Abstract
To study impaired goal-oriented behavior in schizophrenia (SZ), we used a delay discounting task, which consists of a series of choices between receiving a small immediate or larger delayed reward. Few studies of delay discounting have evaluated response consistency (R2), which is especially relevant in SZ because of documented inconsistency in task performance. We calculated the rate of discounting (k) and R2 in SZ (n=35) and healthy controls (HC; n=21). Using a criterion value of R2 > .60 to define consistent performance allowed us to compare discounting in consistent SZ and HC, as well as in inconsistent SZ. Groups did not differ significantly in smoking. Compared to HC, consistent SZ showed greater delay discounting. Both groups exhibited similar patterns of decreasing immediate choices across trial categories, although the decrease was less for SZ. Separate analyses on smokers and non-smokers showed that this group difference was carried by the non-smokers. Inconsistent SZ discounted more than HC and consistent SZ, but their aberrant pattern of choices casts doubt on the validity of their calculated k values.
Keywords: Intertemporal, Executive function, Decision making
1. Introduction
Schizophrenia is a complex mental illness with functional deficits such as the inability to initiate and maintain goal-directed behavior that contribute to the long-term disability associated with the illness (Barch and Dowd, 2010). Goal-directed behavior involves selecting some desired future outcome and making choices that best serve reaching that goal. Such behavior can require choosing between alternative actions that may require short-term sacrifice for long-term gain, similar to choices in the laboratory delay discounting (DD) task. The DD task consists of making a series of choices between receiving a small sooner (usually immediate) reward or a larger delayed reward (Rachlin et al., 1991; Green et al., 1996). Most studies using the DD paradigm characterize an individual;s choices in terms of a discount function that models the effect of delay on subjective value of later rewards (Bickel et al., 1999; Kirby et al., 1999; Heerey et al., 2007). The statistic k indicates the rate at which an individual discounts future rewards, with larger k’s indicating greater DD (Mazur and Coe, 1987; Rachlin et al., 1991). Lower k, or greater willingness to wait for larger but later rewards in DD, has been associated with less impulsivity (Ainslie, 1975) and better cognition and executive function (Shamosh et al., 2008). Individuals with various addictions (Vuchinich and Simpson, 1998; Bickel et al., 1999; Kirby et al., 1999; Mitchell, 1999; Petry and Casarella, 1999; Baker et al., 2003; Robles et al., 2011), some psychiatric conditions (Crean et al., 2000; Petry, 2001; Takahashi et al., 2008), and with poorer treatment outcomes (Sheffer et al., 2012; Stanger et al., 2012) exhibit greater DD; that is, they choose more immediate smaller rewards than controls do. Given the latter, the study of DD in schizophrenia might suggest targeted interventions that could be used to improve decision making.
Patients with schizophrenia (SZ) have neural abnormalities associated with regions of the brain thought to mediate executive functions (Perlstein et al., 2001; Callicott et al., 2003; Manoach, 2003; Tan et al., 2007) and reward (Juckel et al., 2006a; Juckel et al., 2006b; Murray et al., 2007; Jensen et al., 2008; Schlagenhauf et al., 2008; Schlagenhauf et al., 2009; Walter et al., 2009; Koch et al., 2010; Simon et al., 2010; Waltz et al., 2010; Morris et al., 2012). Furthermore, previous studies using functional magnetic resonance imaging (fMRI) to determine the neural bases of the decision making processes involved in DD have revealed activation in executive function regions, among other parts of the brain (McClure et al., 2004; for review, see Avsar et al., 2013). These studies thus provide convergent evidence that the DD task might be particularly relevant to those with SZ.
Previous behavioral studies of DD in patients with SZ have yielded mixed results (Heerey et al., 2007; Heerey et al., 2008; Heerey et al., 2011; Ahn et al., 2011; MacKillop and Tidey, 2011; Wing et al., 2012). Heerey et al. (2007; 2011) reported greater DD in those with SZ, but they did not control for smoking behavior (MacKillop and Tidey, 2011; Wing et al., 2012). Smokers show greater DD than non-smokers (Bickel et al., 1999; Baker et al., 2003), and incidence of smoking is very high in SZ (Hughes et al., 1986; de Leon and Diaz, 2005; Kalman et al., 2005). Two recent studies that took smoking into consideration found no group differences in DD between SZ and healthy controls (HC) (MacKillop and Tidey, 2011; Wing et al., 2012); but see Ahn et al. (2011).
Another important caveat is that, although patients with SZ are more likely to perform tasks inconsistently (Cohen and Servan-Schreiber, 1992; Schooler et al., 2008), inconsistent performance on the DD task has not always been systematically evaluated. R2 has often been used to assess consistency by indexing the degree of correspondence between data points and a mathematical discounting model and then to exclude data from nonsystematic (inconsistent) responders based on R2 less than some criterion value (for review, see Johnson and Bickel, 2008). Other model-driven mathematical measures, such as those based on other indices of goodness-of-fit (e.g., SSE), have also been used occasionally to identify nonsystematic DD data (see Johnson and Bickel, 2008). By contrast, a model-free algorithm was used by Johnson and Bickel (2008) to define nonsystematic data sets as those in which k was not a monotonically decreasing function of delay. Both the R2 and Johnson and Bickel (2008) methods evaluate responses in relation to a monotonically decreasing curve. In previous studies of DD in SZ, Heerey et al. (2007), MacKillop and Tidey (2011) and Wing et al. (2012) used another method, the percentage of responses (< 78%) disagreeing with the participant’s estimated k (Kirby et al., 1999; Heerey et al., 2007; Kirby, 2000) as a measure of inconsistency to exclude discounting data from some SZ participants. In contrast to other measures of consistency, this criterion does not require any systematic relationship between discounting and delay. In the present study, we used regression analysis to calculate each participant’s k and model-fit statistic, R2. Based on the data obtained for R2, all healthy controls (HC) showed R2 > 0.60 and were classified as consistent responders, whereas we divided patients into consistent (R2 > 0.60) and inconsistent (R2 < 0.60) groups. This allowed us to directly compare consistent SZ and HC. Relevant to previous studies of DD, smoking, and SZ (Bickel et al., 1999; Baker et al., 2003; Heerey et al., 2007; MacKillop and Tidey, 2011; Wing et al., 2012), the HC, consistent SZ, and inconsistent SZ groups did not differ significantly in number of cigarettes smoked per day or percentage of smokers and non-smokers. Thus, this is the first paper of DD in SZ to take both consistency of task performance and smoking into consideration. Our paper is also the first to provide details about DD by inconsistent SZ.
The HC and SZ participants of the present study were also studied in our recent fMRI investigation of DD in SZ (Avsar et al., 2013). However, results reported in the fMRI paper were based on a different version of the DD task, individually tailored for each participant based on his or her k, and on smaller subsets of the HC and SZ participants.
2. Materials and methods
2.1 Participants
Thirty-five patients with DSM-IV (2000) schizophrenia or schizoaffective disorder were recruited from the outpatient psychiatric clinics at the University of Alabama at Birmingham (UAB). Diagnoses were established using participants’ medical records and the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994). Diagnostic interviews and clinical evaluations were done by a trained master’s level research coordinator. The diagnosis was made as a consensus reached by a board-certified psychiatrist and the trained research coordinator. Twenty-one HC, not significantly different from the SZ groups (see below) in self-reported number of cigarettes smoked per day and percentages of smokers and non-smokers, nor on age, sex, or parental socioeconomic status, were recruited by advertisements in flyers and the University newspaper. Non-smokers were defined by self-reported number of cigarettes smoked/day = 0–5. Smoking 5 or fewer cigarettes/day is the usual definition of a tobacco or cigarette “chipper” who smokes without becoming dependent, as determined by nicotine dependence scales (Shiffman et al., 1999, 2004). Significantly, cigarette chippers discount like non-smokers, in contrast to the greater delay discounting shown by real smokers (Heyman and Gibb, 2006). Matching on characteristics in addition to smoking is relevant because greater DD has been found related to lower income (Green et al., 1996) and to age (for review, see Samanez-Larkin et al., 2011), as well as smoking (Bickel et al., 1999; Baker et al., 2003). Exclusion criteria were major medical conditions, self-reported substance abuse within the past six months, previous serious head injury, a neurological disorder, and previous loss of consciousness. In addition, because participants were also part of an fMRI study of DD (Avsar et al., 2013), pregnancy or the presence of ferromagnetic material in the body was exclusionary. HC were excluded for any major current or lifetime Axis I diagnosis (e.g., depression, anxiety). The UAB Institutional Review Board approved the study, and all participants gave written informed consent.
General cognitive function of all participants was characterized by the Repeatable Battery of Neuropsychological Status (RBANS) (Randolph et al., 1998). The Brief Psychological Rating Scale (BPRS) (Overall and Gorham, 1962) and its positive and negative subscales were used to assess mental status and symptom severity in SZ. Participants received compensation for participation in the study.
2.2 Delay discounting task
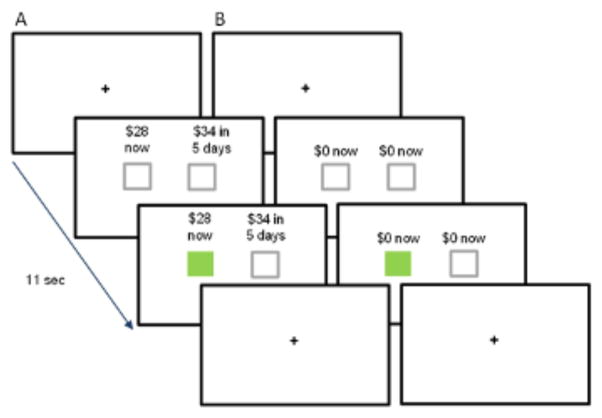
We tested participants on a DD task modified from Kirby et al. (Kirby et al., 1999; Kishinevsky et al., 2012). Participants viewed the 108 trials of the DD task on a computer monitor; 96 trials were divided equally between eight types each with a particular trial k value (see Supplementary Material), interspersed with 12 sensorimotor control (SMC) trials, for which participants arbitrarily made a right or left button response (Figure 1). Each trial consisted of a choice between a unique combination of an immediate reward (IR; Kirby et al., 1999), ranging from $1 to $73, and a delayed reward (DR), ranging from $28 to $86, with delays (D) ranging from 1 to 116 days. Trial k values were used in generating IR and DR for each trial, according to the hyperbolic function, IR = DR/(1 + kD) (Mazur and Coe, 1987). A trial k that matched a participant’s k would be most difficult because the two choices would have equal subjective value and would be equally likely to be chosen (Marco-Pallarés et al., 2010). Trial k’s less than that would result in the immediate rewards having greater subjective value and, therefore, more immediate choices. Conversely, for higher trial k’s, more delayed rewards would be chosen. All rewards were hypothetical. We entered percentages of immediate choices (%Now) versus corresponding trial k values into non-linear (exponential) regression analysis to determine each participant-specific k, defined as a trial k value corresponding to predicted %Now = 50%. The analysis also yielded a model fit statistic, R2, which provided a measurement of how well the participant’s responses fit the expected pattern of decreasing %Now as trial k increased. We used R2 to characterize participants as consistent (R2>0.60) or inconsistent (R2<0.60) (see Results). All HC were consistent, and SZ were divided into consistent and inconsistent groups (see Results).
Fig. 1.
Delay discounting task. (A) Delay discounting task trial; (B) Sensorimotor control trial. All trials were 11 sec in duration, with the initial fixation cross presented for two, four, or six sec, followed by two grey boxes paired with (A) the choice of an immediate or a delayed hypothetical monetary reward ($28 now or $34 in five days; a trial k of 0.041) or (B) the no choice option. Participants had the remainder of the 11-sec trial (nine, seven, or five sec) to indicate their preference by pressing a button on the side corresponding to their choice. The box under the choice turned green, indicating the response selection. A return of the fixation cross indicated the start of the next trial.
2.3 Data analysis
One-way analysis of variance (ANOVA) was used to compare the HC, consistent SZ, and inconsistent SZ groups on rate of discounting and consistency. For the former, we compared log(k) (to the base 10) because distributions of k are severely skewed (Johnson and Bickel, 2002; Heyman and Gibb, 2006). For the latter, R2 values were transformed to Fisher’s R′ values (Howell, 2007). Follow-up comparisons between pairs of means were performed using Fisher’s Least Significant Difference test, which maintains familywise Type I error rate = 0.05 when there are three groups (Howell, 2007). For discounting and consistency data we also performed two-way ANOVA with the factors Diagnosis and Smoking status (see below).
One-way ANOVA was also used to compare groups on age, parental SES, RBANS scales, and BPRS scales. For comparison of number of cigarettes smoked per day, nonparametric ANOVA (Kruskal-Wallis) was used because of the high frequency of 0 values in each group, resulting in positively skewed distributions. The proportion of smokers in each group was compared using the χ2 test of independence. Gender composition of groups was also compared using the χ2 test. Finally, mixed between- and within-group ANOVA was performed to compare groups on %Now across trial k values. For all analyses, α =0.05.
3. Results
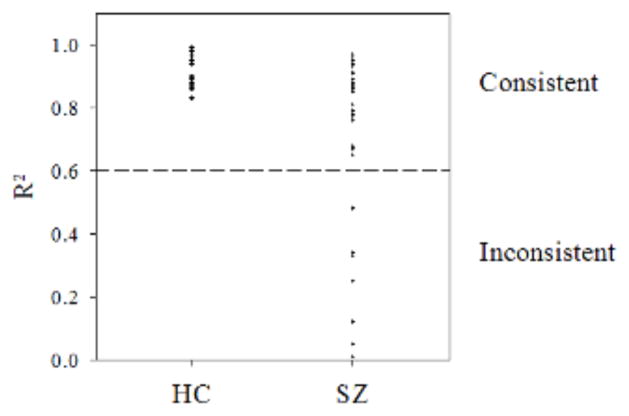
Figure 2 shows the distribution of R2 on the DD task (i.e., model fit) for all participants. Among patients, R2 values formed a quasi-bimodal distribution, with approximately three-quarters displaying R2 values ranging from 0.65 – 0.97 and the remainder showing very low values (≤0.48), suggesting an inability to make consistent choices. Therefore, we defined consistent performance as R2 >0 .60, as we have done previously (Avsar et al., 2013). By this definition, all controls exhibited consistent performance, as did 27 of 35 patients, referred to as the consistent SZ group (Table 1). The remaining eight patients comprised the inconsistent SZ group. Comparisons involving the inconsistent patients should be considered tentative, given their small sample size. When R′ values were compared, HC had significantly higher R′ than both consistent (t[53] = 4.16, p < 0.001) and inconsistent SZ (t[53] = 10.61, p <0 .001). For comparison, we also adapted for our analysis an algorithm proposed by Johnson & Bickel (2008) for identifying non-systematic DD data. Admittedly, their analysis and ours differed in that theirs looked at the relationship between indifference points and delay compared to percentage of immediate choices vs. trial k in our analysis. However, in both situations, systematic, or consistent, data would follow similar patterns by approximately a decreasing monotonic function. Adopting their exclusion criteria for non-systematic data of (1) any subsequent point being greater than the preceding point by >20% or (2) the last point being not greater than 10% less than the first point, five participants, all members of the inconsistent SZ group (see above) were excluded. Thus, in this case, use of our R2 exclusion criterion appeared to be more sensitive in that it identified 8 data sets as inconsistent. Because our rationale entails excluding as many suspect data sets as possible from the SZ vs. HC comparison, we believe the R2 criterion is preferable for these data. However, when group comparisons were performed using SZ groups defined by the Johnson and Bickel (2008) procedure, results were essentially identical to those obtained with our consistent SZ and inconsistent SZ groups. We also found the same results when we applied the Kirby (2000) method to define inconsistent responders (<78% of responses agreeing with their k).
Fig. 2.
Model fit (R2) during estimation of k values for healthy controls (HC; all consistent) and consistent patients and inconsistent patients with schizophrenia (SZ). The reference line at 0.60 indicates the minimum R2 value that was used to define consistent performance.
Table 1.
Demographic data and clinical and behavioral measures from all controls, consistent patients with schizophrenia, and inconsistent patients with schizophrenia.
| Variable | Consistent Controls (n=21) | Consistent Patients (n=27) | Inconsistent Patients (n=8) | ANOVA F | P |
|---|---|---|---|---|---|
| Age (years)a | 37.3 ± 2.7 | 39.0 ± 2.4 | 32.0 ± 3.9 | 0.99 | 0.38 |
| Genderb | 11 Men; 10 Women | 18 Men; 9 Women | 7 Men; 1 Woman | >0.10 | |
| Duration of illness (years)c | 18.8 ± 2.4 | 12.0 ± 2.9 | 1.50 | 0.25 | |
| Parental SESd | 9.8 ± 1.6 | 7.4 ± 1.2 | 13.6 ± 2.7 | 2.73 | 0.074 |
| Smoking, packs/daye | 0.42 ± 0.11 | 0.63 ± 0.11 | 0.52 ± 0.32 | 0.26 | |
| Smokers:non-smokersf | 9:12 | 18:8 | 2:5 | 0.07 | |
| RBANSg | |||||
| Total Index | 92.7 ± 3.h | 74.9 ± 2.2i | 67.5 ± 3.7i | 17.09 | <0.001 |
| Immediate Memory | 95.8 ± 2.9h | 78.0 ± 3.0i | 77.0 ± 3.8i | 10.46 | <0.001 |
| Visuospatial | 92.8 ± 4.1h | 78.1 ± 3.4i | 72.0 ± 7.5i | 5.08 | 0.01 |
| Language | 94.1 ± 3.2h | 89.5 ± 1.6h | 77.0 ± 6.6i | 4.84 | 0.012 |
| Attention | 95.8 ± 4.0h | 81.3 ± 3.1i | 86.8 ± 3.9hi | 4.32 | 0.019 |
| Delayed Memory | 95.6 ± 2.6h | 74.8 ± 3.8i | 58.5 ± 6.0j | 15.02 | <0.001 |
| BPRS | |||||
| Total | 32.8 ± 1.8 | 30.6 ± 2.6 | 0.40 | 0.53 | |
| Positive | 6.2 ± 0.7 | 6.1 ± 1.1 | 0.0003 | 0.99 | |
| Negative | 4.7 ± 0.4 | 5.0 ± 0.8 | 0.12 | 0.74 | |
| Delay Discounting | |||||
| Log10(k) | −1.77 ± 0.16h | −1.20 ± 0.14i | −0.34± 0.27j | 10.70 | <0.001 |
| R2k | 0.94 ± 0.01h | 0.86 ± 0.02i | 0.24 ± 0.06j | 56.30 | <0.001 |
Abbreviations: RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; BPRS, Brief Psychiatric Rating Scale; k, discounting parameter; R2, discounting model fit.
Values are expressed as mean ± standard error unless otherwise indicated.
Values are expressed as number of individuals and χ2 used to test for gender difference.
Data not available for one consistent SZ and three inconsistent SZ. Twenty-four consistent SZ were treated with a second generation antipsychotic (2nd APD), two with a first generation (1st APD), and one with a combination of 1st and 2nd APD. All inconsistent SZ were treated with a 2nd APD.
Parental socioeconomic status (SES) was determined by Diagnostic Interview for Genetic Studies (1–18 score); lower score signifies higher socioeconomic status.
One pack = 20 cigarettes. Data not available for one inconsistent SZ. Data were analyzed with Kruskal-Wallis non-parametric analysis of variance.
Non-smokers were defined as smoking 0–5 cigarettes/day; of the non-smokers, all but one control, two consistent SZ, and one inconsistent SZ smoked 0 cigarettes/day. Data not available for one inconsistent SZ. χ2 used to test for group differences.
RBANS data not available for one healthy control, one consistent patient and two inconsistent patients.
Means with different superscripts are significantly different (p<0.05).
For these statistical comparisons, R′ values were used. See text for details.
3.1 Group comparisons: delay discounting
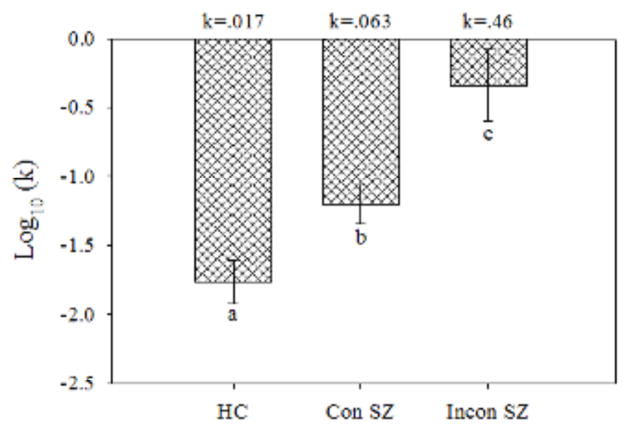
Comparison of log(k) values (Figure 3) revealed greater DD in both the consistent SZ (t[53] = 2.92, p =0.005) and inconsistent SZ (t[53] = 4.70, p =0 .00002) than in HC. Moreover, log(k) was significantly greater in inconsistent compared to consistent SZ (t[53] = 2.68, p =0 .01). These differences remained significant when we controlled for number of cigarettes smoked per day using analysis of covariance. Repeated measures ANOVA comparing %Now values across trial k’s revealed no main effect of Group (F[2,53] = 1.22, p = 0.30) but a Group x Trial k interaction (F[14,371] = 8.87, p <0 .0001). Tests of simple interaction effects comparing groups in pairwise fashion were all significant (p values < 0.05), indicating that the pattern of %Now values across trial k values in each group was significantly different from each of the others. Inspection of mean %Now values (Figure 4) shows that consistent SZ were as likely as HC to choose immediate rewards for low trial k’s, but reduced their frequency of immediate choices less than HC did as trial k increased, which is consistent with their higher rate of discounting. By contrast, inconsistent SZ exhibited a pattern of % Now values which might be best described as aberrant. When data from individual participants were inspected, the pattern of %Now values across trial k’s generally bore little resemblance to the expected decreasing function. Inspection of group results reveals that, while inconsistent SZ did choose immediate rewards more frequently than the other groups for high trial k values (k6 – k8; p values <0 .05), for low trial k’s (k1 – k3), by contrast, they chose immediate rewards less frequently than the other two groups (p values <0 .05), which is inconsistent with a high rate of discounting. This makes the higher average k for the inconsistent SZ group suspect.
Fig. 3.
Mean (± standard error) log(k) for the healthy controls (HC), consistent patients with schizophrenia (Con SZ), and inconsistent patients with schizophrenia (Incon SZ). Smaller negative values indicate greater delay discounting. The values at top above each group are the k values corresponding to the log(k) values on the y-axis and in Table 1. Letters (a, b, c) indicate that all group differences were significant (p < 0.05).
Fig. 4.
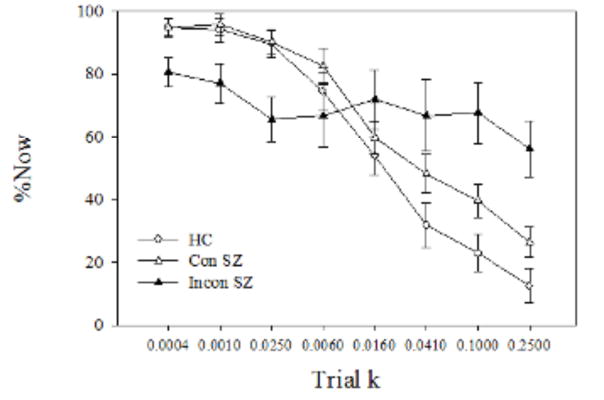
Mean (± standard error) percentage of Now (%Now) choices as a function of the eight trial k s for the healthy controls (HC), consistent patients with schizophrenia (Con SZ), and inconsistent patients with schizophrenia (Incon SZ).
Parenthetically, in the consistent SZ, we found a significant negative correlation between log (k) and R′ (r = −0.66, p <0 .0005). We found no significant correlations in the other two groups.
To investigate whether our observed group difference in delay discounting was related to smoking status, we dichotomized the consistent SZ and HC groups into smokers and non-smokers, with non-smokers defined as those who reported smoking 5 or fewer cigarettes/day (Table 1). (Inconsistent SZ were not included because of small n.). Two-way ANOVA revealed that the SZ vs. HC difference was carried by the non-smokers. The Group x Smoking interaction was significant (F[1,43] = 11.47, p =0.002). Simple-effects tests showed that log(k) was significantly greater in non-smoking SZ than non-smoking HC (t[43] =4.24, p <0.0005) but there was a non-significant difference between SZ and HC smokers (t[43] = 0.34, p =0.74).
Within the HC, smokers had significantly higher log(k) than non-smokers (p =0.009), as expected from the literature on DD and smoking (Bickel et al., 1999; Baker et al., 2003). However, within the consistent SZ, smokers had significantly lower log(k) than non-smokers (p = 0.049).
Because we had also found that discounting was related to consistency in the patients, we asked whether the differences above were paralleled by differences in R2 (with statistical comparisons performed on R′, as previously). The Group x Smoking interaction was significant (F[1,43] = 7.53, p =0.009). Among the non-smokers, SZ were significantly less consistent than HC (t[43] = 4.56, p <0.0005). The analogous difference was not significant among smokers (t[43] = 0.98, p =0.33). When analogous analyses were done with all of our other demographic and clinical assessments, we found no significant effects related to smoking status.
Thus, consistent SZ showed greater discounting and less consistency than controls. Dichotomizing the groups by smoking status revealed that both of these effects were largely carried by the non-smokers. However, the latter finding should be considered in light of the small n’s that followed dividing the SZ and HC groups.
3.2 Group comparisons: demographic variables and clinical assessments
Demographic data and clinical and cognitive assessments (Nurnberger et al., 1994; Randolph et al., 1998) are presented in Table 1. Groups did not differ significantly with regard to number of cigarettes smoked per day or age or percentage of smokers and non-smokers, although there was a trend for the latter. However, subsequent analyses showed that the SZ vs. HC difference in discounting was carried by the non-smokers (see below). Although a higher percentage of inconsistent patients were male than in the other groups, group differences on gender were not significant (although the small sample size limited power), and no consistent relationship between gender and delay discounting has been reported (for review, see Weller et al., 2008). ANOVA revealed no significant group differences on parental socioeconomic status (Table 1).
Inconsistent SZ did not differ significantly from consistent SZ on total RBANS, BPRS, or positive and negative BPRS subscales. However, inconsistent patients had significantly lower scores than consistent patients on the delayed memory and language RBANS scales (p values < 0.05; Table 1).
For HC and consistent SZ, there were no significant within-group correlations between log(k) and RBANS scales. For consistent SZ, there were no significant correlations between log(k) and BPRS scales. For inconsistent SZ, there was a significant negative correlation between log(k) and the RBANS delayed memory scale (r = −0.88, p =0.02) and marginally significant negative correlations between log(k) and total RBANS (r = −0.79, p =0.06), the RBANS language scale (r = −0.66, p =0 .08), and BPRS-positive (r = −0.66; p =0.08).
4. Discussion
In the present study, we used the model-fit statistic, R2, as a method of assessing consistency. The use of R2 provided a useful screening procedure for identifying SZ exhibiting aberrant response patterns. Their k values were very high compared to the other participants’ values but appeared to be artifactual rather than a valid reflection of greater delay discounting. Thus, separating their data from those of SZ exhibiting consistent responding provided a more conservative comparison of DD in SZ vs. HC; including those values in an overall SZ group would have inflated the SZ vs. HC difference. However, we found that, even in the absence of data from inconsistent SZ, consistent SZ showed greater DD than controls.
4.1 Delay discounting results
Overall, consistent SZ showed greater DD than HC. Furthermore, the group difference occurred in the absence of significant differences in percentages of smokers vs. non-smokers or cigarettes smoked per day, and when we controlled for the latter with analysis of covariance. These results appear in contrast to those of two recent studies of DD in SZ and HC when smoking was taken into consideration (MacKillop and Tidey, 2011; Wing et al., 2012). However, the MacKillop and Tidey (2011) study involved all smokers, so their negative findings are actually similar to ours in that we found no difference in DD between consistent SZ and HC who were smokers (with the caveat that our findings are based on small n’s). Wing and colleagues (2012), whose studied smoking and non-smoking controls and SZ, found no overall statistical difference in DD between SZ and HC, unlike our results. Wing and colleagues (2012) defined smokers and non-smokers differently than we did, with their “non-smokers” having expired CO levels < 10 ppm. However, their “smokers” reported smoking at least 10 cigarettes/day, comparable to the reported number of cigarettes/day smoked by our “smoker” consistent HC and SZ. When we divided the HC and consistent SZ groups into smokers and non-smokers, we found that log(k) was higher in non-smoking SZ than non-smoking HC, but there was no difference between smoking SZ and HC. Within the SZ group, smokers actually had lower log (k) than non-smokers. Both sets of results suggest that being a smoker may benefit consistent SZ on DD, improving their performance over non-smoking SZ and “normalizing” their performance compared to smoking HC. These results agree with other literature suggesting that smoking may have such prevalence in SZ because it helps cognition (Kumari & Postma, 2005).
The results of the present behavioral study may appear contrary to those of our recent fMRI study of DD in SZ that did not differ on number of cigarettes smoked/day, in which we reported no differences in mean rate of discounting for smaller subgroups of SZ and HC (Avsar et al., 2013). However, in the Avsar et al. (2013) study, the DD task was a more difficult version than that used in the present study, and SZ and HC groups that were chosen for neuroimaging did not significantly differ in consistency.
Our use of R2 as a measure of consistency in the present study was noteworthy in that it revealed two subgroups within SZ, a majority that performed consistently on the task (although not as consistently as controls) and a minority that performed inconsistently on the task and whose results were excluded from the main comparison consistent SZ and HC. R2 has often been used to assess consistency by characterizing the degree of correspondence between data points and a theoretical mathematical discounting model and then to exclude results from nonsystematic (inconsistent) responders based on R2 < a criterion value (for review, see Johnson and Bickel, 2008). By contrast, Johnson and Bickel (2008) used a model-free algorithm to define nonsystematic data sets as those for which k was not a monotonically decreasing function of delay. Although previous studies of DD in SZ did not systematically evaluate performance consistency using R2 as we did, Heerey and colleagues (2007) excluded DD results for some SZ based on too low a percentage of consistent responses, with consistency defined as in Kirby (2000); that is, >78% of individual trial responses agreeing with the participant’s k. MacKillop and Tidey (2011) and Wing et al. (2012) also used Kirby’s (2000) method to evaluate consistency, but reported high levels of consistency and excluded few participants for inconsistency (although both studies excluded results from outliers). To convince ourselves that our results were not artifacts of our particular definition of consistency, we adapted the Kirby (2000) as well as the Johnson and Bickel (2008) procedures for use with our data and then compared the results on log(k). For both procedures, we obtained the same results as in our original analysis: rate of discounting by inconsistent SZ significantly greater than consistent SZ, who were significantly greater than controls.
As a possibility for the different results of our study and those of MacKillop and Tidey (2011) and Wing et al. (2012) that used the basic Kirby task, it is possible that the Kirby et al. (1999) version of the DD task, which consists of only 27 trials encompassing nine different trial k’s, or only three trials at each trial k, results in a measure of consistency that is less sensitive than our DD task, which presents 12 trials at each trial k.
In the SZ participants, we found a parallel relationship between delay discounting and response consistency. Even in our consistent SZ group, consistency was reduced compared to controls. Moreover, within this patient group, there was a negative correlation between log(k) and R′. Calculated k values of the inconsistent SZ group, although of questionable validity, were substantially higher than those of the other groups. Thus, higher discounting and lower consistency appear to go hand in hand, although the nature of this relationship is not clear.
Concerning previously used measures of consistency and whether we would recommend our R2 over other measures, we think that there is no “gold standard”. As to whether our R2 of >0.60 as the criterion for consistency has clinical significance, it was empirically based on the bimodal distribution of R2 among the patients in our present study, in which there was a clear gap between the values of 0.65 and 0.48, demarcating the two distinct subgroups. (We found a similar distribution when patients were tested in our fMRI study; Avsar et al., 2013.) Also, we document in the current paper that the pattern of responding of the inconsistent SZ was aberrant as compared to the consistent patients and controls, who showed similar patterns despite a modest difference in rate of discounting (Figure 4). Despite the apparent arbitrariness of our R2 criterion, it appeared to have real-world significance in terms of dividing the patient sample into those capable of performing the task and those who could not, possibly because of inattention or some other reason. Thus, our assessment of consistency appeared to appropriately discriminate good vs. poor performers. Also, its use resulted in group comparisons with results very similar to those obtained using previously published criteria (e.g., the Kirby, 2000 method; see above).
4.2 Clinical assessment results in relation to delay discounting
We did not find significant differences between the inconsistent and consistent patient groups on total RBANS or BPRS scores. However, RBANS scores for delayed memory and language were significantly lower in inconsistent patients than in the consistent patients and controls. Thus, deficits in working memory and/or semantic fluency may have contributed to the abnormal responding in the inconsistent group. For example, the task may have been more difficult for them, making it more difficult for them to maintain task engagement. Although there were significant negative correlations between the RBANS delayed memory scale and log(k) in the inconsistent SZ, interpretation is problematic because of concerns about the validity of k in this group.
4.3 Study limitations
A limitation of our study is the small sample size for the inconsistent SZ, but this does not affect the major conclusion of the present study, that consistently performing SZ had greater DD than consistently performing HC. Although the three groups did not differ significantly in self-reported number of cigarettes smoked per day or percentage of smokers and non-smokers, the group differences in percentage of smokers and non-smokers approached significance, and other measures of smoking could have been used that might have revealed group differences in smoking. The latter would be important because of the known relationship between greater delay discounting and smoking (Bickel et al., 1999; Baker et al., 2003). Another possible limitation is that the SZ and HC groups might have differed on IQ, as we did not measure IQ because we believe that assessing parental SES is a better indicator in SZ of premorbid intellectual functioning. Finally, the SZ in this study were on stable doses of antipsychotic medications, although antipsychotic medications have now been shown to not worsen cognitive function (Keefe et al., 2004; Goldberg et al., 2007).
4.4 Conclusions
Compared to controls, individuals with schizophrenia show greater delay discounting, or less willingness to wait for larger but later rewards. Consistent SZ were also less consistent than controls. Greater DD in schizophrenia could stem from several abnormal cognitive or reward related processes. Given our findings of a relationship between DD and consistency in SZ, it is tempting to speculate that inconsistency could be driven by an inability, for any given trial, to keep a mental representation of the choices that were made on previous trials. Indeed, inconsistent patients showed lower scores on delayed memory. Greater DD can be considered a type of deficit in executive function that can result in poor decision making. Even in our fMRI study of DD in SZ and HC who did not differ in mean k or consistency, we found that consistent SZ had reduced activation to DD task versus sensorimotor control trials in executive function, as well as reward, brain areas (Avsar et al., 2013). Impaired decision making in schizophrenia can have grave consequences. Greater delay discounting can contribute to the poor treatment adherence often shown by patients with schizophrenia (Fenton et al., 1997). This outcome underscores the usefulness of the delay discounting task for studying cognitive deficits associated with schizophrenia. In our analysis, the group difference in delay discounting could not be attributed to group differences in smoking–in fact, it was present when non-smoking members of both groups were compared and was found even when patients with suspect high k values were excluded from the comparison.
Inconsistent patients have not been characterized in previous studies of delay discounting in SZ (Heerey et al., 2007; Heerey et al., 2011; MacKillop and Tidey, 2011; Wing et al., 2012). The inconsistent SZ’s aberrant choices on the decision making DD task, as well as their memory and language deficits, suggest that they are a particularly vulnerable subgroup of patients with SZ and might especially benefit from cognitive remediation.
Supplementary Material
Acknowledgments
We want to thank Luke Stoeckel for his assistance in the initial phase of the experiment; Mark Bolding for his assistance in data management and processing; Debbie Lowman for her recruitment expertise; and all our participants who so graciously took part in this project. Acknowledgment of funding: National Institute of Mental Health R01 MH81014 to ACL.
Appendix A. Supporting information
Supplementary material associated with this article can be found in the online version.
Footnotes
5. Author disclosures
Dr. Kathy Burton Avsar, Dr. James Edward Cox, Dr. Rosalyn Eve Weller, Dr. Meredith Amanda Reid and David Matthew White reported no biomedical financial interests or potential conflicts of interest. Dr. Adrienne C. Lahti receives research funding from the University of Alabama Health Services Foundation General Endowment Fund Scholar Award and National Institute of Mental Health R01 MH81014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. 4. APA; Washington, DC: 2000. revised. [Google Scholar]
- Ahn WY, Rass O, Fridberg DJ, Bishara AJ, Forsyth JK, Breier A, Busemeyer JR, Hetrick WP, Bolbecker AR, O’Donnell BF. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. Journal of Abnormal Psychology. 2011;120:911–921. doi: 10.1037/a0023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Avsar KB, Weller RE, Cox JE, Reid MA, White DM, Lahti AC. An fMRI investigation of delay discounting in patients with schizophrenia. Brain and Behavior. 2013;3(4):384–401. doi: 10.1002/brb3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. Journal of Abnormal Psychology. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophrenia Bulletin. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, Weinberger DR. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. The American Journal of Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychological Review. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Crean JP, de Wit H, Richards JB. Reward discounting as a measure of impulsive behavior in a psychiatric outpatient population. Experimental and Clinical Psychopharmacology. 2000;8:155–162. doi: 10.1037//1064-1297.8.2.155. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Research. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophrenia Bulletin. 1997;23 (4):637–651. doi: 10.1093/schbul/23.4.637. [DOI] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophrenia Bulletin. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, Woerner MG, Schooler NR, Kane JM, Robinson DG. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Archives of General Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal discounting in choice between delayed rewards: the role of age and income. Psychology and Aging. 1996;11:79–84. doi: 10.1037//0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biological Psychiatry. 2008;64:62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Matveeva TM, Gold JM. Imagining the future: degraded representations of future rewards and events in schizophrenia. Journal of Abnormal Psychology. 2011;120:483–489. doi: 10.1037/a0021810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cognitive Neuropsychiatry. 2007;12:213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman GM, Gibb SP. Delay discounting in college cigarette chippers. Behavioural Pharmacology. 2006;17:669–679. doi: 10.1097/FBP.0b013e3280116cfe. [DOI] [PubMed] [Google Scholar]
- Howell DC. Statistical Methods for Psychology. 6. Thomson Wadsworth; Belmont, CA: 2007. [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. The American Journal of Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Jensen J, Willeit M, Zipursky RB, Savina I, Smith AJ, Menon M, Crawley AP, Kapur S. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33:473–479. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. An algorithm for identifying nonsystematic delay-discounting data. Experimental and Clinical Psychopharmacology. 2008;16:264–274. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, Knutson B, Kienast T, Gallinat J, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology. 2006a;187:222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006b;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. The American Journal on Addictions. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, Lewine RR, Yurgelun-Todd DA, Gur RC, Tohen M, Tollefson GD, Sanger TM, Lieberman JA. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. The American Journal of Psychiatry. 2004;161:985–995. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kirby KN. Unpublished manuscript. Williams College; Williamstown, MA, USA: 2000. Instructions for inferring discount rates from choices between immediate and delayed rewards. [Google Scholar]
- Kishinevsky F, Cox JE, Murdaugh D, Stoeckel LE, Cook EW, III, Weller RE. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite. 2012;58:582–592. doi: 10.1016/j.appet.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Koch K, Schachtzabel C, Wagner G, Schikora J, Schultz C, Reichenbach JR, Sauer H, Schlosser RG. Altered activation in association with reward-related trial-and-error learning in patients with schizophrenia. NeuroImage. 2010;50:223–232. doi: 10.1016/j.neuroimage.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self-medication hypotheses. Neuroscience and Biobehavioral Reviews. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology. 2011;216:91–99. doi: 10.1007/s00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia Research. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Marco-Pallarés J, Mohammadi B, Samii A, Münte TF. Brain activations reflect individual discount rates in intertemporal choice. Brain Research. 2010;1320:123–129. doi: 10.1016/j.brainres.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Mazur JE, Coe D. Tests of transitivity in choices between fixed and variable reinforcer delays. Journal of the Experimental Analysis of Behavior. 1987;47:287–297. doi: 10.1901/jeab.1987.47-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Lowenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Morris RW, Vercammen A, Lenroot R, Moore L, Langton JM, Short B, Kulkarni J, Curtis J, O’Donnell M, Weickert CS, Weickert TW. Disambiguating ventral striatum fMRI-related bold signal during reward prediction in schizophrenia. Molecular Psychiatry. 2012;17:280–289. doi: 10.1038/mp.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, Jones PB, Bullmore ET, Robbins TW, Fletcher PC. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Molecular Psychiatry. 2007;13, 239:267–276. doi: 10.1038/sj.mp.4002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. The American Journal of Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. Journal of Abnormal Psychology. 2001;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Petry NM, Casarella T. Excessive discounting of delayed rewards in substance abusers with gambling problems. Drug and Alcohol Dependence. 1999;56:25–32. doi: 10.1016/s0376-8716(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. Journal of the Experimental Analysis of Behavior. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Robles E, Huang BE, Simpson PM, McMillan DE. Delay discounting, impulsiveness, and addiction severity in opioid-dependent patients. Journal of Substance Abuse Treatment. 2011;41:354–362. doi: 10.1016/j.jsat.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Mata R, Radu PT, Ballard IC, Carstensen LL, McClure SM. Age differences in striatal delay sensitivity during intertemporal choice in healthy adults. Frontiers in Neuroscience. 2011;5:126. doi: 10.3389/fnins.2011.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf F, Juckel G, Koslowski M, Kahnt T, Knutson B, Dembler T, Kienast T, Gallinat J, Wrase J, Heinz A. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology. 2008;196:673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Sterzer P, Schmack K, Ballmaier M, Rapp M, Wrase J, Juckel G, Gallinat J, Heinz A. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biological Psychiatry. 2009;65:1032–1039. doi: 10.1016/j.biopsych.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Schooler C, Roberts B, Cohen R. Context, complexity, and cognitive processing in schizophrenia. Cognitive Neuropsychiatry. 2008;13:250–266. doi: 10.1080/13546800802058658. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, Deyoung CG, Green AE, Reis DL, Johnson MR, Conway AR, Engle RW, Braver TS, Gray JR. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychological Science. 2008;19:904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Sheffer C, Mackillop J, McGeary J, Landes R, Carter L, Yi R, Jones B, Christensen D, Stitzer M, Jackson L, Bickel W. Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. The American Journal on Addictions. 2012;21:221–232. doi: 10.1111/j.1521-0391.2012.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Kassel M, Gnys M, Zettler-Segal M. Smoking behavior and smoking history of tobacco chippers. Experimental and Clinical Psychopharmacology. 1994;2:126–142. [Google Scholar]
- Shiffman S, Sayette M. Validation of the nicotine dependence scale (NDSS): a criterion-group design contrasting chippers and regular smokers. Drug and Alcohol Dependence. 2005;79:45–52. doi: 10.1016/j.drugalcdep.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, Kaiser S. Neural correlates of reward processing in schizophrenia--relationship to apathy and depression. Schizophrenia Research. 2010;118:154–161. doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK, Budney AJ. Delay discounting predicts adolescent substance abuse treatment outcome. Experimental and Clinical Psychopharmacology. 2012;20:205–212. doi: 10.1037/a0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Oono H, Inoue T, Boku S, Kako Y, Kitaichi Y, Kusumi I, Masui T, Nakagawa S, Suzuki K, Tanaka T, Koyama T, Radford MH. Depressive patients are more impulsive and inconsistent in intertemporal choice behavior for monetary gain and loss than healthy subjects--an analysis based on Tsallis’ statistics. Neuroendocrinology Letters. 2008;29:351–358. [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cerebral Cortex. 2007;17(Suppl 1):i171–181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychopharmacology. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Walter H, Kammerer H, Frasch K, Spitzer M, Abler B. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology. 2009;206:121–132. doi: 10.1007/s00213-009-1586-4. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Ross TJ, Kurup PK, Salmeron BJ, Rose EJ, Gold JM, Stein EA. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology. 2010;35:2427–2439. doi: 10.1038/npp.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RE, Cook EW, 3rd, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51:563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Wing VC, Moss TG, Rabin RA, George TP. Effects of cigarette smoking status on delay discounting in schizophrenia and healthy controls. Addictive Behaviors. 2012;37:67–72. doi: 10.1016/j.addbeh.2011.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.