Abstract
Aims
Bupropion was tested for efficacy to achieve methamphetamine (MA) abstinence in dependent, non-daily users.
Methods
A randomized, double-blind, placebo-controlled trial, with 12-week treatment and 4-week follow-up, was conducted with 204 treatment-seeking participants having MA dependence per DSM-IV, who used MA on a less-than-daily basis. 104 were randomized to matched placebo and 100 to bupropion, sustained-release 150mg, twice daily. Participants were seen three times weekly to obtain urine for MA and bupropion assays, study assessments, and thrice weekly, 90-minute, group psychotherapy. There was no biomarker for placebo adherence. The primary outcome was achievement of abstinence throughout the last two weeks of treatment; ‘success’ requiring at least two urine samples during each of Weeks 11 and 12, and all samples MA-negative (<300ng/mL).
Results
Bupropion and placebo groups did not differ significantly in the percentage achieving abstinence for the last 2 weeks of treatment (chi-square, p=0.32). Subgroup analysis of participants with lower baseline MA use (≤18 of last 30 days before consent) also revealed no difference in success between groups (p=0.73). Medication adherence per protocol (detectable bupropion, >5ng/mL, in ≥50% of urine samples from Study Weeks 1–10 and ≥66% of urine samples from Weeks 11–12) was achieved by 47% of participants taking bupropion.
Conclusions
These data indicate that bupropion did not increase abstinence in dependent participants who were using MA less-than-daily. Medication non-adherence was a limitation in this trial. Psychosocial therapy remains the mainstay of treatment for MA dependence. Further research on subgroups who may respond to bupropion may be warranted.
Trial Registration
Keywords: Bupropion, Methamphetamine, Substance-related disorders, Drug therapy, Medication adherence, Patient acuity
1. INTRODUCTION
Methamphetamine dependence is a complex and severe health problem for individuals and their communities (Berman et al., 2008; Gonzales et al., 2010). Although ‘past month’ methamphetamine (MA) use declined slightly in the US from 2006 to 2012, from 0.3 to 0.2% of the population aged 12 years or older (SAMHSA, 2013), emergency department visits for both illicit and prescribed stimulants increased (up 61% and 85% from 2009 to 2011; SAMHSA, 2013). In spite of numerous trials of psychoactive medications approved for other indications, and a few phase I trials of new entities (Brackins et al., 2011; Karila et al., 2010), the need to find an effective medication persists.
Bupropion, a weak inhibitor of norepinephrine and dopamine uptake, is approved for the treatment of depression and nicotine dependence (GlaxoSmithKline, 2012), and has been shown to improve symptoms of adult Attention-Deficit/Hyperactivity Disorder (ADHD; Wilens et al., 2005).
Previous clinical data suggested that bupropion might be effective in a subgroup with lower baseline MA use (Elkashef et al., 2008). In that trial, males using MA less frequently at baseline achieved more ‘non-use weeks’ with bupropion compared to placebo. That subgroup was also more likely to achieve abstinence throughout the last 2 weeks of the trial, according to a reanalysis of the data using the outcome of ‘terminal abstinence’ (McCann and Li, 2012). Other medication trials have also shown greater treatment effects in participants with less frequent baseline cocaine use (Elkashef et al., 2005). The primary objective of this study was to assess the efficacy of bupropion to increase abstinence in MA-dependent participants who used MA on 29 or fewer days in the month prior to signing consent.
2. METHODS
The protocol and Informed Consent were approved by the Investigational Review Board at each site. The study was monitored by a central Data and Safety Monitoring Board. The bupropion was purchased commercially.
2.1. Study design
This was a randomized, double-blind, placebo-controlled, multi-site study, that provided 12 weeks of treatment with either bupropion SR 150 mg twice daily or matched placebo, and had a four week follow-up.
The methods were nearly identical to our previous study (Elkashef et al., 2008), except we attempted to replicate our finding of bupropion’s reduction in MA use among lower frequency users. To enrich the study population with lower frequency users, we excluded those with daily MA use, only including those who used on ≤ 29 of the 30 days prior to consent. Randomization was balanced on factors of: MA use in the 30 days prior to consent (19–29 days), symptoms of depression (HAM-D >12, Williams, 1988), and (instead of gender) symptoms diagnostic of adult ADHD (Adler et al., 2005). Telephone randomization software incorporated the adaptive “urn” method to balance treatment groups within sites on these three factors (Stout et al., 1994).
2.2. Participants
Treatment-seeking participants were assessed for MA dependence by MINI interview (Sheehan et al., 1998). Participants were required to provide at least one MA-positive urine during screening, but because of slow recruitment the protocol was modified to allow inclusion of those who had no MA-positive urine but “corroboration” of baseline use by a family, medical, or judicial source (see Discussion). Exclusion criteria were described in Elkashef et al (2008), and we also excluded: uncontrolled hypertension (≥Stage 2); history of loss of consciousness greater than 5 minutes; unstable diabetes with hypoglycemia in the past year; and some antiretroviral medications. Twelve outpatient clinic sites recruited participants in cities across the country.
2.3. Psychosocial treatment, bupropion assays, and MA urinalysis
Clinic visits occurred three times per week. At each visit, urine samples were obtained for MA and bupropion assays. Also, research assessments were performed and participants received cognitive-behavioral, relapse-prevention, manual-driven therapy in 90-minute group sessions (Rawson et al., 1995).
Medication adherence in the active group was determined by assay of urine samples for bupropion, with a lower limit of quantification of 5 ng/mL. Adherence was defined as having detectable bupropion in at least 50% of urine samples obtained during Study Weeks 1 through 10 and at least 66% of urine samples obtained during Weeks 11 and 12.
The primary outcome was determined by immunoassay of urine samples for MA and its major metabolite, amphetamine, using a cut-off level of 300 ng/mL for either or both. Only positive immunoassay results were confirmed by gas chromatography/mass spectrometry, with a quantification limit of 78 ng/mL for MA.
2.4. Data Analysis
In the analysis plan, a significant treatment advantage would be demonstrated by using chi-square to compare ‘successful’ proportions of the bupropion vs. placebo groups. Our sample size estimate was obtained by using the last two weeks’ abstinence rates from our earlier trial of bupropion for MA dependence. Those proportions of successful participants who used ≤ 18 days of 30 at baseline were 0.238 for bupropion and 0.057 for placebo. To obtain a power of 95% at a Type I error rate of 5%, using a two-tailed Fisher’s exact test, would require a total sample size of 200 (100 per group).
The primary efficacy outcome measure was success or failure of each individual to achieve abstinence throughout Study Weeks 11 and 12. Success at abstinence required that, (1) at least two urines samples were provided during each of Weeks 11 and 12 and (2) all urine samples in the last two treatment weeks were negative for MA (negative immunoassay or GC/MS quantitative result <300 ng/mL). Any participant who dropped out before the last two weeks of treatment was scored as a failure on the primary outcome.
Interaction effects on the primary outcome were also evaluated, using Cochran-Mantel-Haenszel regression, for the randomization balancing factors as subgroups: i.e., categories of baseline frequency of MA use, HAM-D score, presence of adult ADHD, and gender. Two-sided, type I error rate was controlled at 5%. All analyses were conducted in versions 9.2 and/or 9.3 of SAS (SAS Institute, Cary, NC).
Safety outcomes included vital signs, electrocardiograms, and weekly logs of adverse events.
3. RESULTS
3.1. Screening and treatment retention
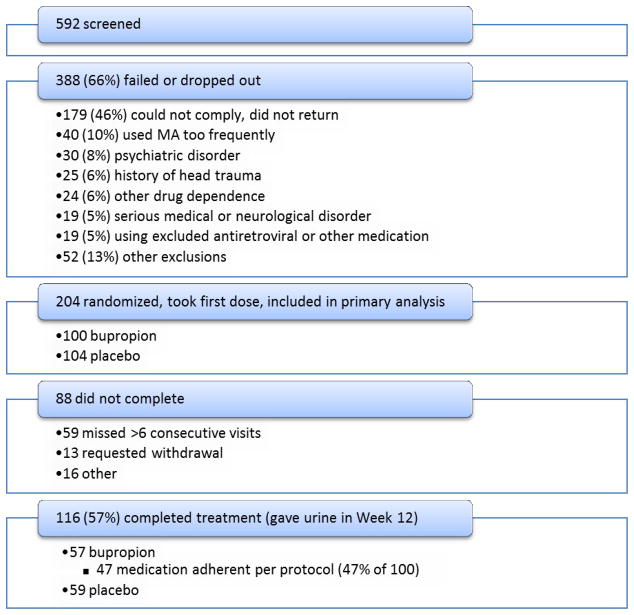
Among 592 participants screened, 388 did not enroll (screen failure = 66%). The main reasons for screen failure are shown in Figure 1, and include: inability to comply/did not return (n=179 (46%)) and too frequent MA use (n=40 (10%)).
Fig. 1.
Flow diagram of participants through the study of bupropion for methamphetamine (MA) dependence in less-than-daily users.
The intent-to-treat analysis utilized 204 randomized participants who took the first dose; 104 participants received placebo, and 100 received bupropion. Fifty nine placebo participants and 57 bupropion participants reached Week 12 (57% completion rate in each group). A log-rank test of retention survival curves showed no group difference (p=0.86).
3.2. Demographics and medication adherence
The two groups had similar demographic and baseline characteristics. The average age was 39.3 years; 65% of participants were male; 69% were white. African Americans accounted for 10%; while 23% had Hispanic ethnicity. Education averaged 13 years. Symptoms of depression (HAM-D >12) occurred in 17%, and adult ADHD in 9%. Higher baseline MA use (19–29 days in past 30) was insignificantly greater among bupropion participants (35%) vs. placebo (27%, chi-square, p=0.21). In the bupropion group, 47% (n=47/100) of participants met protocol criteria (see section 2.3) for medication adherence, while the comparable placebo proportion is unknown.
3.3. Primary Outcome: Efficacy by urine drug screens
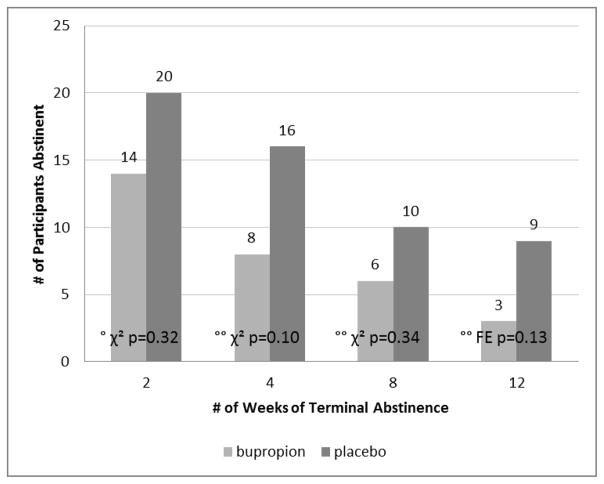
Abstinence success (≥ 2 negative urines in each of Weeks 11 and 12) was achieved by 14% (14/100) of the bupropion group and 19% (20/104) of the placebo group (chi-square, p = 0.32, see Figure 2). Figure 2 also shows rates of abstinence for longer durations, i.e., abstinence for the last four, eight, or even all twelve weeks of treatment. A few individuals in both groups were abstinent for the entire treatment period. Although none of the group differences were significant (chi-square or Fisher’s exact, all p>0.135), the placebo group started with more abstinent in the first week (as well as lower baseline use), and maintained that advantage throughout the trial.
Fig. 2.
Efficacy of bupropion for continuous abstinence from methamphetamine (by ≥ 2 urine samples per week), during the last number of weeks of treatment. Bupropion N=100, placebo N=104. (°p-value for primary outcome, Weeks 11 and 12, °°p-values for secondary outcomes, longer durations of terminal abstinence. χ2 = chi-square, FE = Fisher’s Exact.)
3.4. Subgroup analyses
Without correcting for multiple comparisons, the treatment response (terminal abstinence) was also tested for interactions with the ‘stratification’ factors.
The subgroup having baseline MA use ≤18 days in last 30 included 65 participants treated with bupropion and 76 with placebo (69% of total). There was no significant difference in last-two-weeks abstinence between bupropion and placebo groups (13/65 vs. 17/76, Cochran-Mantel-Haenszel, p=0.73). However, when both treatment groups were combined, the baseline-lower-frequency-use subgroup had a significantly greater proportion of ‘successes’ than the baseline-higher-frequency-use (21% (30/141) vs. 6% (4/63), Cochran-M-H, p=0.015).
There was also no significant difference in last-two-weeks abstinence, between bupropion and placebo treatments, in the following subgroups:
male gender bupropion (n=63) and placebo (n=69) (Cochran-M-H, p=0.31).
more-depressed (HAM-D >12), bupropion (n=15) and placebo (n=20) (Cochran-M-H, p=0.35).
adult ADHD, bupropion (n=10) and placebo (n=8) (Cochran-M-H, p=0.33).
3.5. Safety
The bupropion group demonstrated a profile of adverse events similar to that listed in the FDA-approved label. There were some occasions of non-emergent hypertension or blood pressure increase, but overall there were no significant changes to vital signs or ECG intervals over the course of the study. The most frequently encountered complaints were ‘headache’ and ‘nausea.’ Nausea was more frequent in the placebo (20%) than in the bupropion group (8%, Fisher’s exact, p=0.016), while ‘back pain’ and ‘pain’ were insignificantly more frequent with bupropion. Bupropion was discontinued for a loss of taste, for muscle tension, for insomnia, twice for hives, and twice for seizure. One of the seizures was designated a Serious Adverse Event. That participant was unconscious and taken to the Emergency Department, then revealed (they) had electively discontinued an undisclosed anticonvulsant.
4. DISCUSSION
In this study, bupropion did not significantly increase abstinence in MA-dependent participants, compared to placebo. As in other studies, a lower frequency of MA use at baseline predicted better treatment response among both medication and placebo groups. However, although the study population was limited to less-than-daily users, our previous study finding of some effect in males with lower baseline use was not replicated. No interactions with the primary outcome were detected between bupropion treatment and gender, symptoms of depression, or ADHD.
Regarding the mid-study protocol change, which relaxed the criterion for having a MA-positive urine at baseline, 20 participants were included (10 bupropion and 10 placebo) who had no urines positive for MA, throughout both screening and treatment. Among these, eight were still attending and abstinent during Weeks 11 and 12, four in each treatment group. By chance, this subgroup appeared to be evenly randomized, but their success rate (40%) helped to elevate the overall success rate (17%).
Regarding medication adherence, out of 98 participants in the bupropion group, fewer than half met the protocol criteria for adherence. Plans for future trials include biochemical verification or measurement of exposure in all treatment arms, along with contingency management and/or in-clinic dosing to encourage medication adherence.
Heinzerling et al. (2014) recently compared bupropion to placebo in 84 MA dependent participants. They used a Week 6 plasma bupropion level ≥50 ng/mL to indicate medication adherence. They did not find a significant difference in Weeks 11–12 abstinence rates between the placebo and bupropion groups (p=0.087), despite a lower placebo response rate than ours (their study had 14% placebo abstinence vs. our 19%). Their study also had a similar dropout rate as ours. They reported better abstinence among their 13 bupropion-adherent participants compared to 28 bupropion non-adherent participants. However, as in our study, they did not have a marker that identified placebo adherence, leaving open the probability that a known predictor, such as treatment retention, is a more likely explanation of better abstinence than bupropion efficacy.
Notably, we have seen increasingly higher placebo response rates in 3 MA studies completed prior to this one (6.9% for placebo vs. bupropion (Elkashef et al., 2008); 9.9% vs. topiramate, unpublished outcome; and 17.6% vs. modafinil (Anderson et al., 2012)). In the current study, the placebo response was greater than expected, and medication adherence was not good, which would weaken the statistical power. Using 204 participants and a placebo success rate of 19%, a chi-square estimate of the ability of this dataset to detect ~15% greater success rate (~35% for bupropion) at 5% significance level yielded 73% power. If we also took into account some proportion of inadequate adherence, our power to detect a meaningful difference would be further lowered. Therefore, although this trial does not support bupropion effectiveness, neither can we strongly conclude against it.
Psychosocial therapies (CBT, CM, etc.) remain the mainstay of treatment for MA dependence (e.g., Rawson et al., 2006). But given that we cannot determine whether some reported positive effects of bupropion were spurious, or due to an as-yet-unidentified factor, any decisions about pharmacological treatment of MA dependence must still be guided by clinical experience and evaluation of risk/benefit. That is – as an adjunct to talk therapy for MA dependence, the risk of bupropion’s acute or chronic adverse effects must be weighed against its possible usefulness, e.g., in those with depression, ADHD, or who want to quit smoking. If a clinician did choose a medication, in this population, adherence would need considerable encouragement.
The use of meta-analytic tools, or further research on subgroups looking at pharmacogenomic differences such as slow vs. fast metabolizers, may yet disclose bupropion effectiveness for selected individuals. Adjustments for future trials could include decreasing the amount of psychotherapy to mimic treatment-as-usual, in hopes of having a smaller placebo effect. Also, a placebo run-in period would allow identification and exclusion of placebo responders. Finally, additional measures to enhance adherence, such as a longer-acting formulation, might be employed during drug development.
Supplementary Material
Highlights.
Bupropion SR 150mg BID, vs. matched placebo, for 12 weeks to 204 treatment-seekers.
3x per week urines for meth and bupropion levels. No biomarker for placebo.
No significant difference between treatment groups in % abstinent in Weeks 11–12.
Bupropion adherence per protocol was achieved by just 47%.
Additional data show wide range of adherence patterns, max levels, and clearance rates.
Acknowledgments
Role of the funding source
This study was funded by Nat’l Institutes of Health -- Nat’l Institute on Drug Abuse contract: N01DA-05-8857. NIDA had a major role in study design, analysis and interpretation of the data, and writing and submitting this manuscript for publication. THH received partial salary support from NIDA through the Department of Veterans Affairs Cooperative Studies Program (Interagency Agreement) 1 Y01-DA-40032.
We thank all the study participants, as well as the study staff at each site and at NIDA. We would also like to acknowledge the following Principal Investigators, who conducted this trial at sites supported by their respective Institutions and NIDA:
Jan Campbell, M.D., University of Kansas, Kansas City, KS.
Daniel Dickerson, D.O., M.P.H., Friends Research Institute, Torrance, CA.
Gantt P. Galloway, Pharm.D., St. Luke’s Hospital, San Francisco, CA.
William Haning, M.D., University of Hawaii, Honolulu, HI.
Nishant Kumar, D.O., Friends Research Institute, Torrance, CA.
Robert J. Malcolm, M.D., Medical University of South Carolina, Charleston, SC.
Joseph Mawhinney, M.D., South Bay Treatment Center, San Diego, CA.
Michael McCann, M.A., Matrix Institute on Addictions, Woodland Hills, CA.
Richard A. Rawson, Ph.D., University of California, Los Angeles, CA.
Malcolm Reid, Ph.D., New York University, New York, NY.
John D. Roache, Ph.D., UTX, Health Science Center, San Antonio, TX.
Christopher Stock, Pharm.D., VA Health Care System, Salt Lake City, UT.
Dennis Weis, M.D., Lutheran Hospital, Powell Addiction Research Center, Des Moines, IA.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary materials: Additional data listing all (thrice weekly) urine concentrations of bupropion are available in an online supplement1. These show a wide range of maximum bupropion levels and adherence patterns. Also, there is a graph from a few individuals who stopped bupropion early but stayed in the study, showing (sparse) time curves of elimination after the last dose. Author Disclosures
Contributors
Author Ann Anderson wrote the first and subsequent drafts and edited the final version of the manuscript. Shou-Hua Li, Denka Markova and Tyson Holmes did the statistical design, analyses, reports and editing. Roberta Kahn summarized the adverse events data. Nora Chiang provided chemical assay procedures and reports. Ahmed Elkashef, former Branch Chief at NIDA, and Jan Campbell, Daniel Dickerson, Gantt Galloway and Christopher Stock at their respective sites were Principle Investigators who wrote in debate of our conclusion and considering previous related work. All site Principle Investigators are listed below. All authors have approved the final manuscript.
Conflict of Interest
No pharmaceutical company supported this study. No authors have relevant patent applications or own stock in, are employed or paid for consultations by, or receive other funding from GlaxoSmithKline or relevant pharmaceutical companies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler LA, Spence T, Faraone SV, Reimherr FW, Kelsey D, Michelson D, Biederman J. Training raters to Assess Adult ADHD: reliability of Ratings. J Atten Disord. 2005;8:121–126. doi: 10.1177/1087054705277168. [DOI] [PubMed] [Google Scholar]
- Anderson AL, Li SH, Biswas K, McSherry F, Holmes T, Iturriaga E, Kahn R, Chiang N, Beresford T, Campbell J, Haning W, Mawhinney J, McCann M, Rawson R, Stock C, Weis D, Yu E, Elkashef AM. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120:135–41. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24:541–50. doi: 10.1177/0897190011426557. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–70. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Elkashef A, Holmes TH, Bloch DA, Shoptaw S, Kampman K, Reid MS, Somoza E, Ciraulo D, Rotrosen J, Leiderman D, Montgomery A, Vocci F. Retrospective analyses of pooled data from CREST I and CREST II trials for treatment of cocaine dependence. Addiction. 2005;100(Suppl 1):91–101. doi: 10.1111/j.1360-0443.2005.00986.x. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline. Prescribing Information: Zyban® (bupropion hydrochloride Sustained-Release Tablets) GlaxoSmithKline; Research Triangle Park, N.C: 2012. [accessed 07/30/2014]. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020711s036lbl.pdf. [Google Scholar]
- Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health. 2010;31:385–98. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Hall TM, Ba YY, Wu Y, Shoptaw SJ. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109:1878–86. doi: 10.1111/add.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69:578–92. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18:414–8. doi: 10.1111/j.1755-5949.2011.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Shoptaw SJ, Obert JL, McCann MJ, Hasson AL, Marinelli-Casey PJ, Brethen PR, Ling W. An intensive outpatient approach for cocaine abuse treatment. The Matrix model. J Subst Abuse Treat. 1995;12:117–27. doi: 10.1016/0740-5472(94)00080-b. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw D, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. 34–35. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [accessed 07/30/2014]. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits; p. 58. http://www.samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.pdf. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [accessed 07/30/2014]. p. 15.p. 59. http://www.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/NationalFindings/NSDUHresults2012.pdf. [Google Scholar]
- Wilens TE, Haight BR, Horrigan JP, Hudziak JJ, Rosenthal NE, Connor DF, Hampton KD, Richard NE, Modell JG. Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry. 2005;57:793–801. doi: 10.1016/j.biopsych.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.