Abstract
BACKGROUND:
The use of noninvasive ventilation (NIV) in acute exacerbation of COPD has increased over time. However, little is known about patient factors influencing its use in routine clinical practice.
METHODS:
This was a retrospective cohort study of 723,560 hospitalizations for exacerbation of COPD at 475 hospitals between 2001 and 2011. The primary study outcome was the initial form of ventilation (NIV or invasive mechanical ventilation [IMV]). Hierarchical generalized linear models were used to examine the trends in ventilation and patient characteristics associated with receipt of NIV.
RESULTS:
After adjusting for patient and hospital characteristics, initial NIV increased by 15.1% yearly (from 5.9% to 14.8%), and initial IMV declined by 3.2% yearly (from 8.7% to 5.9%); annual exposure to any form of mechanical ventilation increased by 4.4% (from 14.1% to 20.3%). Among case subjects treated with ventilation, those aged ≥ 85 years had a 22% higher odds of receiving NIV compared with those aged < 65 years, while blacks (OR, 0.86) and Hispanics (OR, 0.91) were less likely to be treated with NIV than were whites. Cases with a high burden of comorbidities and those with concomitant pneumonia had high rates of NIV failure and were more likely to receive initial IMV. Use of NIV increased at a faster rate among the admissions of the oldest patients relative to the youngest.
CONCLUSIONS:
The use of NIV for COPD exacerbations has increased steadily, whereas IMV use has declined. Several patient factors, including age, race, and comorbidities, influenced the receipt of NIV. Further research is needed to identify the factors driving these patterns.
COPD is responsible for approximately 1 million admissions annually to US hospitals and is the third most common cause of death in the United States.1,2 In patients hospitalized for an acute exacerbation of COPD (AE-COPD), usual care consists of supplemental oxygen, bronchodilators, corticosteroids, and antibiotics; in severe cases, ventilatory support with noninvasive ventilation (NIV) or invasive mechanical ventilation (IMV) is required.3
Multiple small randomized controlled trials and two meta-analyses have demonstrated that, when added to standard therapy, NIV reduces the need for IMV and improves survival in carefully selected patients with acute respiratory failure caused by COPD.4‐6 Consequently, NIV has been endorsed by several national and international societies as a first-line treatment in these patients.7‐9 Information about the use of NIV is beginning to emerge, and several studies have reported an increase in NIV use in patients with COPD worldwide.10‐13
Little is known, however, about the patient characteristics associated with NIV use in routine clinical practice and whether factors such as age, sex, race, and comorbidities influence the likelihood that a patient will receive NIV or IMV as an initial ventilation therapy. Using data from a geographically and structurally diverse sample of US hospitals, we examined decade-long trends (2001-2011) in the use of NIV and IMV among patients hospitalized for an AE-COPD and assessed the influence of patient factors on receipt of NIV and IMV.
Materials and Methods
Design, Study Setting, and Subjects
We used data from the Premier Inpatient Database, a dataset that has been used extensively for health services research14,15; its details have been described previously.16,17 In brief, this dataset contains a date-stamped log of all items and services charged to the patient or his/her insurer, including medications, laboratory, and ventilation therapies at the individual patient level. Hospitals in the dataset represent all regions of the United States, are predominantly small- to medium-sized nonteaching facilities, and serve mostly urban patient populations.
We included hospitalizations of patients ≥ 40 years of age with a principal discharge diagnosis of COPD (International Classification of Diseases, Ninth Revision [ICD-9] codes 491.21, 492.22, 491.8, 491.9, 492.8, 496) or a principal diagnosis of acute respiratory failure (ICD-9 codes 518.81, 518.82, 518.84) paired with a secondary diagnosis of COPD who were discharged between January 1, 2001, and June 30, 2011. We excluded admissions transferred from or to another facility (because we did not have information about their initial ventilation therapy or outcomes), hospitalizations of < 2 days, admissions to a hospice bed, and those with a diagnosis of OSA because we could not distinguish whether NIV was used for sleep apnea or for acute respiratory failure.
For each admission, we recorded demographic information, insurance coverage, number of COPD admissions in the previous year, and diagnosed comorbidities. We calculated a single numeric comorbidity score using the method described by Gagne et al,18 and grouped this score into tertiles for analysis. For each hospital, we assessed the number of beds, teaching status, whether it served an urban or a rural population, and geographic region. We included only hospitals that were in the Premier dataset for a minimum of a full year between 2001 and 2011 and had patients eligible for the study.
Outcomes
The primary study outcome was the initial form of ventilation (NIV or IMV) administered at any point during the hospital course. We defined NIV failure as transition to IMV after an initial period of NIV. An additional composite outcome was defined as NIV failure or death.
Administration of NIV and IMV was identified using two approaches. First, we reviewed ICD-9 procedure codes and the date associated with the receipt of each procedure. Second, we used charges generated by the respiratory therapist for NIV or IMV use. We considered a patient to have received NIV or IMV during the admission if there was an ICD-9 procedure code or a charge for the procedure or service. We validated this approach in a single hospital chart audit of 200 patients hospitalized with a diagnosis of acute respiratory failure between 2010 and 2012. We found that ICD-9 procedure codes had a sensitivity of 86% for identifying NIV use, compared with 99% when both ICD-9 and respiratory therapist charge codes were used. Specificity for NIV use was 96% for both procedure and billing codes.19
Data Analysis
We calculated summary statistics at the admission level for all variables, using frequencies and proportions for categorical data, and means, medians, and interquartile ranges for continuous variables. Hierarchical generalized linear models with a random hospital effect using SAS PROC GLIMMIX (SAS Institute Inc) were used to examine relative changes over time in the use and outcomes of NIV and IMV while controlling for several patient and hospital characteristics. The population at risk varied with the outcome. We modeled the use of any ventilation, initial NIV, and initial IMV among all admissions (all admitted patients eligible to receive ventilation therapy) to evaluate trends over time in the use of ventilation and type of ventilation. The use of NIV as the initial ventilation method as a percentage of all ventilation starts was modeled among admissions of patients receiving ventilation to evaluate the relative change over time in the choice of ventilation mode. NIV failure, defined as transition to IMV after a trial of NIV, as well as a composite outcome of NIV failure or death was modeled among admissions started on NIV (eligible for NIV failure) to evaluate trends in outcome following initial NIV use. Year was included as a linear term in all models.
Initial models included prespecified interaction terms of patient characteristics with study year (2001-2011) to identify trends in ventilation use related to patient characteristics that were significantly associated with ventilation outcomes. Interaction terms with P > .05 were dropped from the final models.
All analyses were performed using the Statistical Analysis System (version 9.3; SAS Institute Inc) and STATA (Stata Statistical Software, release 12; StataCorp LP). Because the data do not contain identifiable information, the institutional review board at Baystate Medical Center determined that this study did not constitute human subjects research.
Results
Patient and Hospital Characteristics
A total of 723,560 hospitalizations with an AE-COPD from 475 hospitals were included. The median age at admission was 70 years (interquartile range, 61-79 years), 60% of admissions were women, and 72% were white. The median Gagne comorbidity score was 2 (interquartile range, 1-3), and the most common comorbidities were hypertension, heart failure, and diabetes. One-third of the cases had had at least one admission for COPD during the prior year, and 18% of admissions had a concomitant diagnosis of pneumonia present at admission. Approximately one-half of the admissions were to hospitals located in the south, 68% were to nonteaching facilities, and 84% were to hospitals located in urban areas (Table 1).
TABLE 1 ] .
Characteristics of Admissions for Exacerbation of COPD, 2001 to 2011
| Characteristic | No. | % |
| Total COPD admissions | 723,560 | 100.00 |
| Patient characteristics | ||
| Female | 431,455 | 59.63 |
| Age | ||
| < 65 y | 242,196 | 33.47 |
| 65-74 y | 210,980 | 29.16 |
| 75-84 y | 198,120 | 27.38 |
| ≥ 85 y | 72,264 | 9.99 |
| Race/ethnicity | ||
| White | 524,328 | 72.47 |
| Black | 73,703 | 10.19 |
| Hispanic | 26,010 | 3.59 |
| Other | 99,519 | 13.75 |
| Comorbidity score | ||
| Low: ≤ 1 | 312,439 | 43.18 |
| Median: 2-3 | 233,775 | 32.31 |
| High: ≥ 4 | 177,346 | 24.51 |
| Concomitant pneumonia | 128,464 | 17.75 |
| No. COPD admissions in prior year | ||
| None | 497,223 | 68.72 |
| 1 | 125,365 | 17.33 |
| 2 | 48,797 | 6.74 |
| ≥ 3 | 52,175 | 7.21 |
| Hospital characteristics | ||
| Bed size | ||
| Small (< 200) | 136,463 | 18.86 |
| Medium (200-400) | 301,069 | 41.61 |
| Large (> 400) | 286,028 | 39.53 |
| Region | ||
| Midwest | 144,208 | 19.93 |
| Northeast | 131,441 | 18.17 |
| South | 355,285 | 49.10 |
| West | 92,626 | 12.80 |
| Population served | ||
| Rural | 118,473 | 16.37 |
| Urban | 605,087 | 83.63 |
| Teaching hospital | 229,546 | 31.72 |
Use of NIV and IMV Between 2001 and 2011
Overall, in 18.2% of admissions, patients were mechanically ventilated (IMV or NIV) at some point during their hospitalization; in 10.6% of admissions, patients were initially started on NIV (59.2% of all admissions with ventilation); in 7.3% of admissions, patients were started on IMV; in a small number of admissions (0.4%), the order of ventilation could not be determined. In all, 16.6% of the admissions initially started on NIV resulted in subsequent intubation (ie, NIV failure).
Trends in the Use of NIV and IMV Between 2001 and 2011
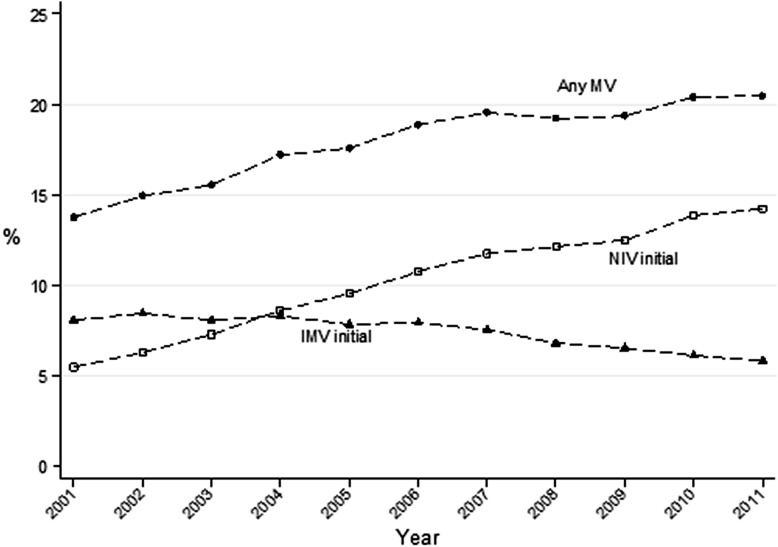
After adjusting for changes in hospital and patient characteristics during the years under study, exposure to any form of mechanical ventilation had a relative increase of 4.4% annually (from 14.1% in 2001 to 20.3% in 2011). The annual rate of initial NIV increased on average by 15.1% (from 5.9% to 14.8%); use of initial IMV declined an average 3.2% per year (from 8.7% to 5.9%); and by 2004, the rate of initial NIV was higher than that of initial IMV (Fig 1). Between 2001 and 2011, the percentage of ventilator starts that were noninvasive increased by an average of 8.0% per year (from 39.9% to 71.7%), and the annual rate of NIV failure declined by 0.7% (from 17.9% to 16.7%). Patient characteristics and ventilation management over time are described in .
Figure 1 –
Trends in initial ventilation strategies and NIV failure. IMV = invasive mechanical ventilation; MV = mechanical ventilation; NIV = noninvasive ventilation.
Patterns of Ventilation Use According to Patient Characteristics
After adjusting for other patient and hospital characteristics, older patients (aged ≥ 75 years) were significantly less likely than the youngest patients (40-64 years of age) to receive any form of mechanical ventilation, and when ventilated, were more likely to be started on NIV; NIV failure was lower in older patients compared with the youngest, but there was no difference in the combined outcome of NIV failure or death. Over the 10 years of the study, the use of NIV increased faster in older, relative to the youngest, patients by a factor of 1.02 times per year, whereas the use of IMV decreased more among the oldest relative to the youngest by a factor of 0.99 times per year. For example, in 2001, the oldest patients were 44% less likely than the youngest to be started on NIV (OR, 0.56), but by 2011, they were 32% less likely to be started on NIV (OR, 0.68) (e-Fig 1 (317.7KB, pdf) , Table 2).
TABLE 2 ] .
Patterns of Ventilation Use According to Patient Characteristics, 2001 to 2011
| Outcome: Ventilation Type/Patient Population | |||||
| Variable | Any Ventilationa/All Patientsb | NIV Initialc/All Patientsb | IMV Initiald/All Patientsb | NIV Proportione/Patients Who Are Ventilatedf | NIV Failureg/Patients on NIVh |
| Year (annual change) | 1.06 (1.05-1.07) | 1.14 (1.12-1.17) | 0.95 (0.94-0.96) | 1.19 (1.17-1.21) | 0.96 (0.93-0.98) |
| Sex | |||||
| Male | Ref | Ref | Ref | Ref | Ref |
| Female | 0.99 (0.98-1.01) | 1.01 (1.00-1.03) | 0.96 (0.95-0.98) | 1.05 (1.02-1.08) | 0.98 (0.94-1.02) |
| Age | |||||
| < 65 y | Ref | Ref | Ref | Ref | Ref |
| 65-74 y | 0.93 (0.89-0.96) | 0.97 (0.92-1.01) | 0.92 (0.88-0.96) | 1.06 (0.99-1.14) | 0.87 (0.76-0.98) |
| 75-84 y | 0.66 (0.64-0.69) | 0.76 (0.72-0.8) | 0.67 (0.63-0.7) | 1.12 (1.04-1.21) | 0.75 (0.64-0.85) |
| ≥ 85 y | 0.43 (0.41-0.46) | 0.56 (0.52-0.6) | 0.45 (0.41-0.48) | 1.22 (1.07-1.38) | 0.50 (0.37-0.62) |
| Age-by-year interaction | |||||
| < 65 y | Ref | Ref | Ref | Ref | Ref |
| 65-74 y | 1.00 (0.99-1.01) | 1.00 (1.00-1.01) | 0.99 (0.98-1.00) | 1.01 (1.00-1.02) | 0.99 (0.97-1.00) |
| 75-84 y | 1.01 (1.00-1.01) | 1.01 (1.00-1.02) | 0.98 (0.98-0.99) | 1.03 (1.02-1.04) | 0.97 (0.95-0.99) |
| ≥ 85 y | 1.02 (1.01-1.03) | 1.02 (1.01-1.03) | 0.99 (0.97-1.00) | 1.04 (1.02-1.06) | 0.98 (0.94-1.01) |
| Race | |||||
| White | Ref | Ref | Ref | Ref | … |
| Hispanic | 0.89 (0.81-0.97) | 0.90 (0.85-0.94) | 0.93 (0.82-1.05) | 0.90 (0.82-0.98) | 0.98 (0.83-1.12) |
| Black | 1.11 (1.05-1.17) | 0.99 (0.96-1.02) | 1.20 (1.12-1.29) | 0.86 (0.82-0.90) | 1.02 (0.94-1.10) |
| Other | 1.13 (1.07-1.20) | 1.00 (0.97-1.04) | 1.15 (1.06-1.24) | 0.91 (0.86-0.96) | 1.01 (0.92-1.10) |
| Race-by-year interaction | |||||
| White | Ref | … | Ref | … | … |
| Hispanic | 1.00 (0.99-1.02) | … | 1.01 (0.99-1.03) | … | … |
| Black | 0.99 (0.98-1.00) | … | 0.99 (0.98-1.00) | … | … |
| Other | 0.99 (0.98-1.00) | … | 0.99 (0.98-1.01) | … | … |
| Comorbidity score | |||||
| Low: ≤ 1 | Ref | Ref | Ref | Ref | Ref |
| Medium: 2-3 | 2.02 (1.95-2.09) | 1.68 (1.60-1.76) | 2.04 (1.99-2.09) | 0.88 (0.81-0.94) | 1.39 (1.19-1.59) |
| High: ≥ 4 | 4.04 (3.89-4.19) | 2.66 (2.53-2.79) | 4.01 (3.91-4.11) | 0.73 (0.68-0.79) | 2.11 (1.81-2.41) |
| Comorbid score-by-year interaction | |||||
| Low: ≤ 1 | Ref | Ref | … | Ref | Ref |
| Medium: 2-3 | 0.98 (0.98-0.99) | 0.99 (0.98-1.00) | … | 0.98 (0.97-0.99) | 1.01 (0.99-1.04) |
| High: ≥ 4 | 0.97 (0.96-0.97) | 0.98 (0.97-0.99) | … | 0.97 (0.96-0.98) | 1.03 (1.01-1.05) |
| Pneumonia | |||||
| No pneumonia | Ref | Ref | Ref | Ref | Ref |
| Pneumonia | 5.62 (5.43-5.81) | 2.75 (2.62-2.88) | 5.88 (5.64-6.13) | 0.55 (0.54-0.57) | 2.6 (2.47-2.72) |
| Pneumonia-by-year interaction | |||||
| No pneumonia | Ref | Ref | Ref | … | … |
| Pneumonia | 0.88 (0.87-0.88) | 0.92 (0.91-0.93) | 0.90 (0.90-0.91) | … | … |
| COPD prior admit | |||||
| None | Ref | Ref | Ref | Ref | Ref |
| 1 | 1.25 (1.21-1.30) | 1.47 (1.44-1.50) | 1.04 (0.99-1.10) | 1.39 (1.28-1.49) | 0.85 (0.80-0.90) |
| 2 | 1.39 (1.32-1.47) | 1.79 (1.74-1.84) | 0.96 (0.89-1.04) | 1.71 (1.52-1.90) | 0.86 (0.79-0.93) |
| ≥ 3 | 1.66 (1.58-1.75) | 2.21 (2.15-2.27) | 1.16 (1.07-1.24) | 1.70 (1.52-1.89) | 0.72 (0.66-0.78) |
| COPD prior admit-by-year interaction | |||||
| None | Ref | … | Ref | … | … |
| 1 | 1.01 (1.01-1.02) | … | 1.00 (1.00-1.01) | … | … |
| 2 | 1.02 (1.01-1.03) | … | 1.02 (1.01-1.03) | … | … |
| ≥ 3 | 1.02 (1.01-1.03) | … | 1.00 (0.99-1.01) | … | … |
Data are presented as OR (95% CI). Ventilation type refers to the ventilation therapy administered during admission. Patient population refers to the population eligible for each type of ventilation. All models were adjusted for year, sex, age group, race, combined comorbidity score, concomitant pneumonia, COPD prior admission, and hospital characteristics (ie, number of beds, teaching status, urban/rural geographical area). Initial models evaluated interaction terms for year with sex, age group, race, comorbidity score, concomitant pneumonia, and prior COPD admissions. Nonsignificant interaction terms (P > .05) were dropped from final models reported here. For NIV proportion and NIV failure, we also adjusted for annual hospital ventilation rate. Main effect OR estimates apply to baseline year 2001. Multiply by the year interaction effect for each subsequent year. For example, the OR 4.04 (3.89-4.19) indicates that in 2001, patients with a high comorbidity burden were 4.04 times more likely to be ventilated than were patients with a low comorbidity burden. The interaction effect with year, OR 0.97 (0.96-0.97), indicates that the odds of ventilation for patients with a high comorbidity burden decreased by a factor of 0.97 times per year, so that 10 years later, by 2011, they were 4.04 × 0.9710 = 2.98 times more likely to be ventilated than were those with a low comorbidity burden. IMV = invasive mechanical ventilation; NIV = noninvasive ventilation; Ref = reference.
Any NIV or IMV during hospitalization.
All admissions included in the analysis.
NIV was the ventilation type that was used first.
IMV was the ventilation type that was used first.
NIV was used first as the ventilatory therapy.
Admissions among all patients who were ventilated.
Transition to IMV after a trial of NIV.
Admissions among all patients ventilated with NIV initially.
In 2001, compared with admissions of white patients, Hispanics were less likely to receive any ventilation (OR, 0.89). In contrast, blacks were more likely than whites to be ventilated (OR, 1.11 in 2001) and were more likely to be started on IMV (OR, 1.20 in 2001). However, across the 10-year period, the use of IMV decreased among black relative to white patients and increased among Hispanic relative to white patients, so that by 2011, use was similar in Hispanic and white patients (e-Fig 2 (317.7KB, pdf) , Table 2). NIV failure rates were similar across race groups.
Admissions of patients with high levels of comorbidity (Gagne comorbidity score ≥ 4) were four times more likely to lead to any form of ventilation compared with those of patients with the lowest levels of comorbidity (Gagne comorbidity score ≤ 1) in 2001. Among hospitalizations with ventilation, those of patients with high comorbidity scores were less likely to lead to NIV starts and more likely to have NIV failure than were those of patients with lower comorbidity burdens. Although NIV use increased over time in all hospitalizations, the annual rate of increase among those with a higher comorbidity burden was lower than in those with the lowest comorbidity burden. For example, patients with a high comorbidity score were 4.04 times more likely to be ventilated in 2001 than those with low score, but only 2.98 times more likely to get ventilated than those with a low score in 2011 (e-Figs 3, 4 (317.7KB, pdf) , Table 2).
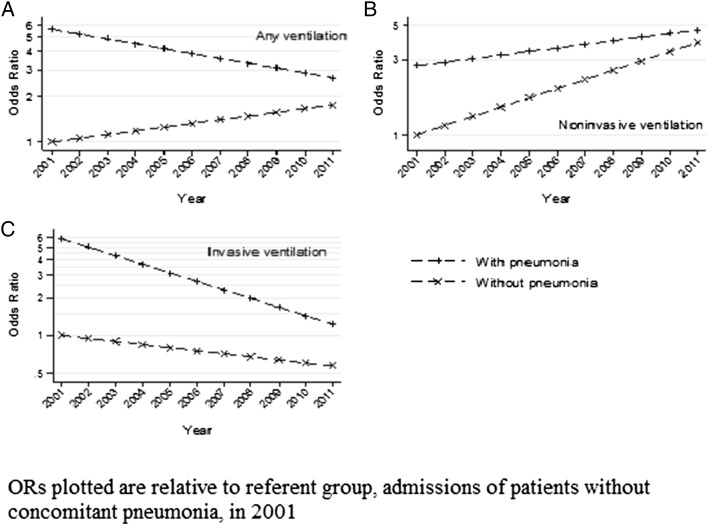
Compared with admissions without pneumonia, those with AE-COPD and pneumonia were more likely to lead to any ventilation, especially IMV initiation, and to experience NIV failure. Over the 10-year study period overall ventilation use decreased among admissions of COPD with concomitant pneumonia, whereas ventilation use increased among those without pneumonia, although overall odds of ventilation were still higher in those with pneumonia than in those without (OR = 1.57 in 2011). Although NIV use increased among both groups of patients, the rate of increase was greater among those without pneumonia. At the same time, IMV use decreased in both groups, but the rate of decline was greater among admissions of pneumonia patients. (Fig 2, Table 2)
Figure 2 –
Trends in ventilation use in COPD with and without pneumonia, 2001 to 2011. A, Any ventilation. B, Noninvasive ventilation. C, Invasive ventilation.
Discussion
The results of this large observational study of hospitalizations of patients with COPD demonstrate a steady and dramatic increase in the use of NIV, a substantial decline in the use of IMV, an overall increase in the use of mechanical ventilation, and a slight decrease in the frequency of NIV failure. Advanced age, race, comorbidity burden, and concomitant pneumonia were important determinants of whether a patient received mechanical ventilation and influenced the annual rate of change in the use of various ventilation strategies.
Our results are consistent with trends in ventilation use reported in a study by Chandra et al11 that analyzed data from the Nationwide Inpatient Sample and found a significant increase in NIV use from 1998 to 2008. The NIV rates reported in our study are considerably higher than those rates (eg,14.0% vs 4.5% in 2008), most likely because of the more sensitive approach we used for identifying NIV and a more specific set of ICD-9 codes that restricted the cohort to admissions with more severe exacerbations of COPD. When limiting the identification of NIV to the same methodology used in the study by Chandra et al,11 we obtained comparable rates (eg, 5.4% in 2008). Our observed rates are more similar to those from an audit in the United Kingdom that assessed the in-hospital care of 9,700 patients with COPD in 2008 and found that NIV was used in 11% of these patients.20
In this study, the overall increase in NIV as an initial ventilation strategy between 2001 and 2011 was offset mostly by a decrease in IMV use. However, there was also an increase in all ventilator starts, suggesting that NIV was progressively used in patients who may not in the past have received any ventilation.
By providing data on patient factors related to the use of NIV, and more precise estimates of NIV use, our results extend the findings of earlier studies. We have demonstrated that factors such as age, race, comorbidity burden, and concomitant pneumonia influence whether a patient receives NIV or IMV as initial ventilator therapy. Although there was an increase in the use of NIV and corresponding declines in the use of IMV during the years under study in all hospitalizations, the rate of change varied by patient characteristics. Patients > 85 years of age had 57% lower odds of receiving any form of mechanical ventilation compared with the youngest patients and were more likely to receive NIV than IMV when they were ventilated. These results are consistent with the findings of other studies suggesting that the intensity of treatment decreases with advancing age and is likely influenced by patient and/or physician preference to withhold more aggressive treatments such as IMV.21‐23 The NIV growth rate was highest in the admissions of the oldest compared with the youngest patients; these trends may reflect the diffusion of the results of several studies reporting that NIV has a good success rate in older patients.24‐28
Black patients had higher rates of ventilation compared with white patients and were more likely to be invasively ventilated, even after adjusting for the hospital where care was received. Several prior studies have shown that black patients, compared with white patients, are more likely to prefer and receive life-sustaining treatments, including IMV.21,29,30 It is also possible that black patients delay hospitalization and present with more severe disease than do white patients.
Our results provide new information about the role of comorbidity burden and concomitant pneumonia in the ventilatory management of patients with COPD. We found that patients with a higher disease burden were more likely to be ventilated and to receive initial IMV treatment than were patients with a lower comorbidity burden; they also had the highest rate of NIV failure. Similar ventilation patterns were observed for patients with pneumonia. The respiratory deterioration found in these patients may be less easily reversible, and these patients may more often deteriorate to the point where they have to be intubated. Of interest is the fact that, although the use of NIV increased in all admissions with COPD during the years under study, the annual rate of increase was lower for those with a high comorbidity burden and for those with pneumonia. These findings suggest that providers may have become more cautious about NIV use among patients at high risk of requiring subsequent intubation.
The strengths of our study include the large and diverse set of hospitals included in the analysis, increasing the generalizability of our findings, and the detailed charge data to which we had access, which enabled us to provide more accurate estimates of NIV use than would be obtained from ICD-9 procedure codes only. We also examined trends over time, which provided perspective on how the ventilatory management of patients with COPD is evolving. In addition, we used robust statistical methods to identify patient factors associated with the adoption of NIV over time and possible changes in these factors over a decade-long perspective.
Our study has a number of limitations. First, we relied on ICD-9 codes to identify cases of acute COPD exacerbation and, therefore, we cannot exclude disease misclassification. However, we used a previously validated set of ICD-9 codes that were shown to have reasonable sensitivity and specificity. Second, hospitals that participate in the Premier database are not completely representative of US hospitals (predominantly small to medium size and serving urban populations), and the results may not be entirely generalizable. Third, we did not have physiology data on the severity of acute respiratory failure, and therefore, we could not determine if the threshold for applying mechanical ventilation has changed over time. Forth, we excluded patients with a diagnosis of OSA because we were not able to distinguish if NIV was used for sleep apnea or for respiratory failure. Finally, the dataset does not contain information on patients’ decisions about life-sustaining treatments, and the decrease in NIV failure may reflect a larger number of patients withholding aggressive treatment at the end of life.
Conclusions
The use of NIV as an initial ventilation strategy for patients with COPD has increased steadily over the past 10 years, whereas the use of IMV has declined. Patient age and race, comorbidities, and coexisting pneumonia influence the receipt and success rates of NIV. Further research is needed to identify the factors driving these patterns.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: M. S. S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M. S. S., P. S. P., N. H., M. B. R., and P. K. L. contributed to the conception and design of the study; M. S. S., M.-S. S., P. S. P., M. B. R., and P. K. L. contributed to the analysis and interpretation of the data; and M. S. S., M.-S. S., P. S. P., N. H., M. B. R., and P. K. L. contributed to the drafting and review of the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Hill has received research grant support and has served on advisory boards for Breathe Technologies, Inc, and Fisher Paykel. He has also consulted for Phillips Respironics and ResMed. Drs Stefan, Shieh, Pekow, Rothberg, and Lindenauer have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figures and e-Table can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- AE-COPD
acute exacerbation of COPD
- ICD-9
International Classification of Diseases, Ninth Revision
- IMV
invasive mechanical ventilation
- NIV
noninvasive ventilation
Footnotes
FUNDING/SUPPORT: This research was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) [Grant 1R18HL108810-01]. Dr Stefan is supported by the NHLBI of the NIH [Grant 1K01HL114631-01A1].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.FastStats. Chronic obstructive pulmonary disease (COPD) includes: chronic bronchitis and emphysema. CDC website. http://www.cdc.gov/nchs/fastats/copd.htm. Centers for Disease Control and Prevention website. Accessed December 10, 2013.
- 2.NHLBI morbidity and mortality chartbook. National Heart, Lung, and Blood Institute website. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf. Accessed March 11, 2014.
- 3.Todisco T, Baglioni S, Eslami A, et al. Treatment of acute exacerbations of chronic respiratory failure: integrated use of negative pressure ventilation and noninvasive positive pressure ventilation. Chest. 2004;125(6):2217-2223. [DOI] [PubMed] [Google Scholar]
- 4.Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817-822. [DOI] [PubMed] [Google Scholar]
- 5.Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ. 2003;326(7382):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keenan SP, Kernerman PD, Cook DJ, Martin CM, McCormack D, Sibbald WJ. Effect of noninvasive positive pressure ventilation on mortality in patients admitted with acute respiratory failure: a meta-analysis. Crit Care Med. 1997;25(10):1685-1692. [DOI] [PubMed] [Google Scholar]
- 7.Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute Respiratory failure. Am J Respir Crit Care Med. 2001;163(1):283-291. [DOI] [PubMed] [Google Scholar]
- 8.Keenan SP, Sinuff T, Burns KE, et al. ; Canadian Critical Care Trials Group/Canadian Critical Care Society Noninvasive Ventilation Guidelines Group. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ. 2011;183(3):E195-E214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57(3):192-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demoule A, Girou E, Richard JC, Taillé S, Brochard L. Increased use of noninvasive ventilation in French intensive care units. Intensive Care Med. 2006;32(11):1747-1755. [DOI] [PubMed] [Google Scholar]
- 11.Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. 2012;185(2):152-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walkey AJ, Wiener RS. Use of noninvasive ventilation in patients with acute respiratory failure, 2000-2009: a population-based study. Ann Am Thorac Soc. 2013;10(1):10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai CL, Lee WY, Delclos GL, Hanania NA, Camargo CA., Jr Comparative effectiveness of noninvasive ventilation vs invasive mechanical ventilation in chronic obstructive pulmonary disease patients with acute respiratory failure. J Hosp Med. 2013;8(4):165-172. [DOI] [PubMed] [Google Scholar]
- 14.Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894-903. [DOI] [PubMed] [Google Scholar]
- 15.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361. [DOI] [PubMed] [Google Scholar]
- 16.Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA. 2004;291(17):2092-2099. [DOI] [PubMed] [Google Scholar]
- 17.Safavi KC, Dharmarajan K, Kim N, et al. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States. Circulation. 2013;127(8):923-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefan MS, Nathanson BH, Higgins T, Steingrub JS, Lagu T. Validity of noninvasive and invasive ventilation billing and procedure codes in patients with acute respiratory failure PKL [abstract]. Am. J. Respir. Crit. Care Med. 2014;189:A1187. [Google Scholar]
- 20.Roberts CM, Stone RA, Buckingham RJ, Pursey NA, Lowe D; National Chronic Obstructive Pulmonary Disease Resources and Outcomes Project implementation group. Acidosis, non-invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66(1):43-48. [DOI] [PubMed] [Google Scholar]
- 21.Lagu T, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS, Lindenauer PK. Variation in the care of septic shock: the impact of patient and hospital characteristics. J Crit Care. 2012;27(4):329-336. [DOI] [PubMed] [Google Scholar]
- 22.Hamel MB, Teno JM, Goldman L, et al. ; SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. Patient age and decisions to withhold life-sustaining treatments from seriously ill, hospitalized adults. Ann Intern Med. 1999;130(2):116-125. [DOI] [PubMed] [Google Scholar]
- 23.Hamel MB, Phillips RS, Davis RB, et al. Are aggressive treatment strategies less cost-effective for older patients? The case of ventilator support and aggressive care for patients with acute respiratory failure. J Am Geriatr Soc. 2001;49(4):382-390. [DOI] [PubMed] [Google Scholar]
- 24.Riario-Sforza GG, Scarpazza P, Incorvaia C, Casali W. Role of noninvasive ventilation in elderly patients with hypercapnic respiratory failure. Clin Ter. 2012;163(1):e47-e52. [PubMed] [Google Scholar]
- 25.Scarpazza P, Incorvaia C, Amboni P, et al. Long-term survival in elderly patients with a do-not-intubate order treated with noninvasive mechanical ventilation. Int J Chron Obstruct Pulmon Dis. 2011;6:253-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scarpazza P, Incorvaia C, di Franco G, et al. Effect of noninvasive mechanical ventilation in elderly patients with hypercapnic acute-on-chronic respiratory failure and a do-not-intubate order. Int J Chron Obstruct Pulmon Dis. 2008;3(4):797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarpazza P, Incorvaia C, Melacini C, et al. Shrinking the room for invasive ventilation in hypercapnic respiratory failure. Int J Chron Obstruct Pulmon Dis. 2013;8:135-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nava S, Grassi M, Fanfulla F, et al. Non-invasive ventilation in elderly patients with acute hypercapnic respiratory failure: a randomised controlled trial. Age Ageing. 2011;40(4):444-450. [DOI] [PubMed] [Google Scholar]
- 29.Cannon KT, Sarrazin MV, Rosenthal GE, Curtis AE, Thomas KW, Kaldjian LC. Use of mechanical and noninvasive ventilation in black and white chronic obstructive pulmonary disease patients within the Veterans Administration health care system. Med Care. 2009;47(1):129-133. [DOI] [PubMed] [Google Scholar]
- 30.Bayer W, Mallinger JB, Krishnan A, Shields CG. Attitudes toward life-sustaining interventions among ambulatory black and white patients. Ethn Dis. 2006;16(4):914-919. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement