastic anemia by donor leukocyte telomere length
Longer donor leukocyte telomere length was associated with a similar survival advantage (p =0.94) in young (HR=0.61, 95% CI=0.40–0.93, p=0.02) and relatively older (HR=0.61, 95% CI=0.29–1.25, p=0.18) recipients in this study when multivariable models were stratified by recipient age (≤19 and >19 years old). Stratified analyses of survival outcome in severe aplastic anemia patients by HCT preparative regimen, HLA matching revealed a similar pattern. Similarly, analysis stratified on the combined effect of recipient age and HLA matching was consistent with previous observation except for an attenuated inverse association in relatively older patients with HLA matching ≤7/8. No statistical significant difference was observed when stratifying the analysis by calendar year of transplant (HR=0.60, 95% CI=0.38–0.96 and 0.69, 95% CI=0.39–1.21, for patients who received transplant between 1988–1999 and 2000–2007, respectively, p =0.88) (Table 3).
Table 3.
Donor leukocyte telomere length (LTL) and HCT overall survival in patients with severe aplastic anemia stratified by disease subtype and selected transplant covariables
| Variables | Number of Events/sample size in long LTL | Number of events/sample size in short LTL | Adjusted HR (95% CI) ^ | p-value |
|---|---|---|---|---|
| Disease Subtype | ||||
| Acquired aplastic anemia | 28/70 | 93/164 | 0.64 (0.41–1.0) | 0.05 |
| Inherited aplastic anemia (Fanconi anemia and Diamond Blackfan anemia combined) | 25/43 | 41/51 | 0.61 (0.36–1.03) | 0.06 |
| Conditioning Regimen | ||||
| Myeloablative | 21/36 | 61/84 | 0.56 (0.31–1.0) | 0.05 |
| Reduced Intensity or Non-myeloablative | 28/69 | 66/113 | 0.62 (0.38–1.0) | 0.05 |
| HLA matching | ||||
| 8/8 matching | 15/48 | 51/103 | 0.51 (0.26–0.98) | 0.04 |
| ≤7/8 matching | 38/65 | 83/112 | 0.61 (0.40–0.94) | 0.02 |
| Recipient Age | ||||
| ≤19 years old | 39/84 | 85/138 | 0.61 (0.40–0.93) | 0.02 |
| >19 years old | 14/29 | 49/77 | 0.61 (0.29–1.25) | 0.18 |
| Combined effect of Recipient age and HLA matching | ||||
| ≤19 years old and 8/8 matching | 11/36 | 27/62 | 0.59 (0.26,1.37) | 0.22 |
| ≤19 years old and ≤7/8 matching | 28/48 | 58/76 | 0.58 (0.33,1.01) | 0.06 |
| >19 years old and 8/8 matching | 4/12 | 24/41 | 0.25 (0.04,1.42) | 0.12 |
| >19 years old and ≤7/8 matching | 10/17 | 25/36 | 0.94 (0.34,2.57) | 0.90 |
| Calendar Year at Transplant | ||||
| 1988–1999 | 32/47 | 84/111 | 0.60 (0.38–0.96) | 0.03 |
| 2000–2007 | 21/66 | 50/104 | 0.69 (0.39–1.21) | 0.19 |
All models compared long (T/S≥0.81) to short LTL (T/S<0.81); hazard ratio and 95% confidence interval adjusted for donor age, Karnofsky performance score, prior therapy for aplastic anemia, race, graft type, calendar year of transplant, disease subtype (except for model stratified on it), and HLA matching (except for model stratified on it).
Donor leukocyte telomere length was not associated with neutrophil recovery at 28 days post-HCT (86 vs. 85%, p=0.90) or at 100 days (95% and 91%, p=0.13), or with platelet engraftment at 100 days (67% and 59%, p=0.19), acute GvHD grades III–IV at 100 days (22% vs. 28%, p=0.20), or chronic GvHD at 1-year (28% vs. 30%, p=0.62) and at 2 years (32% and 34%, p=0.72) for the long versus short donor leukocyte telomere length, respectively (Figure 2). Table 2 summarizes results from multivariable models.
Figure 2. Hematopoietic cell transplant outcomes for patients with severe aplastic anemia by donor leukocyte telomere length.
A) Cumulative incidence of neutrophil recovery; B) Cumulative incidence of platelet recovery; C) Cumulative incidence of acute graft-versus-host disease grade III/IV; D) Cumulative incidence of chronic graft-versus-host disease
Review of causes of death reported by centers did not identify a statistically significant difference of reported mortality causes by donor leukocyte telomere length (See eTable 1 for cause of death by tertiles of donor leukocyte telomere length.)
Discussion
Although survival after HCT has improved over the last decade, the survival outcomes of unrelated donor transplants for severe aplastic anemia are still unsatisfactory with a 5-year survival probability of 39% to 62%.17–20 Previous studies suggest that optimal donor selection is a key parameter for improving survival in those patients.21,22 In our study, longer donor leukocyte telomere length was associated with a higher overall survival probability in severe aplastic anemia patients who underwent allogeneic unrelated HCT (5-year OS=56% vs. 40%, in the long vs. short donor leukocyte telomere length group, respectively). After adjusting for donor age and clinical factors associated with survival following HCT in severe aplastic anemia, the risk of post-HCT all-cause mortality remained approximately 40% lower in patients receiving HCT from donors with long versus short leukocyte telomere length. We observed similar patterns in patients with acquired severe aplastic anemia (5-year OS=61% vs. 46%), Fanconi anemia and Diamond Blackfan anemia combined (5-year OS=47% vs. 23%), and by disease subtype (5-year OS in Fanconi anemia=46% vs. 22%; in Diamond Blackfan anemia=50% vs. 33%).
Telomere length is a biological marker for cellular aging and replicative capacity, and thus could explain, in part, the previously reported association between donor age and outcomes after HCT. For example, in a large study of 6,978 unrelated HCT recipients, a significant decline in survival was noted with higher donor age23. Donor age has also been reported to predict recipient risk of post-HCT obstructive lung disease,24 secondary graft failure,25 and B-cell lymphoproliferative disorder.26 Of note, no association between donor leukocyte telomere length and incidence of secondary graft failure was present in our study at 42 days post-HCT in the long and short leukocyte telomere length, respectively (8% vs. 7%, p=0.9.) In solid organ transplantation, donor age is one of the main variables guiding graft selection.27 Biological markers of graft aging including telomere length or POT1 (protection of telomeres 1) gene expression have been associated with recipient’s outcomes after both liver28 and kidney transplantation.29,30
Our study showed no statistically significant association between donor leukocyte telomere length and post-HCT primary engraftment. This results are in agreement with previously reported data31 on 47 transplant recipients that found a positive correlation between donors’ hematopoietic stem cell telomere length and cellular regenerative capacity in vitro, but no association with recipient hematopoietic recovery. On the other hand, donor telomere length correlated with time to graulocyte recovery in a small study including 19 children.32
Our data show that severe aplastic anemia patients have shorter pre-transplant age-adjusted leukocyte telomere length than their donors but there was no association between their pre-transplant leukocyte telomere length and post-HCT outcomes. Our results are in agreement with a previous report of 178 patients (86% of them transplanted for a hematological malignancy)33 that found no statistically significant association between recipient pre-HCT leukocyte telomere length and overall survival, engraftment, acute or chronic GvHD. However, a statistically significant association was observed with transplant-related mortality (TRM) in the same study (17% vs. 33% in the long vs. short recipient leukocyte telomere length, respectively, p=0.02). TRM is an important HCT outcome parameter in hematological malignancies in which relapse is treated as a competing risk event, but this is not a concern in severe aplastic anemia.
Strengths of this study include the relatively large sample size, the prospective assessment of leukocyte telomere length with blood samples collected prior to HCT and the availability of detailed covariate data known to influence transplant outcome.
The study was limited by restricting participants to young severe aplastic anemia patients, and therefore might not be applicable to patients > 40 years of age or those with different indications for HCT. Validating our findings in a different HCT patient population, particularly in patients with hematologic malignancies, is warranted. Our study did not have sufficient statistical power to identify cause-specific deaths associated with short donor leukocyte telomere length; a larger study would be necessary to answer this question. The statistically significant association between donor leukocyte telomere length and patient survival after HCT in this study was limited to the longest tertile; another reason for the need of a larger sample size to allow for the re-evaluation of this association in more categories of telomere length. The use of qPCR in this study provides an average measure of telomere length in all white blood cell subsets. Since leukocyte telomere length varies by leukocyte subset,34 the average value across cell subtypes could be affected by their differential leukocyte proportions in the sample at blood collection. A future study with a cell-specific method, such as flow cytometry with fluorescence in situ hybridization, would avoid this limitation. Additionally, qPCR telomere length assay may be limited by possible sensitivity to DNA quality35. We have not compared results obtained from qPCR assay in this study to other know standard techniques such as Southern blots; however, high correlation between the two methods has been reported earlier (R2=0.6815, 0.8436)
Conclusions
Among patients with severe aplastic anemia who received unrelated donor allogeneic HCT, longer donor leukocyte telomere length was associated with higher overall survival at 3 and 5 years. There was no association between donor leukocyte telomere length and engraftment or graft versus host disease. On the other hand, recipient telomere length was not associated with patient overall survival. This observational study suggests that donor leukocyte telomere length may have a role in long-term post-transplant survival.
Supplementary Material
Acknowledgments
The study was supported by a Public Health Service grant (U24-CA76518) from the National Cancer Institute, the National Heart Lung and Blood Institute and the National Institute of Allergy and Infectious Diseases, Heath Resources (T.W., M.H., S.S., S.J.L.) and Services Administration (HHSH234200637015C), two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research (T.W., M.H. and S.S.), NIH grant 2R01 CA082838 (I.D.), and the Intramural Research Program of the National Cancer Institute (S.M.G., and S.A.S.). We thank Mrs. Heather Severance, M.S., of the Center for International Blood and Marrow Transplant Research for assistance in manuscript preparation. Mr. Stephen Spellman and Dr. Shahinaz Gadalla had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Footnotes
Conflict of interest: None
Role of the Sponsors: The above-mentioned funding agencies were not responsible for the design and conduct of the study, for the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
References
- 1.Lin J, Kaur P, Countryman P, Opresko PL, Wang H. Unraveling secrets of telomeres: One molecule at a time. DNA repair. 2014 Feb 22; doi: 10.1016/j.dnarep.2014.01.012. Epub 2014 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khincha PP, Savage SA. Genomic characterization of the inherited bone marrow failure syndromes. Seminars in hematology. 2013 Oct;50(4):333–347. doi: 10.1053/j.seminhematol.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2013;2013:76–81. doi: 10.1182/asheducation-2013.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greim H, Kaden DA, Larson RA, et al. The bone marrow niche, stem cells, and leukemia: impact of drugs, chemicals, and the environment. Annals of the New York Academy of Sciences. 2014 Mar;1310(1):7–31. doi: 10.1111/nyas.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheinberg P. Aplastic anemia: therapeutic updates in immunosuppression and transplantation. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2012;2012:292–300. doi: 10.1182/asheducation-2012.1.292. [DOI] [PubMed] [Google Scholar]
- 6.Gadalla SM, Savage SA. Telomere biology in hematopoiesis and stem cell transplantation. Blood reviews. 2011 Nov;25(6):261–269. doi: 10.1016/j.blre.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baerlocher GM, Rovo A, Muller A, et al. Cellular senescence of white blood cells in very long-term survivors after allogeneic hematopoietic stem cell transplantation: the role of chronic graft-versus-host disease and female donor sex. Blood. 2009 Jul 2;114(1):219–222. doi: 10.1182/blood-2009-03-209833. [DOI] [PubMed] [Google Scholar]
- 8.Akiyama M, Asai O, Kuraishi Y, et al. Shortening of telomeres in recipients of both autologous and allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2000;25(4):441–447. doi: 10.1038/sj.bmt.1702144. [DOI] [PubMed] [Google Scholar]
- 9.Akiyama M, Hoshi Y, Sakurai S, Yamada H, Yamada O, Mizoguchi H. Changes of telomere length in children after hematopoietic stem cell transplantation. Bone Marrow Transplant. 1998;21(2):167–171. doi: 10.1038/sj.bmt.1701060. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Kook H, Chung I, et al. Telomere length changes in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24(4):411–415. doi: 10.1038/sj.bmt.1701923. [DOI] [PubMed] [Google Scholar]
- 11.Scheinberg P, Cooper JN, Sloand EM, Wu CO, Calado RT, Young NS. Association of telomere length of peripheral blood leukocytes with hematopoietic relapse, malignant transformation, and survival in severe aplastic anemia. JAMA: the journal of the American Medical Association. 2010 Sep 22;304(12):1358–1364. doi: 10.1001/jama.2010.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995 Jun;15(6):825–828. [PubMed] [Google Scholar]
- 13.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematology/oncology clinics of North America. 1999 Oct;13(5):1091–1112. viii–ix. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 14.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2009 Dec;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong JY, De Vivo I, Lin X, Fang SC, Christiani DC. The Relationship between Inflammatory Biomarkers and Telomere Length in an Occupational Prospective Cohort Study. PloS one. 2014;9(1):e87348. doi: 10.1371/journal.pone.0087348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima S, Matsuyama T, Kato S, et al. Outcome of 154 patients with severe aplastic anemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Blood. 2002 Aug 1;100(3):799–803. doi: 10.1182/blood.v100.3.799. [DOI] [PubMed] [Google Scholar]
- 18.Deeg HJ, O’Donnell M, Tolar J, et al. Optimization of conditioning for marrow transplantation from unrelated donors for patients with aplastic anemia after failure of immunosuppressive therapy. Blood. 2006 Sep 1;108(5):1485–1491. doi: 10.1182/blood-2006-03-005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Albuerne ED, Eapen M, Klein J, et al. Outcome of unrelated donor stem cell transplantation for children with severe aplastic anemia. British journal of haematology. 2008 Apr;141(2):216–223. doi: 10.1111/j.1365-2141.2008.07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szpecht D, Gorczynska E, Kalwak K, et al. Matched sibling versus matched unrelated allogeneic hematopoietic stem cell transplantation in children with severe acquired aplastic anemia: experience of the polish pediatric group for hematopoietic stem cell transplantation. Archivum immunologiae et therapiae experimentalis. 2012 Jun;60(3):225–233. doi: 10.1007/s00005-012-0174-1. [DOI] [PubMed] [Google Scholar]
- 21.Viollier R, Socie G, Tichelli A, et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone marrow transplantation. 2008 Jan;41(1):45–50. doi: 10.1038/sj.bmt.1705894. [DOI] [PubMed] [Google Scholar]
- 22.Maury S, Balere-Appert ML, Chir Z, et al. Unrelated stem cell transplantation for severe acquired aplastic anemia: improved outcome in the era of high-resolution HLA matching between donor and recipient. Haematologica. 2007 May;92(5):589–596. doi: 10.3324/haematol.10899. [DOI] [PubMed] [Google Scholar]
- 23.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 24.Schultz KR, Green GJ, Wensley D, et al. Obstructive lung disease in children after allogeneic bone marrow transplantation. Blood. 1994;84(9):3212–3220. [PubMed] [Google Scholar]
- 25.Davies SM, Kollman C, Anasetti C, et al. Engraftment and survival after unrelated-donor bone marrow transplantation: a report from the national marrow donor program. Blood. 2000;96(13):4096–4102. [PubMed] [Google Scholar]
- 26.Gross TG, Steinbuch M, DeFor T, et al. B cell lymphoproliferative disorders following hematopoietic stem cell transplantation: risk factors, treatment and outcome. Bone Marrow Transplant. 1999;23(3):251–258. doi: 10.1038/sj.bmt.1701554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akkina SK, Asrani SK, Peng Y, Stock P, Kim WR, Israni AK. Development of organ-specific donor risk indices. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012 Apr;18(4):395–404. doi: 10.1002/lt.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aini W, Miyagawa-Hayashino A, Tsuruyama T, et al. Telomere shortening and karyotypic alterations in hepatocytes in long-term transplanted human liver allografts. Transplant international: official journal of the European Society for Organ Transplantation. 2012 Sep;25(9):956–966. doi: 10.1111/j.1432-2277.2012.01523.x. [DOI] [PubMed] [Google Scholar]
- 29.Koppelstaetter C, Schratzberger G, Perco P, et al. Markers of cellular senescence in zero hour biopsies predict outcome in renal transplantation. Aging Cell. 2008;7(4):491–497. doi: 10.1111/j.1474-9726.2008.00398.x. [DOI] [PubMed] [Google Scholar]
- 30.McGlynn LM, Stevenson K, Lamb K, et al. Cellular senescence in pretransplant renal biopsies predicts postoperative organ function. Aging Cell. 2009;8(1):45–51. doi: 10.1111/j.1474-9726.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 31.Widmann TA, Willmann B, Pfreundschuh M, Beelen DW. Influence of telomere length on short-term recovery after allogeneic stem cell transplantation. Experimental hematology. 2005 Oct;33(10):1257–1261. doi: 10.1016/j.exphem.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Mangerini R, Lanino E, Terranova P, et al. Telomere length of donors influences granulocyte recovery in children after hematopoietic stem cell transplantation. Annals of hematology. 2009 Oct;88(10):1029–1031. doi: 10.1007/s00277-009-0712-z. [DOI] [PubMed] [Google Scholar]
- 33.Peffault de Latour R, Calado RT, Busson M, et al. Age-adjusted recipient pretransplantation telomere length and treatment-related mortality after hematopoietic stem cell transplantation. Blood. 2012 Oct 18;120(16):3353–3359. doi: 10.1182/blood-2012-01-403337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS genetics. 2012;8(5):e1002696. doi: 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aubert G, Hills M, Lansdorp PM. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutation research. 2012 Feb 1;730(1–2):59–67. doi: 10.1016/j.mrfmmm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic acids research. 2009 Feb;37(3):e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.