Abstract
There is an age-associated reduction in the bone healing activity of bone morphogenetic protein -2 (BMP-2) that is currently addressed by administering higher doses of BMP-2 in elderly patients. The unwanted medical complications from high dose BMP-2 motivated this investigation to determine whether the addition of a low dose of fibroblast growth factor 2 (FGF-2) could enhance the ability of a lower dose of BMP-2 to heal calvarial bone defects in old mice (18-20 months old). FGF-2 (5 ng) and BMP-2 (2 μg) were administered by a controlled release two-phase biomaterial scaffold placed into the bone defect. FGF-2 released more rapidly and completely in vitro than BMP-2 (40% vs 2%). In vivo, both BMP-2 and FGF-2+BMP-2 groups formed more new bone in calvarial defects than scaffold alone (p <0.001) or FGF-2 only groups (p < 0.01). The overall total volume of new bone was not statistically increased by the addition of FGF-2 to BMP-2 as measured by microCT, but the pattern of bone deposition was different. In old mice, but not young, there was enhanced bony fill in the central bone defect area when the BMP-2 was supplemented with FGF-2. Histological analysis of the center of the defect revealed an increased bone volume (%BV/TV (p = 0.004)) from the addition of FGF-2. These studies suggest that combining a low dose of FGF-2 with a low dose of BMP-2 has the potential to increase bone healing in old mice relative to BMP-2 alone.
1. Introduction
As animals age, their potential for repair and regeneration after injury dramatically decreases. One of the organ systems in which this is most apparent is the skeleton. With aging, bone mass declines and so does the ability of the bone to regenerate in response to injury (Aalami et al. 2004; Justesen et al. 2002; Stolzing et al. 2008; Zhang et al. 2004). Bone morphogenetic protein -2 (BMP-2) is an osteogenic protein known to positively affect fracture healing and bone regeneration in both elderly animals and humans (Govender et al. 2002; Howell et al. 1997; Kenley et al. 1994; Yasko et al. 1992). While BMP-2 has been demonstrated to significantly enhance bone regeneration in human patients, the complication profile, likely related to the supraphysiologic dose of BMP-2 delivered in the current formulation (> 40 mg), has lead to safety concerns that now limit its clinical use (Carragee et al. 2011; Glassman et al. 2011). Reported complications include early inflammatory reaction and osteolysis, ectopic bone formation sometimes leading to compression of neural elements, seroma formation and a possible increase in the risk of malignancy (Carragee et al. 2011; Glassman et al. 2011). Thus, there is a need to refine the delivery and improve the efficacy of BMP-2 so that it can be delivered in lower doses with less risk of complications to patients.
The age-associated reduction in osteoblast progenitor cell number, and the decline in proliferation and response to growth factors with age is a major reason for the decreased bone regeneration capability of BMP-2 in the elderly (Bergman et al. 1996; Heersche et al. 1998). Based on the proliferative effects and the synergistic interactions of fibroblast growth factor-2 (FGF-2) with BMP-2 (Canalis et al. 1988; Globus et al. 1988; Kotev-Emeth et al. 2000; Marie et al. 2012; Naganawa et al. 2008) we hypothesized that the addition of FGF-2 would increase the bone healing capability of BMP-2 in old mice. This hypothesis is supported by previous FGF-2 and BMP-2 combination studies in young animals (Fujimura et al. 2002; Hanada et al. 1997; Kakudo et al. 2006; Minamide et al. 2007; Nakamura et al. 2005; Ono et al. 1996; Takita et al. 1997; Wang et al. 2010), post-puberty rats (Tanaka et al. 2006), and in cell culture studies using cells obtained from young and old mice and humans (Fakhry et al. 2005; Kuhn et al. 2013; Luong et al. 2012; Maegawa et al. 2007; Varkey et al. 2006). These studies have shown that successful bone regeneration from the combination of FGF-2 and BMP-2 requires using a low dose of FGF-2, optimizing the ratio of FGF-2 relative to BMP-2, and administering FGF-2 before BMP-2 (Fakhry et al. 2005; Fujimura et al. 2002; Kuhn et al. 2013; Varkey et al. 2006; Wang et al. 2010).
Guided by the previous studies, this study examined if the delivery of combined low doses of FGF-2 and BMP-2 from a two-phase biomaterial scaffold made of collagen/hydroxyapatite (Col-HA) infused with a polyethylene glycol (PEG) hydrogel could enhance calvarial bone defect healing in old mice. This biomaterial combination has successfully been used for the delivery of BMP-2 to achieve bone regeneration in a rat mandible (Wen et al. 2011). FGF-2 has been previously delivered from PEG hydrogels and shown to enhance proliferation of in vitro human mesenchymal stromal cell cultures (King et al. 2010). Given the strong affinity of BMP-2 for hydroxyapatite (Wang et al. 2014) and the weak association of FGF-2 with the PEG hydrogel (King et al. 2010), it was additionally hypothesized that this composite biomaterial would enable the desired biomimetic sequential delivery of FGF-2 followed by BMP-2.
There are only a few studies that have demonstrated a benefit of FGF-2 and BMP-2 co-delivery on bone healing in older animals (Tanaka et al. 2006) or in cell culture using cells from older rodents or humans (Kuhn et al. 2013; Varkey et al. 2006). Notably, the only previous in vivo calvarial defect studies (Tanaka et al. 2006) were conducted in rats of two ages: pre-puberty and post-puberty. Their studies demonstrated a slight positive effect on bone defect healing in the older adult rats from a 25 ng dose of FGF-2 combined with BMP-2, but not in the younger rats. However, as per the guidelines from the National Institutes of Health (NIH) - National Institute on Aging (NIA) (Nadon 2006), to be considered old, rats must be 24 months old (corresponding to a 50% survival age, http://www.nia.nih.gov/research/dab/aged-rodent-colonies-handbook/available-strains). The capability of FGF-2 to increase the bone regeneration capability of BMP-2 in truly advanced age rodents was thus unknown and provided further impetus for the present studies in old BALB/c mice 18-22 months of age.
2.0 Materials and Methods
2.1 Scaffold Preparation
FGF-2 and BMP-2 were delivered into the mouse calvarial defect via a two-phase biomaterial scaffold prepared as described previously (Wen et al. 2011). A 0.5-1 mm thick × 3.5 mm diameter disk of collagen/hydroxyapatite (Col-HA) (Healos™, DePuy, Johnson & Johnson, Raynham, MA) was cut from the as-supplied rectangular block. The Col-HA was wetted with sterile, deionized water and dried before cutting to minimize shrinkage during BMP-2 application. BMP-2 (e. coli expressed non-glycosylated recombinant human BMP-2) was prepared as previously described (Sachse et al. 2005). The lyophilized BMP-2 was reconstituted in 2 ul of sterile deionized water and placed directly on the Col-HA and allowed to dry. Simple adsorption and drying of BMP-2 does not negatively impact the biological activity of BMP-2 (Sachse et al. 2005). FGF-2 was obtained from R&D Systems, Minneapolis, MN (recombinant human FGF basic, 223-FB/CF) and was mixed with 5 μL of PEG hydrogel phase and then infiltrated into the spongy Col-HA scaffold and allowed to set for at least 10 min prior to implantation.
A dose of 2 μg of BMP-2 was selected for these studies based on our previous in vivo BMP-2 dose response studies with this same scaffold (Wen et al. 2011). A dose of 5 ng of FGF-2 was selected for these studies based on a previous study in mice which demonstrated that 1-10 ng of FGF-2 generated the most ectopic bone in a subcutaneous site when combined with 5 μg of BMP-2 (Nakamura et al. 2005).
2.2 FGF-2 and BMP-2 in vitro release studies
In vitro release studies were completed to evaluate the release of FGF-2 from the two-phase scaffold, with and without BMP-2 present. Previous studies had confirmed BMP-2 release from the Col-HA/PEG scaffold used in these studies (Wen et al. 2011). The scaffolds and the FGF-2 and BMP-2 doses tested in vitro were the same as those used in the in vivo studies (5 ng FGF-2, 2 μg BMP-2). Scaffolds with growth factors were placed in 2 ml of cell culture medium (MEM with 10% FBS, 10 mM HEPES, 2 mM L-glutamine, 1% penicillin-streptomycin and 1% non-essential amino acids) and incubated at 37°C to initiate growth factor release. Samples were collected every 24 hrs with complete replacement of the medium until the PEG-hydrogel alone sample was totally degraded on day 15. The samples were frozen immediately and only thawed once prior to analysis to avoid degradation. Repeated freezing and thawing was found to greatly reduce the measurable amount of both growth factors in the samples. To determine if growth factor quantitation was affected by the presence of scaffold degradation products, additional experiments were conducted in which fresh FGF-2 or BMP-2 was spiked into medium that Col-HA/PEG hydrogel scaffold had been incubated in for 12 days at 37°C. The pH was monitored throughout the study and was slightly reduced from pH =7.40 to pH=7.16 over the 12 days. Released growth factors in the supernatants were analyzed with ELISA kits (for BMP-2: AntigeniX America Inc, Huntington Station, NY and for FGF-2: R+D Systems, USA). Each assay was performed with strict adherence to the manufacturer's instructions.
2.3 Mouse Calvarial Defect Model
The study protocol was approved by the Animal Care and Use Committee at the University of Connecticut Health Center prior to initiating the studies. The surgical site was prepared by shaving and disinfecting the exposed skin while the animals were anesthetized by inhalation of 1% isoflurane in O2. Prior to making incisions, each animal received 90 mg/kg ketamine and 9 mg/kg xylazine via intraperitoneal injection. An incision of approximately 1 cm in length was made above the calvaria and the parietal bones were exposed. A circular, full-depth defect of 3.5 mm in diameter was made in the left parietal bone using a trephine drill and care was taken to avoid contact with any of the bone suture lines or the underlying dura. Previous work has demonstrated that adult mice (60 days old) show less that 5% healilng of a calvarial defect as small as 3 mm over an 8-week period when left untreated (Aalami et al. 2004).One defect site per animal was made since our experience has been that adjacent scaffolds with growth factors can influence each other in the calvariae (e.g. cross-over effects). After scaffold placement, the skin incision was closed using resorbable polylactide co-glycolide (90/10) suture material (Vicryl 6-0, Ethicon) attached to a P-1 reverse cutting needle. Mice were given 0.08 mg/kg buprenorphine via intramuscular injection for pain management for three days post-surgery. At the end of the study, mice were euthanized by CO2 asphyxiation to collect calvarial bone defect tissues for analysis. The surrounding soft tissue was removed and the calvariae were harvested and fixed in 10% formalin for 24 hours at 4 °C.
2.4 Animal Study Design
A pilot study to evaluate the effects of FGF-2 and BMP-2 on calvarial bone defect healing was completed using 7 young CD-1 mice (2-4 months of age) and 13 old CD-1 mice (24 months of age) with 3-4 mice per group before completing the main study. The groups tested included control mice with scaffold only, BMP-2 only, FGF-2 only, and test mice with 2 μg of BMP-2 combined with 5 ng of FGF-2. The pilot study was used to determine expected differences between the groups in order to use power analysis to determine the number of mice needed per group in the main study to achieve statistical significance. Based on the power analysis with the pilot data, n=8 observations per treatment were required and used in the main study for the test groups close in performance such as BMP-2 vs FGF-2 and BMP-2 assuming an effect size of 0.5, α= 0.05 and power of 0.90. For the groups with large differences in calvarial bone defect healing, such as scaffold alone (that does not heal) vs BMP-2, or FGF-2 vs BMP-2, power analysis indicated a sample size of 3 or more was adequate. Thus, n=4 mice were used for the scaffold only group and an n=5 was used for the FGF-2 group in the main study thereby refining and reducing unnecessary use of animals. In total, 25 young (3-5 months) and 25 old (18-22 months) female BALB/c mice (obtained from NIH/National Institute on Aging aged rodent colony, Bethesda, MD) were used for the primary study. While 8 mice underwent surgery in each of the BMP-2 or FGF-2+BMP-2 test groups, one mouse from each of these groups (except the young BMP-2 only group) was eliminated from the final analysis due to excessive implant movement out of the defect site that was discovered at the time of euthanasia.
The optimal tissue harvest time point for old mice (i.e. when at least some of the test group defects in old mice had completely healed) was determined through live VIVA CT computed tomography imaging as described below. Based on the lack of substantial bone healing observed at 3 weeks (Appendix/Supplementary Material Figure), the old mice were allowed to heal for a total of five weeks prior to tissue harvest. In young mice, tissues were collected at the standard 4 week time point since healing was complete by that time point.
2.5 Micro-Computed Tomography (microCT) Analysis
Mineralized bone volume within the retrieved calvarial defect sites was quantified using conebeam micro-focus X-ray computed tomography (μCT40, Scanco Medical AG, Bassersdorf, Switzerland). Serial tomographic images were acquired at 55 kV and 145 μA, collecting 1000 projections per rotation at 300 msec integration time on fixed tissue samples prior to decalcification for histology. Three-dimensional 16-bit grayscale images were reconstructed using standard convolution back-projection algorithms with Shepp and Logan filtering, and rendered within a 16.4 mm field of view at a discrete density of 244,141 voxels/mm3 (isometric 16-μm voxels). Segmentation of bone from marrow and soft tissue was performed in conjunction with a constrained Gaussian filter to reduce noise, applying a hydroxyapatite-equivalent density threshold of 530 mg/cm3. Volumetric regions were selected manually by visual inspection of two-dimensional cross sections to include all new bone formation, whether it formed within the circular defect space or extended away from the defect onto the intact calvarial bone surface. Two-dimensional cross sections were also used to determine percent of mice with complete defect fill and to investigate the pattern of bone deposition within the defect. Due to a sample processing error, one third of the young mouse samples were accidentally decalcified for histology prior to microCT analysis and thus fewer data points are available for some of the young mouse groups.
For live imaging of old mice, an in vivo conebeam micro-focus x-ray computed tomography system (VivaCT40; Scanco Medical AG) was used. Anesthesia was induced and maintained using 2-3% isoflurane in oxygen. Serial tomographic images of the skull were acquired at 55 kV and 145 μA, collecting 2000 projections per rotation at 250 msec integration time. Three-dimensional 16-bit grayscale images were reconstructed using standard convolution back-projection algorithms with Shepp and Logan filtering, and rendered within a 38.9 mm field of view at a discrete density of 145,794 voxels/mm3 (isometric 19-μm voxels).
2.6 Histological Analysis
Retrieved, fixed calvariae were decalcified with 10% ethylenediaminetetraacetic acid (EDTA) at pH 7.4 for 2-3 days at 4 °C, with one replacement of the solution. Once decalcified, samples were cut in half along the frontal plane directly through the center of the defect. Calvariae were rinsed twice with 10 mM phosphate buffer and then dehydrated with a graded ethanol series, and stored in 70% ethanol at 4 °C until being processed for paraffin embedding. Calvariae were infiltrated with xylene and both halves were embedded with the cut side down so that the center of the defect was sectioned first and reproducibly in all samples. Six μm thick serial sections were cut with a total of six sections per slide and five slides per sample. Samples were deparaffinized, treated with Histo-Clear™ followed by a series of graded alcohols, rehydrated and stained with hematoxylin and eosin.
2.7 Histological Scoring of Bone Defect Filling and Histomorphometry
For each sample, the six serial sections cut through the center of the defect were examined under a light microscope to assess the extent of complete defect healing resulting in new bone filling the entire defect in the central region. The defect area was viewed and if the new bone was continuous across the entire defect a score of ‘1’ was recorded. Those samples with gaps and incomplete bony fill into the center of the defect bone were scored as ‘0’. Results were obtained from the assessment of three individuals operating in a blinded, non-biased manner. The results were graphed and the percentage of mice with central defect fill was calculated and analyzed for statistical significance.
If differences in the percentage of mice with central defect fill were detected by the histology scoring technique, bone histomorphometric measurements were completed to quantify the differences using the OsteoMeasure (OsteoMetrics Inc., Nashville, TN) software. A 1200 μm × 400 μm rectangle was placed equidistant from the edges of the defect within the region of regenerated tissue for each sample. The size of the rectangle was determined by creating the largest box that would fit the defect region in all sections. The bone tissue within the assigned rectangle was traced and the bone volume as a percentage of total tissue volume (BV/TV) was recorded and reported. The terminology and units used are those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (Dempster et al. 2013; Parfitt et al. 1987)
2.8 Statistical Analysis
Data plotted as bar graphs are presented as mean ± standard error. Statistical analysis of group mean differences was performed using one-way ANOVA with a Bonferroni post-test to conduct multiple comparisons and significance set at p < 0.05. The microCT and histological data for percentage of mice with central defect fill vs partial defect fill was analyzed using the Fisher's Exact Test because it is categorical data.
3.0 Results
3.1 In vitro growth factor release profiles
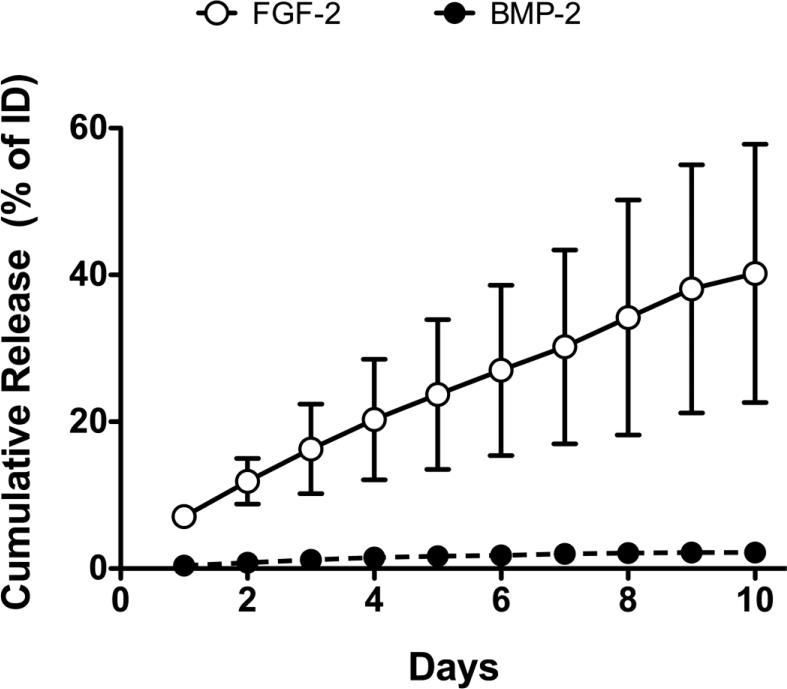
The cumulative in vitro release data of the two growth factors from the Col-HA/PEG hydrogel scaffold versus time are shown in Figure 1. BMP-2 release reached a maximum of 2% of the total loaded dose (2 μg) under these conditions. FGF-2 release was more complete with first order release kinetics and reached a maximum of 40% release at 10 days. When 250 pg/ml fresh FGF-2 was spiked into the medium that had been incubated with Col-HA/PEG for 12 days and measured after 2 hrs, only 83.8 ± 11.6 pg/ml (or 33% of the total dose) was detected using ELISA suggesting that the majority of the FGF-2 could bind to the material degradation products and render it unmeasurable. This result was not used to adjust the ELISA values shown in Figure 1. There was no effect from the scaffold degradation products on the BMP-2 ELISA measurements.
Fig. 1.
In vitro release of FGF-2 and BMP-2 from the controlled release Col/HA/PEG hydrogel scaffold. Cumulative release is shown as a function of days for both growth factors as a percentage release relative to initial dose (ID) loaded. FGF-2 was loaded in the PEG hydrogel and released more rapidly and completely than the BMP-2 that was directly bound to the collagen/hydroxyapatite. Open circle: FGF-2, filled circle: BMP-2.
3.2 Micro-CT analysis of FGF-2 and BMP-2 effects on Calvarial Bone Defect Healing
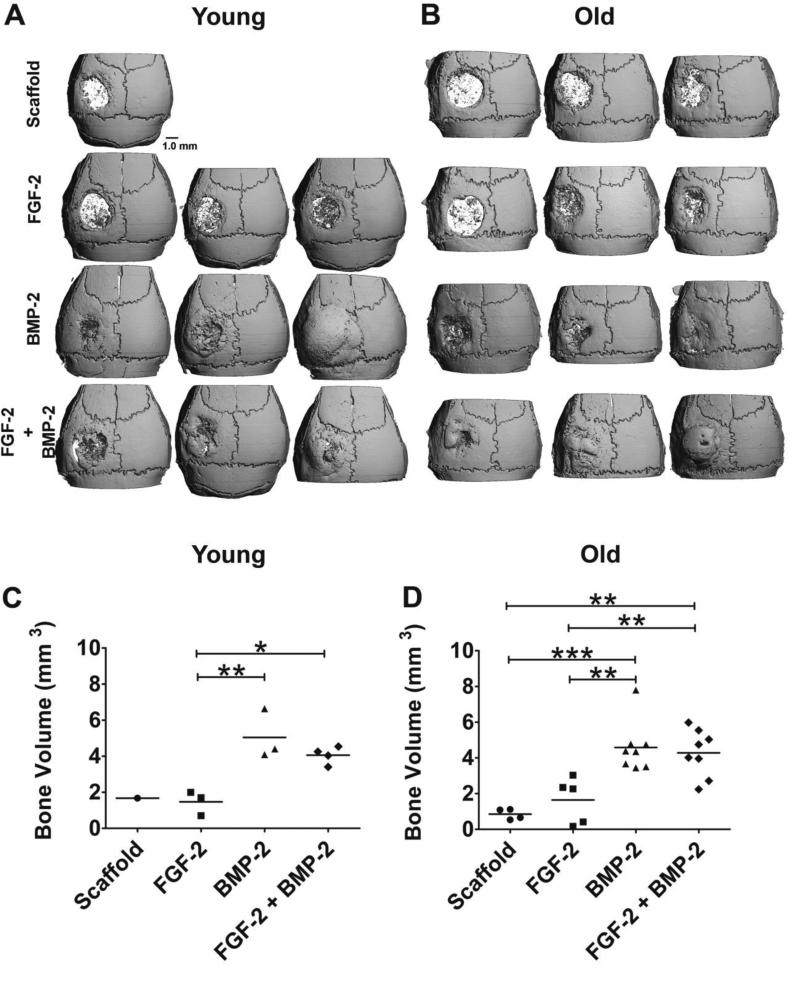
Representative 3-D microCT reconstructions of the calvarial bones of each test group for both age groups are shown in Figure 2 A, B. At this threshold setting, the Col/HA implant is radiolucent as compared to bone enabling a clear visualization of the extent of bone healing within the calvarial bone defect. Representative images were selected to show the range of healing observed. At this dose of BMP-2 complete defect healing was sometimes observed in the BMP-2 only group in both young and old animals. Statistical analysis of the bone volume determined by microCT demonstrated that bone healing in both young and old mice was significantly increased when BMP-2 or FGF-2+BMP-2 was administered relative to FGF-2 or scaffold alone (Fig. 2 C, D). In young mice, both the bone volume from BMP-2 alone (5.0 ± 0.8 mm3) and FGF-2+BMP-2 (4.1 ± 0.2 mm3) groups were statistically greater than FGF-2 only or scaffold only groups (p < 0.01 and p < 0.05 respectively). In old mice, the bone volume in the BMP-2 only group (4.6 ± 0.5 mm3) and the FGF-2+BMP-2 (4.3 ± 0.5 mm3) group was also statistically greater than scaffold alone (0.8 ± 0.1 mm3) (p <0.001) or FGF-2 alone treatments (1.6 ± 0.6 mm3) (p < 0.01). MicroCT measurements of total new bone volume within and around the defect did not reveal any significant increase due to the addition of FGF-2 to BMP-2 as compared to BMP-2 only in both young and old mice.
Fig. 2.
Three-D microCT reconstructions of mouse calvarial bones from young (A) and old (B) mice after treatment with: control scaffold with no growth factors (scaffold), FGF-2 (5 ng), BMP-2 (2 μg), or both factors in combination (FGF-2+BMP-2). Three representative images were selected from each group to illustrate the range of healing observed (and are shown in this order: worse, mid-range, best). MicroCT data for young animals was only obtained for two thirds of the animals in the study due to accidental decalcification during processing. MicroCT quantitation of total new bone volume within and adjacent to the bone defect: (C) young mice and (D) old mice. New bone volume was greatest in the BMP-2 and FGF-2+BMP-2 groups in both young and old test groups. No statistical difference was detected between the microCT data for FGF-2 + BMP-2 group relative to BMP-2 only in either young or old mice. * = p ≤ 0.05; ** = p ≤ 0.01; *** = p ≤ 0.001.
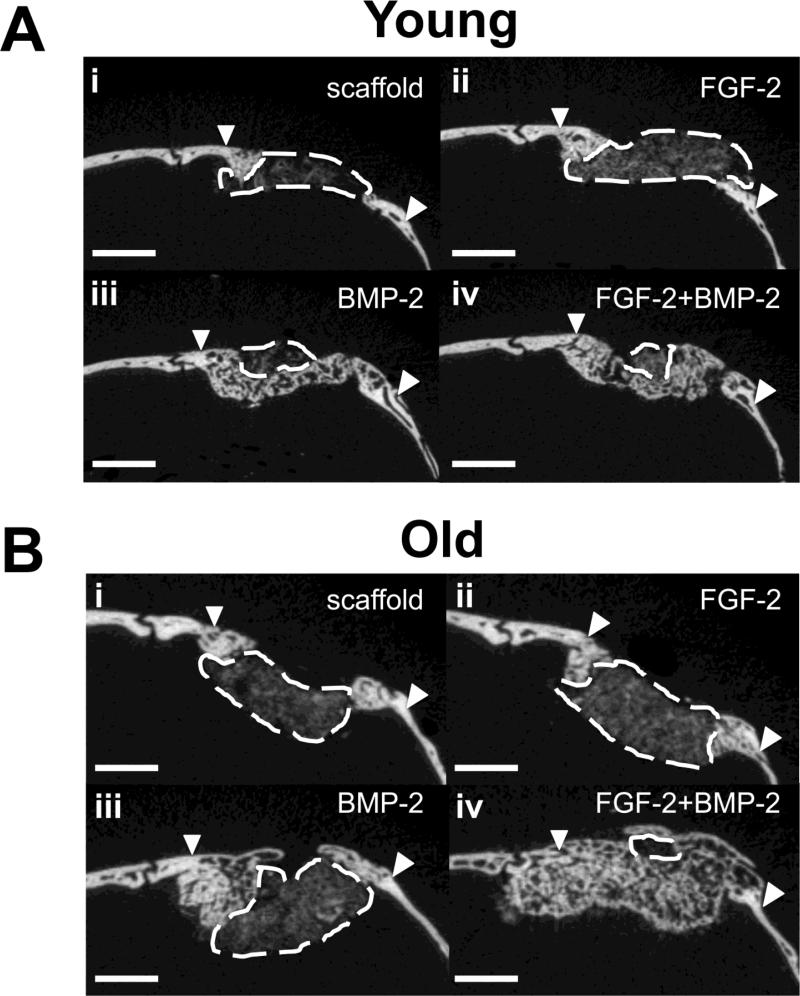
An examination of the 2-D microCT cross-sections of the calvarial bone defects revealed a subtle effect of FGF-2 on the extent of healing in the central bone defect area in old mice treated with FGF-2 and BMP-2, but not the young mice (Fig. 3). The 2-D cross sections of calvarial defects shown in Fig. 3 are taken from the scans of same young and old mice shown in Fig. 2 representing the mid-range of healing. At this dose of BMP-2, some defects were completely filled with new bone, so the examination of 2-D cross sections focused on the mice with incomplete healing. At this threshold setting, the porous structure of the Col-HA/PEG scaffold can be visualized by microCT, but still discriminated from bone. The area of the Col-HA/PEG scaffold that was not infiltrated with new bone is outlined with a dashed white line. As seen in Fig. 3 A i and ii, large amounts of scaffold remained unfilled with new bone in the control scaffold group and the FGF-2 group in young mice. The scaffolds containing BMP-2 were nearly completely filled with new bone in the young mice and addition of FGF-2 did not increase bone defect healing (Fig. 3 A iii, iv). In the old mice, a large amount of scaffold remained unfilled with new bone in the central defect area of both the scaffold control group and FGF-2-only group, (Fig. 3 B i, ii). Addition of FGF-2 to BMP-2 in old mice had a positive effect and led to more complete defect filling as shown in Fig. 3 B iii, iv. From examination of all of the 2-D microCT cross sections from old mice treated with BMP-2, it was observed that new bone did not completely span the entire calvarial defect in 6 out of 7 old mice. In the old mice treated with FGF-2+BMP-2, the addition of FGF-2 reduced the number of incomplete defect fills to 3 out of 7. These differences were not statistically significant according to Fisher's Exact Test for categorical data due to a low sample number.
Fig. 3.
Two-dimensional microCT scans of young (A) and old (B) mouse calvarial defects with non-healed defect areas outlined shows a difference in the pattern of bone regeneration when FGF-2 was added to BMP-2: (A i) scaffold only without growth factors, (A ii) 5 ng FGF-2, (C) 2 μg BMP-2, (D) 5 ng FGF-2 + 2 μ g BMP-2. The area of defect that is not filled with bone is outlined in dashed white lines. Arrows point to the original defect edges. The addition of FGF-2 to BMP-2 increased the amount of new bone formation in the center of the defect leading to more complete defect fill. Scale bar = 1 mm.
3.3 Histological Assessment of Calvarial Bone Defect Healing
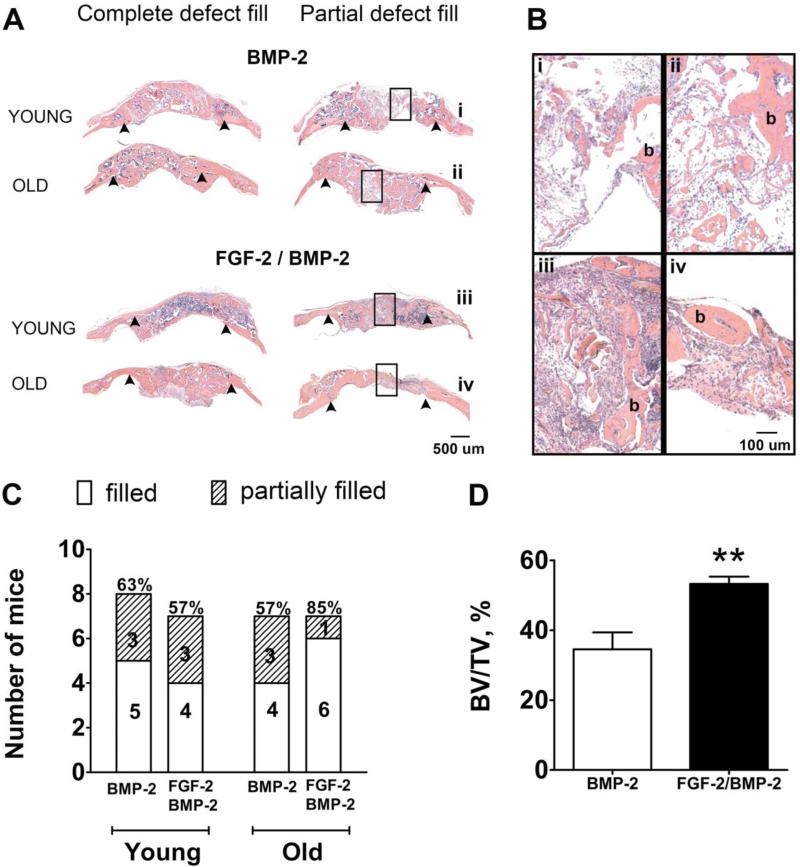
To investigate differences in bone defect healing between test groups, H+E stained histological sections of the bone defects taken from the mid-section of the defect were analyzed and scored in a binary manner based on whether the center of the defect was filled with new bone (scored as 1) or not completely filled (scored as 0). Fig. 4 A shows histology images of completely filled defects and partially filled defects for BMP-2 alone and FGF-2+BMP-2 groups in both young and old mice. Higher magnifications of the bone discontinuities present in the partially filled defects are shown in Fig. 4 B which clearly demonstrate lack of continuous bone (marked with “b”) in the center region of the defects in these animals. Incomplete healing in some mice was also observed in the microCT images (Fig. 2 and Fig. 3). The percentage of mice with new bone completely spanning the central area of the defect was calculated for each group based on 1 vs 0 histological scoring. In young mice, 63% (5/8) of the mice that received BMP-2 treatment alone had new bone spanning the defect center and this value did not increase with addition of FGF-2 (4/7, 57%) (Fig. 4 C). In old mice, 57% (4/7) of the mice had new bone spanning the defect center when treated with BMP-2 alone (Fig. 4 C). Adding FGF-2 increased the percentage of old mice with healed central defects to 85% (6/7). This was not a significant outcome according to Fisher's Exact test for categorical data, but was very similar to microCT analysis and motivated the use of standard quantification techniques: bone histomorphometry.
Fig. 4.
Histological analysis of mouse calvarial bone defect healing. (A) Representative decalcified H+E stained tissue cross-sections taken through the center of the defect for the BMP-2 and FGF-2+BMP-2 groups (shown for both young and old mice). The left column shows examples of defects that are completely healed and filled with new bone and the right column shows examples of partial bone defect fill. Arrowheads mark the edges of the original defect. Rectangular boxes in the center of the defect identify the higher magnification images shown in panel (B). (B) Magnified histology images that verify incomplete fill: discontinuous islands of new bone (marked with “b”) are found in the central area of the partially filled defects. (C) A comparison of the number of mice with completely filled central defect areas vs those that were partially filled for the BMP-2 group and the FGF-2+BMP-2 group (p = 0.55). One mouse was excluded from each of these groups, except the young BMP-2 group, due to movement of the scaffold out of the defect. (D) Histomorphometric calculations of percent bone volume per total volume (BV/TV) in the central region of the defect area in old mice showing an increase in the mice treated with FGF-2 and BMP-2 compared to BMP-2 alone. ** = p ≤ 0.01.
To further quantify the observed difference in bone defect filling that was seen in the old mice only, the percent of bone volume to total volume (BV/TV) in the central defect area of old mice was quantified using bone histomorphometry. BV/TV increased significantly in old mice from 35 ± 5 % when treated with BMP-2 alone to 53 ± 2 % with co-administration of FGF-2+BMP-2 (p = 0.004) (Fig. 4 D). Histomorphometry calculations were not completed for the young mice because no positive effect was observed from the addition of FGF-2 in either the microCT images or the histological sections.
4.0 Discussion
These studies suggest that controlled delivery of FGF-2 in combination with a low dose of BMP-2 can improve old mouse calvarial bone defect healing as compared to treatment with BMP-2 alone. In old mice, the addition of FGF-2 significantly increased the extent of central defect bone fill (% BV/TV) as shown through histological measurements, leading to better bone healing than BMP-2 alone, or FGF-2 alone (p = 0.004). The enhancement of BMP-2 associated bone healing in old mice by the combination of a low dose of FGF-2 with BMP-2 is an important finding because it offers a strategy to reduce the high concentrations of BMP-2 typically used clinically for bone regeneration in the elderly. High doses of BMP-2 are associated with a number of negative side effects that motivated the present study (Carragee et al. 2011; Pradhanet al. 2006; Zara et al. 2011). The use of a combination of low doses of multiple growth factors, such as FGF-2 and BMP-2, is one way to overcome the need for a higher dose of a single bone growth factor.
In contrast to the positive effects of FGF-2 addition in old mice, calvarial bone defect healing by BMP-2 was not enhanced in young mice by FGF-2 administration at these doses. In young mice, new bone had completely spanned the defects by 4 weeks at this dose of BMP-2 in nearly every mouse; however, in old mice additional time was required and still only one old mouse had complete defect healing. This is consistent with previous studies in old rats (Hak et al. 2006). Similar calvarial bone defect studies with FGF-2 and BMP-2 have been conducted in rats that were 8 weeks (pre-puberty) or 16 weeks (post-puberty) old (Tanaka et al. 2006). They tested a range of FGF-2 doses (0, 25, and 250 ng) with a BMP-2 dose approximately twice that of the present study. All of the rat defects in all of the groups they tested were healed at the 4 week time point due to the use of a non-critical defect size and higher BMP-2 dose. However, they also observed a subtle positive effect of FGF-2: the new bone density was slightly increased with FGF-2 in the older rats, but not the young rats. The similarities between our results with old mice and their studies in adult post-puberty rats, further supports the use of FGF-2 as an anti-aging strategy.
In the present studies, microCT and histological analysis were used to quantify newly formed bone within the defect. The total bone volume determined by microCT analysis was not significantly increased from the addition of FGF-2 to BMP-2 in both young and old mice. This was most likely due to the inclusion in the analysis of the larger bone callous formed in the BMP-2 only-treated defects that is especially prominent at the edges of the defect that sometimes extends backwards onto original calvarial bone (Fig. 3). It is also due to the fact that BMP-2 at this dose was sufficient to heal the bone defects in some of the mice, but not all. Examination of 2-D microCT cross sections showed qualitatively that FGF-2 increased the ability of new bone formation in the center of the defect (Fig. 3). Likewise, histological analysis which was focused on quantifying healing in the central region of the defect could distinguish the benefit of FGF-2 on increased central bone defect fill. The pattern of bone deposition was different with FGF-2. FGF-2 combined with BMP-2 stimulated bone formation throughout the defect in contrast to the more restricted bone formation on the edges and defect periphery in the BMP-2 only group. Histologic evaluation of woven and mature bone with characteristic osteoblasts lining their surfaces provides a more accurate measurement of newly formed bone than microCT which relies on density measurements, but has the advantage of evaluating healing throughout the whole defect region.
The mechanism behind the FGF-2 associated bone defect healing is likely due to proliferative effects of FGF-2 on bone progenitor cells (Chen et al. 2004) and because of synergistic signaling mechanisms of FGF-2 with BMP-2 (Farhadi et al. 2005; Nakamura et al. 2005; Singhatanadgit et al. 2006). In our previous in vitro studies with cells derived from both calvaria and long bones of old mice and from osteoblast cultures from aging humans, the combination of a low dose of FGF-2+BMP-2 was able to increase cell proliferation, maintain mesenchymal progenitor marker expression, and enhance mineralization (Kuhn et al. 2013; Ouet al. 2010). FGF-2 has also been shown by itself to stimulate proliferation in vivo during fracture healing (Behr et al. 2010; Chen et al. 2004; Kodama et al. 2009). FGF-2 caused an increase in proliferating cell nuclear antigen (PCNA) staining at the fracture site up to 2 weeks post-fracture in the metaphyseal region of rabbit tibiae (Chen et al. 2004). In another study, FGF-2 increased PCNA staining in mesenchymal progenitor cells, as well as upregulation of BMP-2 expression at 7, and 14 days after implantation of FGF-2 loaded gelatin hydrogel under the maxillary periosteum (Kodama et al. 2009). The increased central bone defect fill seen in the old mice with FGF-2 addition to BMP-2 may thus be due in large part to these proliferative effects of FGF-2 on the slowed, aged progenitor cells in the old mice. Increased proliferation would lead to more robust cellular infiltration throughout the scaffold and subsequent larger regions of osteoblast differentiation and matrix mineralization which was observed in this study.
Another reason for the positive effects from exogenous administration of FGF-2 in old mice seen in this study may be due the effect of FGF-2 on BMP-2 receptors and signaling that are impaired in older mice. In Fgf2 knockout mice that resemble an aging phenotype, both Fgf2 −/− calvarial osteoblasts and bone marrow stromal cells showed an impaired ability to respond to BMP-2, as measured by alkaline phosphatase activity and Runx2 accumulation and nuclear localization (Naganawa et al. 2008). This inability to respond was corrected when cells were transfected with an Fgf2 construct suggesting that FGF-2 plays a critical role in the maximal response to BMP-2 in bone (Naganawa et al. 2008) and provides a rationale for continued studies with these two factors. FGF-2 also increases BMP-2 levels and BMP-2 receptor expression, which would increase cellular responses to the addition of BMP-2 (Farhadi et al. 2005; Singhatanadgit et al. 2006). FGF-2 led to an increase of BMPR-1B expression in muscle-derived primary cell cultures in a BMP-2 induced ectopic bone formation model (Nakamura et al. 2005). The possible mechanisms of the enhanced bone defect healing in old mice by FGF-2 are therefore: (a) an increase in the population of progenitor cells throughout the defect due to proliferative effects of FGF-2, (b) an increase in differentiation of progenitor cells into mineralizing osteoblasts in response to a low dose of BMP-2 because of increased BMP-2 receptors and their own production of BMP-2 due to FGF-2.
The literature paradoxically contains several reports of a negative effect of FGF-2 on BMP-2 stimulated bone healing, although in most cases there appears to be a correlation between high doses of FGF-2 and negative bone healing outcomes(Fujimura et al. 2002; Nakamura et al. 2005; Takita et al. 1997). In the studies with negative outcomes, the growth factors were delivered from single phase carrier made of Type I collagen, gelatin or calcium phosphate or PLA/PGA incapable of biomimetic sequential delivery of FGF-2 followed by BMP-2 (Fujimura et al. 2002; Hanada et al. 1997; Nakamura et al. 2005; Ono et al. 1996; Takita et al. 1997; Tanaka et al. 2006). The controlled release biomaterial scaffold used in the present study was designed so that FGF-2 was released earlier due to its incorporation in the quickly degrading PEG hydrogel phase and this was confirmed by the in vitro release studies (Fig. 1). The majority of the BMP-2 was retained for long periods on the Col-HA scaffold requiring close cell proximity for osteogenic induction. While the in vitro release studies showed that up to 40% of the initially loaded FGF-2 dose was released, the remaining 60% of FGF-2 could have been rendered non-measurable or inactive due to the PEG hydrogel degradation product interference discovered during ELISA testing of spiked release medium. The incomplete FGF-2 release could also be due, in part, to the adsorption of the FGF-2 on the Col-HA scaffold rather than outwards diffusion since both FGF-2 and BMP-2 have a high affinity to HA (JeonJang 2009; Luong et al. 2012). In any event, to further maximize the positive effects of low dose FGF-2 on BMP-2 associated bone repair in old mice, alternate delivery systems should be investigated that are capable of distinct, step-wise sequential release. Recently, biomimetic delayed in vivo release of BMP-2 relative to FGF-2 was achieved with a mixture of colloidal gelatin gels with different cross-linking densities (van der Stok et al. 2013; Wang et al. 2013). The most effective femoral defect bridging in rats occurred in their studies with a BMP-2/FGF-2 ratio of 5:1 and the doses were 3 ug BMP-2 and 600 ng FGF-2 (van der Stok et al. 2013). This is more than a 100x higher dose of FGF-2 than that tested in the present study.
In summary, these studies show a modest, positive effect from the combination of a low dose of FGF-2 (5 ng) with 2 μg of BMP-2 on calvarial bone defect healing in old mice when delivered from a bi-phasic Col-HA/PEG hydrogel. The BMP-2 dose used in these studies was slightly too high and led to complete healing in a few of the old mice and nearly all of the young mice thereby making it difficult to demonstrate improvement due to FGF-2 on total bone volume. However, analysis of 2-D cross sections revealed areas of residual scaffold that did not contain new bone in the BMP-2 samples without FGF-2. Addition of FGF-2 enhanced the central bone defect filling. So while total bone volume was not statistically increased by FGF-2, the pattern of deposition became more favorable. Based on the known proliferative effects of FGF-2 and its synergistic reaction with BMP-2, the likely mechanism for the enhanced central defect filling is due to an increased number of progenitor cells that more robustly infiltrated the scaffold and were more effectively differentiated. The inactivation of FGF-2 by the Col-HA/PEG hydrogel scaffold degradation products and lack of sequential delivery is a concern and should be addressed by future studies with alternate delivery systems.
Supplementary Material
Highlights.
Staged delivery of FGF-2 and BMP-2 via a composite scaffold.
Low dose FGF-2+BMP-2 increased calvarial bone defect repair in old mice.
FGF-2+BMP-2 did not improve bone repair in young mice as compared to BMP-2 alone.
Acknowledgments
Research reported in this publication was supported by the International Team for Implantology (ITI; Basel, Switzerland) grant # 348/2004 and the National Institute Of Dental & Craniofacial Research of the National Institutes of Health under award number R01DE021103. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Dr. Qin Amy Wang, Dr. Jin Pan, and Dr. Roberta Kelm for assistance in conducting animal surgeries. We would also like to acknowledge Dr. Doug Adams and the MicroCT Imaging Facility at the UConn Health Center for the microCT imaging and Dr. James Grady, UCONN Health Center for help with the statistical analysis. The non-glycosylated human recombinant BMP-2 was kindly provided by Dr. Peter Hortschansky from the Hans Knöll Institute, Jena, Germany and the PEG-hydrogel kindly provided by Institut Straumann.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure statement
No competing financial interests exist.
References
- Aalami OO, Nacamuli RP, Lenton KA, Cowan CM, Fang TD, Fong KD, Shi YY, Song HM, Sahar DE, Longaker MT. Applications of a mouse model of calvarial healing: differences in regenerative abilities of juveniles and adults. Plast Reconstr Surg. 2004;114:713–720. doi: 10.1097/01.prs.0000131016.12754.30. [DOI] [PubMed] [Google Scholar]
- Behr B, Panetta NJ, Longaker MT, Quarto N. Different endogenous threshold levels of Fibroblast Growth Factor-ligands determine the healing potential of frontal and parietal bones. Bone. 2010;47:281–294. doi: 10.1016/j.bone.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ. Age-related changes in osteogenic stem cells in mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1996;11:568–577. doi: 10.1002/jbmr.5650110504. [DOI] [PubMed] [Google Scholar]
- Canalis E, Centrella M, McCarthy T. Effects of basic fibroblast growth factor on bone formation in vitro. The Journal of clinical investigation. 1988;81:1572–1577. doi: 10.1172/JCI113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. The spine journal : official journal of the North American Spine Society. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Chen W-J, Jingushi S, Aoyama I, Anzai J, Hirata G, Tamura M, Iwamoto Y. Effects of FGF-2 on metaphyseal fracture repair in rabbit tibiae. Journal of Bone and Mineral Metabolism. 2004;22:303–309. doi: 10.1007/s00774-003-0487-6. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. Journal of Bone and Mineral Research. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhry A, Ratisoontorn C, Vedhachalam C, Salhab I, Koyama E, Leboy P, Pacifici M, Kirschner RE, Nah HD. Effects of FGF-2/-9 in calvarial bone cell cultures: differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential. Bone. 2005;36:254–266. doi: 10.1016/j.bone.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Farhadi J, Jaquiery C, Barbero A, Jakob M, Schaeren S, Pierer G, Heberer M, Martin I. Differentiation-dependent up-regulation of BMP-2, TGF-beta1, and VEGF expression by FGF-2 in human bone marrow stromal cells. Plast Reconstr Surg. 2005;116:1379–1386. doi: 10.1097/01.prs.0000182355.67397.5a. [DOI] [PubMed] [Google Scholar]
- Fujimura K, Bessho K, Okubo Y, Kusumoto K, Segami N, Iizuka T. The effect of fibroblast growth factor-2 on the osteoinductive activity of recombinant human bone morphogenetic protein-2 in rat muscle. Archives of oral biology. 2002;47:577–584. doi: 10.1016/s0003-9969(02)00046-8. [DOI] [PubMed] [Google Scholar]
- Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine. 2011;36:1849–1854. doi: 10.1097/BRS.0b013e3181d133d0. [DOI] [PubMed] [Google Scholar]
- Globus RK, Patterson-Buckendahl P, Gospodarowicz D. Regulation of bovine bone cell proliferation by fibroblast growth factor and transforming growth factor beta. Endocrinology. 1988;123:98–105. doi: 10.1210/endo-123-1-98. [DOI] [PubMed] [Google Scholar]
- Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Hak DJ, Makino T, Niikura T, Hazelwood SJ, Curtiss S, Reddi AH. Recombinant human BMP-7 effectively prevents non-union in both young and old rats. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2006;24:11–20. doi: 10.1002/jor.20022. [DOI] [PubMed] [Google Scholar]
- Hanada K, Dennis JE, Caplan AI. Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1997;12:1606–1614. doi: 10.1359/jbmr.1997.12.10.1606. [DOI] [PubMed] [Google Scholar]
- Heersche JN, Bellows CG, Ishida Y. The decrease in bone mass associated with aging and menopause. The Journal of prosthetic dentistry. 1998;79:14–16. doi: 10.1016/s0022-3913(98)70187-8. [DOI] [PubMed] [Google Scholar]
- Howell TH, Fiorellini J, Jones A, Alder M, Nummikoski P, Lazaro M, Lilly L, Cochran D. A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int J Periodontics Restorative Dent. 1997;17:124–139. [PubMed] [Google Scholar]
- Jeon E, Jang JH. Protein engineering of a fibroblast growth factor 2 protein for targeting to bone mineral hydroxyapatite. Protein and peptide letters. 2009;16:664–667. doi: 10.2174/092986609788490267. [DOI] [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Eriksen EF, Kassem M. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int. 2002;71:36–44. doi: 10.1007/s00223-001-2059-x. [DOI] [PubMed] [Google Scholar]
- Kakudo N, Kusumoto K, Kuro A, Ogawa Y. Effect of recombinant human fibroblast growth factor-2 on intramuscular ectopic osteoinduction by recombinant human bone morphogenetic protein-2 in rats. Wound Repair and Regeneration. 2006;14:336–342. doi: 10.1111/j.1743-6109.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- Kenley R, Marden L, Turek T, Jin L, Ron E, Hollinger JO. Osseous regeneration in the rat calvarium using novel delivery systems for recombinant human bone morphogenetic protein-2 (rhBMP-2). J Biomed Mater Res. 1994;28:1139–1147. doi: 10.1002/jbm.820281004. [DOI] [PubMed] [Google Scholar]
- King WJ, Jongpaiboonkit L, Murphy WL. Influence of FGF2 and PEG hydrogel matrix properties on hMSC viability and spreading. Journal of biomedical materials research Part A. 2010;93:1110–1123. doi: 10.1002/jbm.a.32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama N, Nagata M, Tabata Y, Ozeki M, Ninomiya T, Takagi R. A local bone anabolic effect of rhFGF2-impregnated gelatin hydrogel by promoting cell proliferation and coordinating osteoblastic differentiation. Bone. 2009;44:699–707. doi: 10.1016/j.bone.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Kotev-Emeth S, Savion N, Pri-chen S, Pitaru S. Effect of maturation on the osteogenic response of cultured stromal bone marrow cells to basic fibroblast growth factor. Bone. 2000;27:777–783. doi: 10.1016/s8756-3282(00)00389-6. [DOI] [PubMed] [Google Scholar]
- Kuhn LT, Ou G, Charles L, Hurley MM, Rodner CM, Gronowicz G. Fibroblast growth factor-2 and bone morphogenetic protein-2 have a synergistic stimulatory effect on bone formation in cell cultures from elderly mouse and human bone. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68:1170–1180. doi: 10.1093/gerona/glt018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong LN, Ramaswamy J, Kohn DH. Effects of osteogenic growth factors on bone marrow stromal cell differentiation in a mineral-based delivery system. Biomaterials. 2012;33:283–294. doi: 10.1016/j.biomaterials.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegawa N, Kawamura K, Hirose M, Yajima H, Takakura Y, Ohgushi H. Enhancement of osteoblastic differentiation of mesenchymal stromal cells cultured by selective combination of bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-2 (FGF-2). J Tissue Eng Regen Med. 2007;1:306–313. doi: 10.1002/term.41. [DOI] [PubMed] [Google Scholar]
- Marie PJ, Miraoui H, Severe N. FGF/FGFR signaling in bone formation: progress and perspectives. Growth Factors. 2012;30:117–123. doi: 10.3109/08977194.2012.656761. [DOI] [PubMed] [Google Scholar]
- Minamide A, Yoshida M, Kawakami M, Okada M, Enyo Y, Hashizume H, Boden SD. The effects of bone morphogenetic protein and basic fibroblast growth factor on cultured mesenchymal stem cells for spine fusion. Spine. 2007;32:1067–1071. doi: 10.1097/01.brs.0000261626.32999.8a. [DOI] [PubMed] [Google Scholar]
- Nadon NL. Exploiting the rodent model for studies on the pharmacology of lifespan extension. Aging cell. 2006;5:9–15. doi: 10.1111/j.1474-9726.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- Naganawa T, Xiao L, Coffin JD, Doetschman T, Sabbieti MG, Agas D, Hurley MM. Reduced expression and function of bone morphogenetic protein-2 in bones of Fgf2 null mice. J Cell Biochem. 2008;103:1975–1988. doi: 10.1002/jcb.21589. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Tensho K, Nakaya H, Nawata M, Okabe T, Wakitani S. Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone. 2005;36:399–407. doi: 10.1016/j.bone.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Ono I, Tateshita T, Takita H, Kuboki Y. Promotion of the osteogenetic activity of recombinant human bone morphogenetic protein by basic fibroblast growth factor. J Craniofac Surg. 1996;7:418–425. doi: 10.1097/00001665-199611000-00006. [DOI] [PubMed] [Google Scholar]
- Ou G, Charles L, Matton S, Rodner C, Hurley M, Kuhn L, Gronowicz G. Fibroblast growth factor-2 stimulates the proliferation of mesenchyme-derived progenitor cells from aging mouse and human bone. The journals of gerontology Series A, Biological sciences and medical sciences. 2010;65:1051–1059. doi: 10.1093/gerona/glq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units: Report of the ASBMR histomorphometry nomenclature committee. Journal of Bone and Mineral Research. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Pradhan BB, Bae HW, Dawson EG, Patel VV, Delamarter RB. Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine. 2006;31:E277–284. doi: 10.1097/01.brs.0000216442.12092.01. [DOI] [PubMed] [Google Scholar]
- Sachse A, Wagner A, Keller M, Wagner O, Wetzel WD, Layher F, Venbrocks RA, Hortschansky P, Pietraszczyk M, Wiederanders B, Hempel HJ, Bossert J, Horn J, Schmuck K, Mollenhauer J. Osteointegration of hydroxyapatite-titanium implants coated with nonglycosylated recombinant human bone morphogenetic protein-2 (BMP-2) in aged sheep. Bone. 2005;37:699–710. doi: 10.1016/j.bone.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Singhatanadgit W, Salih V, Olsen I. Up-regulation of bone morphogenetic protein receptor IB by growth factors enhances BMP-2-induced human bone cell functions. Journal of Cellular Physiology. 2006;209:912–922. doi: 10.1002/jcp.20799. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Takita H, Tsuruga E, Ono I, Kuboki Y. Enhancement by bFGF of osteogenesis induced by rhBMP-2 in rats. Eur J Oral Sci. 1997;105:588–592. doi: 10.1111/j.1600-0722.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Ishino Y, Sasaki A, Hasegawa T, Watanabe M, Dalla-Bona DA, Yamano E, van Eijden TM, Tanne K. Fibroblast growth factor-2 augments recombinant human bone morphogenetic protein-2-induced osteoinductive activity. Ann Biomed Eng. 2006;34:717–725. doi: 10.1007/s10439-006-9092-x. [DOI] [PubMed] [Google Scholar]
- van der Stok J, Wang H, Amin Yavari S, Siebelt M, Sandker M, Waarsing JH, Verhaar JA, Jahr H, Zadpoor AA, Leeuwenburgh SC, Weinans H. Enhanced bone regeneration of cortical segmental bone defects using porous titanium scaffolds incorporated with colloidal gelatin gels for time- and dose-controlled delivery of dual growth factors. Tissue engineering Part A. 2013;19:2605–2614. doi: 10.1089/ten.TEA.2013.0181. [DOI] [PubMed] [Google Scholar]
- Varkey M, Kucharski C, Haque T, Sebald W, Uludag H. In vitro osteogenic response of rat bone marrow cells to bFGF and BMP-2 treatments. Clinical orthopaedics and related research. 2006;443:113–123. doi: 10.1097/01.blo.0000200236.84189.87. [DOI] [PubMed] [Google Scholar]
- Wang H, Zou Q, Boerman OC, Nijhuis AW, Jansen JA, Li Y, Leeuwenburgh SC. Combined delivery of BMP-2 and bFGF from nanostructured colloidal gelatin gels and its effect on bone regeneration in vivo. Journal of controlled release : official journal of the Controlled Release Society. 2013;166:172–181. doi: 10.1016/j.jconrel.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen Y, Zhu X, Yuan T, Tan Y, Fan Y, Zhang X. Effect of phase composition on protein adsorption and osteoinduction of porous calcium phosphate ceramics in mice. Journal of biomedical materials research Part A. 2014 doi: 10.1002/jbm.a.35102. [DOI] [PubMed] [Google Scholar]
- Wang L, Huang Y, Pan K, Jiang X, Liu C. Osteogenic responses to different concentrations/ratios of BMP-2 and bFGF in bone formation. Ann Biomed Eng. 2010;38:77–87. doi: 10.1007/s10439-009-9841-8. [DOI] [PubMed] [Google Scholar]
- Wen B, Karl M, Pendrys D, Shafer D, Freilich M, Kuhn L. An evaluation of BMP-2 delivery from scaffolds with miniaturized dental implants in a novel rat mandible model. J Biomed Mater Res B Appl Biomater. 2011;97:315–326. doi: 10.1002/jbm.b.31817. [DOI] [PubMed] [Google Scholar]
- Yasko AW, Lane JM, Fellinger EJ, Rosen V, Wozney JM, Wang EA. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992;74:659–670. [PubMed] [Google Scholar]
- Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, Li W, Chiang M, Chung J, Kwak J, Wu BM, Ting K, Soo C. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue engineering Part A. 2011;17:1389–1399. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lewis CG, Aronow MS, Gronowicz GA. The effects of patient age on human osteoblasts’ response to Ti-6Al-4V implants in vitro. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2004;22:30–38. doi: 10.1016/S0736-0266(03)00155-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.