Abstract
Various neutrophil functions such as phagocytosis, superoxide production, and survival are regulated by integrin signaling. Despite the essential role of focal adhesion kinase (FAK) in mediating this signaling pathway, its exact function in neutrophils is ill defined. In this study, we investigated the role of FAK in neutrophils using a myeloid-specific conditional FAK knockout mouse. As reported in many other cell types, FAK is required for regulation of focal adhesion dynamics when neutrophils adhere to fibronectin or ICAM-1. Adhesion on VCAM-1-coated surfaces and chemotaxis after adhesion were not altered in FAK null neutrophils. In addition, we observed significant reduction in NADPH oxidase-mediated superoxide production and complement-mediated phagocytosis in FAK null neutrophils. As a result, these neutrophils displayed decreased pathogen killing capability both in vitro and in vivo in a mouse peritonitis model. In adherent cells, the defects associated with FAK deficiency are likely due to suppression of phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3) signaling and chemoattractant-elicited calcium signaling. Disruption of FAK also reduced chemoattractant-elicited superoxide production in suspended neutrophils in the absence of cell adhesion. This may be solely caused by suppression of PtdIns(3,4,5)P3 signaling in these cells, because the fMLP-elicited calcium signal was not altered. Consistent with decreased PtdIns(3,4,5)P3/Akt signaling in FAK null neutrophils, we also observed accelerated spontaneous death in these cells. Taken together, our results revealed previously unrecognized roles of FAK in neutrophil function and provided a potential therapeutic target for treatment of a variety of infectious and inflammatory diseases.
Neutrophils are the most abundant cell type among circulating white blood cells and constitute the first line of host defense against invading microorganisms. In response to inflammatory stimuli, neutrophils migrate from the circulating blood to infected tissues, where they protect their host by phagocytosing, killing, and digesting bacterial and fungal pathogens (1–3). A variety of neutrophil functions are mediated by integrin, a superfamily of transmembrane heterodimeric glycoproteins composed of one α and one β subunit. Neutrophil recruitment to sites of infection or inflammation involves sequential interactions with vascular endothelial and extravascular compartments. Integrin receptors expressed on neutrophils play a central role in these interactions, mediating linkages between the cellular cytoskeleton and the external environment. The migrating neutrophils must establish transient and dynamic adhesive contacts with extracellular matrix proteins, and the integrin activity should be tightly regulated during neutrophil locomotion (4–8). Lack of functional β2 integrin is associated with leukocyte adhesion deficiency type 1 immunodeficiency in which neutrophils fail to migrate to sites of inflammation.
In resting neutrophils, integrins are in a conformationally inactive state, unable to bind their extracellular ligands. Upon stimulation with cytokines, chemokines, or anti-integrin Abs, the integrins are primed to an active form, which can be activated by ligand binding and subsequently initiates downstream signaling (5–8). Integrin ligation, either by cell-matrix or cell-cell interaction, elicits outside-in signaling in neutrophils that leads to cellular responses such as cell spreading, firm adhesion, cell survival, degranulation, and reactive oxygen species (ROS)3 production. Some neutrophil responses, particularly NADPH oxidase-mediated ROS production, can be elicited solely by integrin ligation in the absence of any other inflammatory stimuli (9–11).
The ability of neutrophils to generate ROS after adherence to extracellular matrices varies depending on the nature of the substrate. For example, adherence to fibronectin results in a more pronounced ROS production and shorter lag period compared with adherence to laminin or HUVEC surface (12). Although engagement of integrin receptor can induce ROS production by itself, it suppresses ROS production induced by soluble cytokine stimuli, e.g., fMLP or TNF-α (12–14). Integrin also plays an essential role in phagocytosis of pathogens by neutrophils (15, 16). To be recognized and engulfed by neutrophils, foreign particles (e.g., invading pathogens) must be coated with either IgG or complement fragment C3bi, a process known as opsonization. IgG and C3bi then bind to FcγR and complement receptor 3 (CR3), respectively, and initiate a complex series of cellular events including actin polymerization, membrane remodeling, extension of pseudopods, phagosome closure, and particle engulfment (17). CR3 (CD11b/CD18, αMβ2, Mac-1) is one of the members of β2 integrin subfamily and is necessary for productive phagocytic signals. Blockade of CR3 receptor using mAbs greatly impairs the ability of neutrophils to engulf complement-opsonized bacteria (18). β1 integrin is also involved in the phagocytosis of certain bacteria via regulating phagosome maturation through Rac expression (19).
Although it is well accepted that integrins transmit various activating signals in neutrophils, the underlying pathways are still not completely understood. At sites of integrin adhesion to the extracellular matrix, a multiprotein focal adhesion complex is formed. This complex contains clustered integrins and numerous cytoplasmic proteins, such as talin, paxillin, vinculin, focal adhesion kinase (FAK), and zyxin. FAK was first identified in 1992 as a 125-kDa, highly tyrosine-phosphorylated protein associated with the v-Src oncogene and localized within integrin-enriched focal adhesion contact sites (20–23). It is an important mediator functioning between cells and the extracellular matrix and has been implicated in controlling several integrin-dependent biological processes, including cell spreading, migration, and regulation of cell survival (24, 25).
Despite the essential role of FAK in mediating integrin signaling, the exact function of FAK in neutrophils has not yet been clearly defined. This is partially due to the embryonic lethality of FAK knockout mice. In this study, we have investigated the role of FAK in the regulation of neutrophil function by using a myeloid-specific FAK knockout mouse. We found that FAK is required for fibronectin- and ICAM-1-, but not VCAM-1-mediated neutrophil adhesion. It also plays a crucial role in NADPH oxidase-mediated ROS production and complement-mediated phagocytosis. Disruption of FAK leads to reduced pathogen killing capability by neutrophils. In addition, the phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3)/Akt prosurvival pathway in FAK null neutrophils was significantly reduced, leading to elevated death of these cells.
Materials and Methods
Mice
The conditional FAK knockout mice (FAKloxP/loxP) were gifts from Dr. Louis F. Reichardt (University of California, San Francisco, CA; Ref. 26), and the myeloid-specific Cre mice (B6.129P2-Lyz2tm1(cre)Ifo/J) were purchased from The Jackson Laboratory. In all experiments, wild-type littermate (FAKwt/wtCre+/+) mice were used as experimental controls. All procedures involving mice were conducted in accordance with the Animal Welfare Guidelines of Children’s Hospital (Boston, MA) and were approved and monitored by the Children’s Hospital Animal Care and Use Committee. Bone marrow-derived mouse neutrophils were isolated from femurs and tibias as previously described (27). We routinely obtain 4–8 million cells per mouse, and >90% of them are morphologically mature neutrophils (27).
Chemotaxis assay
The EZ-TAXIScan MIC-1000 (Hirata Corp. of America) was used to investigate real-time horizontal chemotaxis of mouse neutrophils. The EZ-TAXIScan consists of an etched silicon substrate and a flat glass plate, both of which form two compartments. Glass coverslips (Corning) with or without fibronectin coating were placed on the glass plate at the bottom of the compartment. Purified bone marrow-derived wild-type and FAK−/− neutrophils in RPMI 1640 containing 0.1% BSA (1 μd of 3 × 106 cells/ml) were placed into the single hole at the bottom of a 4-μm depth × 260-nm width microchip. One microliter of medium with or without 1 μM fMLP was loaded into the contrahole. Cells were allowed to adhere for 3 min before starting experiment. Chemotaxis was recorded at 37°C for 20 min with a 30-s interval using a charge-coupled device camera. Outlines of migrating cells were traced using DIAS software (Solltech), and tracks were generated using centroid-based methods. Chemotactic parameters were then analyzed from the cell tracks using an in-house Matlab program as previously described. A description of chemotactic parameters is described in supplemental Fig. 5.4
Random migration
Freshly prepared bone marrow-derived wild-type and FAK−/− mouse neutrophils in RPMI plus 2% FCS were plated onto fibronectin (10 μg/ml) precoated glass-bottom dishes (MatTek) and allowed to adhere for 3 min. Cells were then uniformly stimulated with 100 nM fMLP, and images were captured from multiple fields every 10 s for 20 min using a ×60 objective on an inverted microscope (Olympus IX17).
Flow-based adhesion assay
The flow-based adhesion assay was conducted essentially as previously described for leukocyte interactions with adhesion molecules (28). Briefly, Dia glass coverslips (25 mm; Carolina Biological Supply) were coated with fibronectin (5 μg/ml; Sigma-Aldrich), BSA (20 μg/ml; Sigma-Aldrich), murine ICAM-1 (20 μg/ml; R&D Systems), or murine VCAM-1 (20 μg/ml; R&D Systems). Mouse neutrophils were pretreated with 1 μM fMLP for 1 min or PMA for 15 min before being loaded. Neutrophil adhesion was examined under conditions of fluid shear stress in a parallel-plate flow chamber. Neutrophils (0.5 × 106/ml) suspended in flow buffer (Dulbecco PBS-0.1% human serum albumin) were drawn through the chamber at decreasing flow rates corresponding to an estimated shear stress of 1 dyne/cm2, 0.8 dyne/cm2, and 0.5 dyne/cm2. Neutrophil accumulation was determined after the initial minute of each flow rate by counting the number of cells in four different fields. Polymorphonuclear neutrophil interactions with the coated surfaces were recorded using a ×20 phase contrast objective, a videomicroscopy, and VideoLab software. Data are means ± SEM of three experiments. A value of p < 0.05 was considered statistically significant using the paired t test or one-way ANOVA for multiple groups.
Cremaster muscle preparation
The surgical procedure to expose the cremaster muscle was performed as described previously (29) with slight modification. Mice were anesthetized i.p. with a mixture of 200 μg of ketamine and 20 μg of xylazine per mouse. The hair covering the right testes area was shaved with an electric razor followed by application of hair removal lotion. A diagonal incision was made starting at the tip of the testis, which was exposed on a Plexiglas custom-built stage. A longitudinal incision was made at the ventral side of the muscle using heat cauterizer, and the muscle was spread using 6–0 sutures. The muscle was continuously superfused with 35°C-thermostable 0.09% saline. Temperature at the muscle surface was frequently monitored by a digital thermometer probe. The surgical procedure takes ~13 min.
Intravital video microscopy acquisition
The muscle preparation was transferred to an intravital microscope (IV-500; Mcron Instruments) with epifluorescence capability. Digital recording of videos was acquired by QED Imaging software (MediaCybernetics). At least three vessels with diameters of 20–40 μm were recorded per time point. A waiting period of 15 min was observed to stabilize blood flow before any recording was taken. The mouse was injected intrascrotally with 300 μl of sterile saline alone (control) or saline with 1 μg of MIP-2, 2 h before video acquisition. To measure the blood flow centerline velocity, 100 μl of rhodamine 6G (2 μg/ml) were injected i.v. Videos of 30–60 s were recorded under a ×40 water immersion objective with a frame rate of 10 frames per second (fps), and 1 or 2 binning. Epifluorescent videos were taken at 96 fps/4 × 4 binning (for rhodamine 6G).
Intravital video microscopy analysis
Offline analysis of recorded videos was performed by frame-by-frame playback using Quicktime software. Rolling cells were defined as the cells moving over a 100-μm-long vessel with a velocity below the velocity of RBCs. Adherent cells were defined as cells staying still for at least 30 s. Emigrating cells were calculated as the number of cells that left the blood vessel and still within a distance of 50 μm on both sides, thus in an area of 100 μm × 50 μm × 2 = 104 μm2. Rolling velocity (10 cells/vessel) was measured as the distance (micrometers) between two points over time (seconds). Centerline blood velocity (Vrbc) was measured for 20 cells/vessel under a high recording rate of 96 fps. Mean blood flow velocity was calculated as centerline velocity × 0.625. Wall shear rate was calculated as 8 × 2.12 (mean blood velocity/diameter) (30).
Superoxide production
Determination of superoxide production was performed as previously described (31). Briefly, reaction mixture in HBSS containing 0.4 × 106 mouse neutrophils, 4 U/ml HRP (type XII; Sigma-Aldrich), and 5.5 μM luminol was allowed to equilibrate to 37°C for 4 min in a 1420 Wallac Victor multilabel counter. fMLP (20 μd of 10× concentrated) was then injected into the mixture via the injection port of the luminometer and luminescence was recorded (for 2 s) at the fixed time intervals.
In vitro bacterial killing assay
Bacteria (Escherichia coli strain 19138; American Type Culture Collection) was subcultured at 37°C to logarithmic growth from an overnight culture; 2.5 × 107 bacteria were opsonized with 12.5% mouse serum at 37°C for 30 min. Opsonized bacteria (12.5 × 105 E. coli) were added to 2.5 × 105 purified wild-type or FAK null neutrophils and incubated at 37°C for 30 and 120 min. After each time point, 900 μl of ice-cold distilled water were added to the sample to lyse neutrophils. Viable bacterial counts from each mixture were measured by plating serial dilutions of bacteria onto Luria-Bertani (LB) plates in triplicate. Bacteria survival was assessed by comparing colony number (CFU) to identically handled and plated untreated bacteria (no neutrophils).
Mouse peritonitis model
Wild-type and FAK−/− mice were injected i.p. with 200 μd of 1 × 107 E. coli (strain 19138; American Type Culture Collection) in 0.9% saline. After 4 h, mice were sacrificed, and the peritoneal cavity was flushed with 10 ml of ice-cold PBS. Dilutions of peritoneal lavage were plated onto LB agar, and the number of bacterial colonies was enumerated to present the in vivo killing capability. The number of cells in peritoneal cavity was quantified by using a hemocytometer, and the differential cell count was obtained by microscopic analysis of Wright-Giemsa-stained cytospins. The total number of neutrophils was determined as previously described (32).
IgG- and complement-mediated phagocytosis
FITC-labeled E. coli particles (K-12 strain; Molecular Probes) were opsonized with either 50% mouse serum or 20 μg/ml purified mouse IgG (Sigma-Aldrich) at 37°C for 30 min. For C3 complement-mediated phagocytosis, E. coli particles were incubated with purified mouse IgM (10 μg/ml; Accurate Chemical and Scientific) for 1 h at 37°C, followed by an incubation with 10% C5-deficient serum (Sigma-Aldrich) for another 30 min. The opsonized E. coli particles were washed twice and resuspended in PBS. Mouse neutrophils (4 × 106 cells) were incubated with either serum-opsonized or IgG-opsonized E. coli (1.7 × 108) at 37°C for the indicated time points. For C3 complement-mediated phagocytosis, neutrophils were treated with 100 ng/ml PMA (Sigma-Aldrich) for 15 min at 37°C before E. coli addition. At each time point, phagocytosis was terminated by putting the tubes on ice and quantified using an inverted fluorescence microscope (Olympus IX17; ×100 objective); >100 cells were counted from random fields per coverslip. Results are presented as the phagocytosis index which is defined as the total number of internalized particles per 100 neutrophils.
Measurement of calcium flux in nonadherent cells
Mouse neutrophils (5 × 106/ml) were loaded with 5 μM fura 2-AM (Invitrogen) for 45 min at room temperature, then washed twice with warm PBS to remove the extracellular dye, and resuspended with HBSS buffer containing Ca2+ and Mg2+ at a density of 5 × 106/ml. Neutrophils were left at room temperature for at least 15 min before the start of the experiment. Fura-2-loaded neutrophils were transferred to a cuvet, resuspended in 3.6 ml of HBSS buffer, and allowed to equilibrate for at least 10 min before the addition of 1 μM fMLP. Fluorescence changes were recorded for 3 min in QuantaMaster Spectrofluorometer QM-8/2005 (Photon Technology International) using 340 and 380 nm for excitation and 510 nm for emission wavelengths. All experiments were performed at room temperature with continuous stirring. The mean of the first 10 points of fluorescence ratios was taken as a baseline for each experiment and subtracted from subsequent fluorescence ratios to allow generation of changes in fluorescence ratio (ΔF340/380) values. The maximum increase of intracellular calcium induced by fMLP was calculated as the peak of the response.
Calcium flux in adherent cells
Mouse neutrophils (5 × 106/ml) were loaded with 2 μM fura 2-AM (Invitrogen) as described above. The final pellets were resuspended in HBSS buffer containing Ca2+, Mg2+, 20 mM HEPES, and 1% BSA at a density of 5 × 106/ml. Fura-2-loaded neutrophils (100 μd) were plated onto fibronectin (10 μg/ml)-precoated glass-bottom dishes (MatTek) and allowed to loosely adhere for 1.5 min. Cells were then uniformly stimulated with 1 μM fMLP. Emitted fluorescences were collected simultaneously at 340 and 380 nm excitation and 510 nm emission for 6 min with a 2-s interval using an inverted fluorescence microscope (Olympus IX17; ×40 oil immersion objective). The changes in fluorescence ratio in each cell were analyzed by IPLab software (Scanalytics).
FACS analysis of neutrophil spontaneous death
Bone marrow-derived mouse neutrophils were cultured in RPMI 1640 with 10% FCS for the indicated time and stained with annexin V-FITC and 7-aminoactinomycin D-PE staining. FACS analysis was performed by using a FACScan flow cytometer (BD Biosciences) equipped with a 488-nm argon laser. Ten thousand cells were collected and analyzed by FlowJo software.
Results
Myeloid-specific FAK knockout mice
Conventional FAK knockout mice die in the early stage of embryonic development (E8.5) due to defective gastrulation events (33). To investigate the role of FAK in neutrophils, we generated myeloid-specific conditional FAK knockout mice by crossing a FAK-floxed mouse with a mouse expressing Cre recombinase under the control of lysozyme M promoter which is activated only in the myeloid-specific linage including mature macrophages, monocytes and neutrophils (supplemental Fig. 1). Western blotting analysis showed that FAK protein expression levels were significantly reduced in bone marrow neutrophils isolated from Cre+/−FAKloxP/loxP or Cre+/+ FAK loxP/loxP mice compared with Cre−/−FAKloxP/loxP littermates (supplemental Fig. 2 and data not shown). Homozygous mice (Cre+/−; FAKloxP/loxP or Cre+/+; FAKloxP/loxP) were viable, fertile, and normal in size and displayed no physical or behavioral abnormalities. The number of neutrophils in peripheral blood was also normal in these mice (supplemental Fig. 3). In addition, microscopic examination of blood smears did not show any morphological abnormality in these neutrophils (data not shown).
Disruption of FAK impairs fibronectin and ICAM-1-, but not VCAM-1-mediated neutrophil adhesion
FAK is a crucial component of focal adhesion complex and has been implicated in integrin-mediated cellular signaling (34–36). FAK null fibroblasts show enhanced focal-contact formation. It has been hypothesized that FAK signaling is associated with the disassembly of integrin-based adhesion sites (33). Accordingly, we first addressed whether FAK is involved in the regulation of integrin-mediated adhesion in neutrophils. To mimic the physiological flow condition and to investigate whether there is a change in the dynamics of integrin activation, we examined neutrophil adhesion using a flow-based adhesion assay (Fig. 1). Bone marrow-derived neutrophils were stimulated or unstimulated with chemoattractant and then plated on fibronectin (ligand for α9β1, α4β1/VLA-4, αMβ2/Mac-1), VCAM-1 (another ligand for α4β1/VLA-4)- or ICAM-1 (ligand for αLβ2/LFA-1 and αMβ2/Mac-1)-coated surface. Neutrophil adhesion was examined under conditions of fluid shear stress in a parallel plate flow chamber. In the absence of any extracellular stimuli, only a small percentage of cells were adherent (data not shown). Upon stimulation with peptide fMLP (100 nM and 1 μM), a tripeptide widely used as a model chemoattractant in studies of neutrophil chemotaxis (37), neutrophil adhesion was much enhanced compared with cells that were not stimulated at all. A significant reduction was observed in the FAK null neutrophils when cells were plated on fibronectin or ICAM-1-coated surface, no matter whether the assay was conducted in the absence (Fig. 1, B and D) or presence of chemoattractant fMLP (Fig. 1, F and H). Interestingly and unexpectedly, FAK−/− neutrophils showed an adhesion efficiency similar to that of wild-type cells when the surface was coated with VCAM-1, suggesting that the effect of FAK on cell adhesion is ligand specific (Fig. 1, C and G). In addition, disruption of FAK did not impair cell adhesion induced by PMA, indicating that FAK might not directly regulate cell adhesion; instead, it may be involved in chemoattractant-elicited signaling pathways leading to firm adhesion (Fig. 1E).
FIGURE 1.
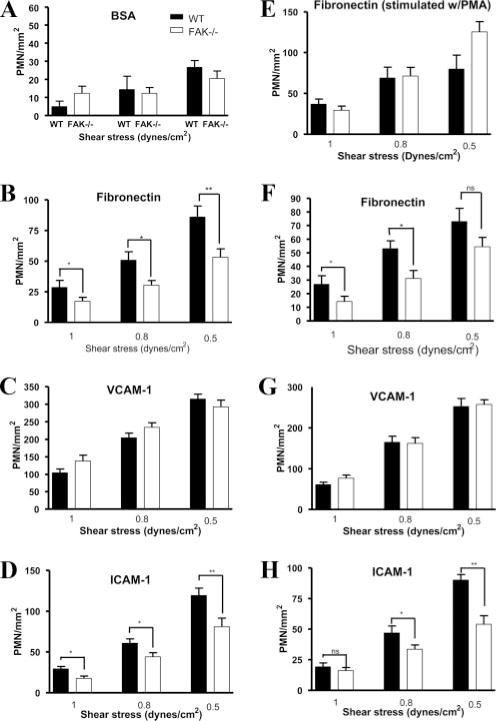
FAK−/− neutrophils exhibit impaired adhesion to fibronectin and ICAM-1, but not VCAM-1, under flow conditions. Wild-type (WT) and FAK−/− (KO) neutrophils (PMN; 0.5 × 106/ml) suspended in flow buffer (PBS, 0.1% BSA) were drawn through the flow chamber at decreasing flow rates corresponding to an estimated shear stress of 1, 0.8, and 0.5 dyne/cm2. Neutrophils were pretreated with (w/) 1 μM fMLP for 1 min (A–D and F–H), or PMA for 15 min (E) before being loaded. Data represent the accumulation of wild-type and FAK-deficient (FAK−/−) neutrophils on glass coverslips coated with BSA (A), fibronectin (B and F), recombinant VCAM-1 (C and G), or ICAM-1 (D and H) under the indicated shear stresses. Data are means ± SEM of three experiments. A value of p < 0.05 was considered statistically significant using the paired t test or one-way ANOVA for multiple groups. A–E, No extra fMLP was added during the assay. F–G, The assay was conducted in the presence of fMLP (100 nM).
Disruption of FAK did not affect neutrophil migration
FAK also plays an essential role in regulating adhesion turnover in migrating cells. Disruption of FAK in fibroblasts and macrophages lead to defects in cell motility (33, 36, 38). We therefore investigated whether deletion of FAK leads to migration defect in neutrophils. We first performed a chemotaxis assay using an EZ-Taxiscan chemotaxis device in which a stable chemoattractant gradient was formed in a 260-μm-wide channel (supplemental Fig. 4). In the absence of fMLP gradient, chemotaxis was barely observed in neutrophils isolated from wild-type and FAK−/− bone marrow (data not shown). In the presence of fMLP gradient, migration of FAK−/− neutrophils on either uncoated or fibronectincoated coverslips was similar in speed and directionality index to that of wild-type neutrophils (Fig. 2, A and B; supplemental Fig. 4; and supplemental Movies 1 and 2). Similar results were observed when higher concentrations (25, 50, and 100 μg/ml) of fibronectin were used (supplemental Movies 3–5). We next examined the speed and morphology of neutrophils during chemoattractant-elicited random migration (chemokinesis). Neutrophils were plated on fibronectin (10 μg/ml)-coated glass-bottom dishes and uniformly stimulated with 100 nM fMLP. Random migrations were monitored by time lapse imaging for a period of 10 min at 10 s intervals. Wild-type and FAK−/− neutrophils were predominantly round before fMLP stimulation (Fig. 2C). Upon stimulation, both wild-type and FAK−/− neutrophils polarized, form distinct pseudopods and uropods, and move randomly (Fig. 2C and supplemental Movie 6). No detectable impairment in polarization and random migration speed was observed in FAK−/− neutrophils (Fig. 2, C and D). Similar results were obtained when higher concentration of fibronectin (50 μg/ml) was used (supplemental Movie 7). We therefore concluded that FAK is not essential for chemotaxis and chemokinesis in neutrophils.
FIGURE 2.
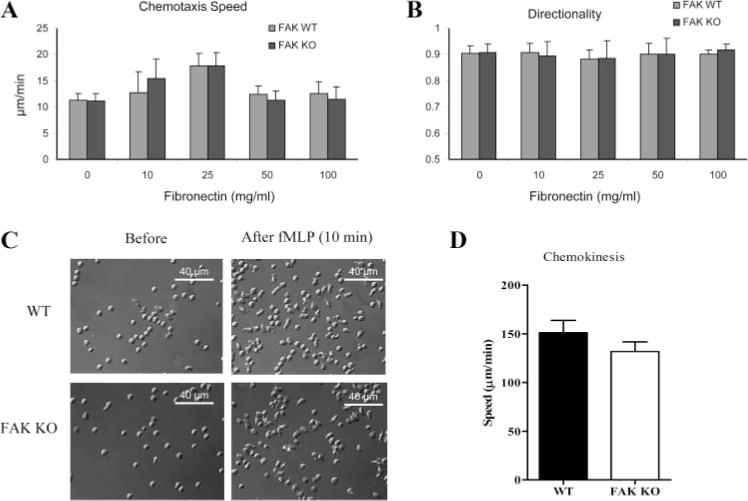
FAK−/− neutrophils exhibit normal chemotaxis and chemokinesis upon fMLP stimulation. A and B, Wild-type (WT) and FAK−/− (KO) neutrophils were plated onto uncoated or fibronectin-coated coverslips. Chemotaxis was visualized using an EZ-TAXIScan device (supplemental Fig. 4). Chemotaxis speed and directionality were quantified using DIAS imaging software as described in supplemental Fig. 5. Results are presented as means ± SD of >20 neutrophils. C, Bone marrow-derived wild-type and FAK−/− neutrophils were plated onto fibronectin (10 μg/ml)-coated MatTek dish and uniformly stimulated with 100 nM fMLP. Shown are representative images of neutrophils before (left) and 10 min after fMLP stimulation (right). Two videos of the experiment described in this figure are included in supplemental Movie 6). Images were captured using a ×40 optical lens (recorded at 1 frame/10 s). D, Migration speeds of wild-type and FAK−/− neutrophils during chemokinesis were calculated as total distance of migration from origin divided by total time. Results are means ± SD of 10 neutrophils. ■, Wild type; □, FAK−/−.
Disruption of FAK did not affect in vivo neutrophil trafficking
Circulating neutrophils initiate tissue entry through a complex series of interactions with the endothelial cells that line local postcapillary venules. The process can be divided into at least four discrete phases: capture and rolling; activation; arrest or firm adhesion; and diapedesis or transmigration from circulation across endothelium into tissues (39–41). We investigated the role of FAK in neutrophil transendothelial migration in live animals using a cremaster muscle model and intravital microscopy. Wild-type and FAK−/− mice were intrascrotally injected with either saline (control) or MIP-2, 2 h before microscopy analysis. We detected a similar number of rolling neutrophils in MIP-2-challenged FAK−/− and wild-type mice (Fig. 3A). In addition, similar rolling velocity, adhesion, and emigration were also observed in these mice (Fig. 3, B–D and supplemental Movies 8 and 9). Consistently, in a mouse peritonitis inflammation model, we observed similar degree of recruitment of neutrophils to inflamed peritoneal cavity in FAK−/− and wild-type mice (Fig. 3E). Collectively, these results suggest that FAK is not essential for neutrophil transendothelial migration in vivo.
FIGURE 3.
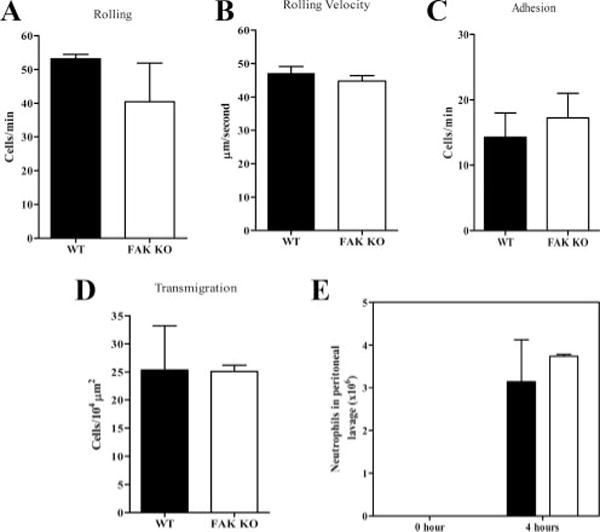
The in vivo neutrophil trafficking is normal in FAK−/− mice. Wild-type (WT) and FAK−/− (KO) mice were intrascrotally injected with MIP-2 (1 μg/mouse). The contralateral cremaster muscle was externalized for observation. The indicated parameters were assessed by intravital video microscope. A, The number of cells rolling per minute; B, rolling velocity; C, number of adherent cells per minute; D, transmigration. Results are means ± SD from two to three experiments. Two videos of the experiment described in this figure are included in supplemental Movie 9). E, Wild-type and FAK−/− mice were injected i.p. with 2 × 106 E. coli cells in 0.9% NaCl. After 4 h, mice were killed, and the peritoneal lavage fluid was collected. The total number of neutrophils in peritoneal cavity was determined using microscopic examination of Wright-Giemsa-stained cytospins. Results are means ± SD of four mice. ■, Wild type; □, FAK−/−.
Disruption of FAK led to reduced complement-mediated phagocytosis
Phagocytosis of invading pathogens is mediated by either IgG or complement fragment C3bi. Integrin plays an essential role in complement-mediated phagocytosis by neutrophils (15, 16). CR3 is one of the members of β2 integrin subfamily, and blockade of CR3 receptor greatly impairs the ability of neutrophils to engulf complement opsonized bacteria (18). Consistently, FAK has been suggested to be involved in the process of E. coli phagocytosis by insect hemocytes (42). However, the role of FAK in pathogen phagocytosis by mammalian neutrophils has not been fully investigated. Therefore, we examined whether deletion of FAK affects phagocytosis in neutrophils. Wild-type and FAK−/− bone marrow-derived neutrophils were incubated with serum-opsonized FITC-labeled E. coli for either 30 or 120 min. In both cases, the efficiency of phagocytosis was significantly decreased in FAK−/− neutrophils (Fig. 4A). The phagocytosis index in FAK−/− neutrophils (number of ingested particles per 100 neutrophils) was reduced by 45% at 30 min and ~35% at 120 min, compared with wild-type neutrophils. The augmented phagocytosis was likely a result of enhanced engulfment, given that there was essentially no difference in the initial bacteria-binding capability between wild-type and FAK null neutrophils (Fig. 4B). To investigate whether complement- or IgG-mediated phagocytosis was affected, we opsonized bioparticles using either purified IgG or IgG-depleted C3i-rich serum. As expected, FAK−/− neutrophils showed a clear defect in C3 complement-mediated phagocytosis, but not in IgG-mediated phagocytosis (Fig. 4, C and D). These results demonstrated that FAK plays an essential role in complement-mediated phagocytic signaling pathway.
FIGURE 4.
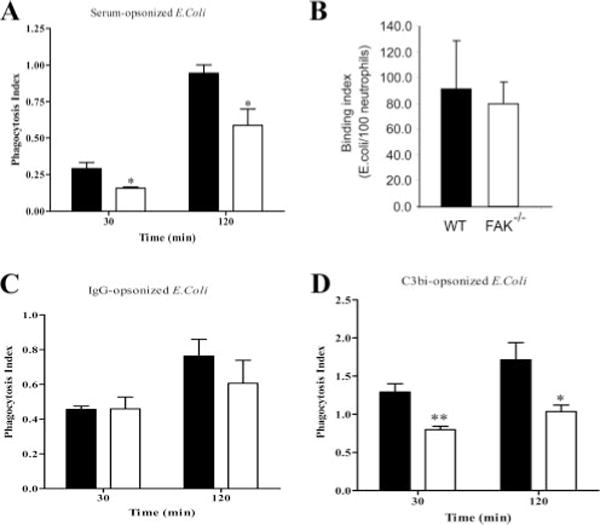
FAK−/− neutrophils show decreased complement-mediated phagocytosis. FITC-labeled E. coli bioparticles were opsonized with mouse serum (A and B), IgG (20 μg/ml; C), or C3 complement (D). Bone marrow-derived wild-type (WT) and FAK−/− neutrophils were incubated with opsonized E. coli for 30 and 120 min (approximate E. coli-neutrophil ratio, 46:1). Phagocytosis of E. coli particles was determined under the inverted fluorescence microscope. The phagocytosis index was expressed as the number of bioparticles engulfed by 100 neutrophils (A, C, and D). Binding index was expressed as the number of bioparticles bound to 100 neutrophils (B). More than 200 neutrophils were counted in each group. Results are means ± SD from at least three independent experiments. *, p < 0.05, **, p < 0.01 vs wild-type neutrophils by Student’s t test. ■, Wild type; □, FAK−/−.
Disruption of FAK reduced both adhesion and chemoattractant-elicited superoxide production
Neutrophil adhesion to extracellular matrix or the surface of endothelial cells elicits integrin-mediated signaling pathway leading to NADPH oxidase activation and subsequent release of superoxide. Although FAK was identified as a key component in integrin signaling, whether it is essential for adhesion-elicited superoxide production is still largely unknown. To test this, we conducted an in vitro luminal chemiluminescence assay using bone marrow-derived neutrophils. Cell adhesion-induced superoxide production in FAK null neutrophils was significantly reduced at each time points examined, whereas the time course for the increase and subsequent decrease in superoxide production was not altered (Fig. 5A). When adherent neutrophils were stimulated with chemoattractant fMLP, a much higher level of superoxide was produced. Under this condition, similar inhibition was observed in FAK null neutrophils (>70% reduction compared with wild-type neutrophils; Fig. 5B).
FIGURE 5.
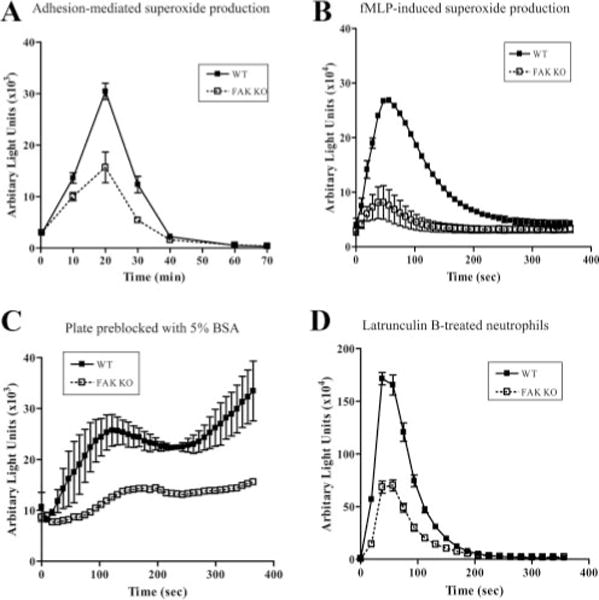
Superoxide production is reduced in FAK−/− neutrophils. Bone marrow-derived wild-type (WT) and FAK−/− (KO) neutrophils were used in a luminol-dependent chemiluminescence assay to investigate superoxide productions. Superoxide production was monitored in a luminometer at 37°C. Chemiluminescence (arbitrary unit lights) was recorded (for 2 s) at indicated time points. A, Adhesion-mediated superoxide production. B, fMLP-induced superoxide production. C, fMLP-induced superoxide production in plate preblocked with 5% BSA. D, fMLP-induced superoxide production in latrunculin B-treated neutrophils. Results are means ± SD of duplicated samples from one experiment representative of three. ■, Wild-type; □, FAK−/−.
We next examined whether this effect occurred only in adherent neutrophils. We suppressed neutrophil adhesion by preblocking the assay plate with a high concentration of BSA. Reduced adhesion was confirmed by microscopy examination (supplemental Fig. 6). Wild-type and FAK−/− neutrophils were then stimulated with fMLP, and superoxide products were measured using a luminal chemiluminescence assay as described in Materials and Methods. Surprisingly, we could still detect a pronounced reduction of superoxide production in the FAK−/− neutrophils, suggesting that FAK might also play a role in chemoattractant-elicited NADPH oxidase activation independent of cell adhesion (Fig. 5C). To confirm this unexpected result, we examined superoxide production in neutrophils treated with latrunculin B, which rapidly and specifically disrupted the actin cytoskeleton and completely prevented cell adhesion (supplemental Fig. 7). Disruption of actin cystoskeleton with latrunculin B markedly enhanced fMLP-stimulated superoxide production in neutrophils (Fig. 5D) as previously reported (43). Consistent with what was observed in Fig. 5C, disruption of FAK in these neutrophils decreased the fMLP-elic-ited superoxide production by >60% compared with wild-type neutrophils (Fig. 5D). Although many cellular processes can be affected by latrunculin, in this study we examined ROS production by wild-type and FAK KO neutrophils under exactly the same condition, and thus the comparison of interest should most likely be the effect of genetic deficiency rather than effects of latrunculin treatment. Altogether, these results suggest that FAK is involved not only in integrin-mediated but also in chemoattractant receptor-mediated NADPH oxidase activation independent of cell adhesion.
FAK is a component in chemoattractant-elicited cellular signal pathway leading to NADPH oxidase activation in both adherent and suspended neutrophils
FAK activation and autophosphorylation creates the binding sites for p85 subunit of PI3K and phosphatidylinositol-specific phospholipase C (PLC; Refs. 44–46). Association of FAK with these proteins leads to activation of the corresponding enzymes and the subsequent generation of signal molecules PtdIns(3,4,5)P3, IP3, and calcium, which have all been implicated in the activation of NADPH oxidase. Nevertheless, FAK role and relative contribution to chemoattractant-elicited NADPH oxidase activation signaling have not been fully investigated. To examine this, we explored whether loss of FAK can lead to down-regulation of fMLP-induced productions of PtdIns(3,4,5)P3 and intracellular calcium transient. Phosphorylation of Akt was used as an indicator of the PtdIns(3,4,5)P3 signaling pathway. Akt contains a PtdIns(3,4,5)P3-specific pleckstrin homology domain and can be recruited onto the plasma membrane via its specific binding to PtdIns(3,4,5)P3. Only the Akt molecules on the plasma membrane can be phosphorylated by two phosphatidylinositol-dependent protein kinases and become activated (47, 48). Akt phosphorylation was monitored by Western blot analysis using a phospho-Akt-specific Ab. In both adherent (Fig. 6, A and B) and suspended (Fig. 6, C and D) neutrophils, Akt phosphorylation was hardly detected before challenging with chemoattractant. Levels of phospho-Akt increased substantially after fMLP stimulation and reached the maximum value between 1 and 3 min. Disruption of FAK significantly decreases the level of Akt phosphorylation after fMLP stimulation in adherent and suspended neutrophils. These results demonstrated that FAK is involved in chemoattractant-elicited PtdIns(3,4,5)P3 signaling pathway activation independent of cell adhesion.
FIGURE 6.
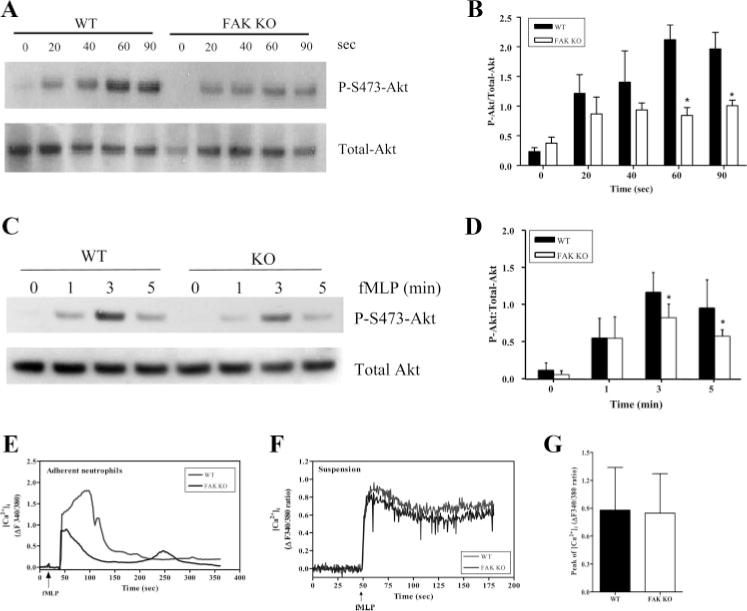
fMLP-mediated signaling pathways are reduced in FAK−/− neutrophils. A and B, fMLP-induced Akt phosphorylation in adherent neutrophils. Bone marrow-derived wild-type (WT) and FAK−/− (KO) neutrophils were allowed to adhere at 37°C for 4 min and then stimulated with 1 μM fMLP for the indicated time (0–90 s). A, Western blotting analysis. B, Results of densitometry. C and D, fMLP-induced Akt phosphorylation in nonadherent neutrophils. Wild-type and FAK−/− neutrophils in suspension were stimulated with 1 mM fMLP for the indicated time (0–5 min). C, Western blotting analysis. D, Results of densitometry. Expression levels of phosphorylated Akt and total Akt were detected by Western blotting analysis using antiphos-phorylated Akt (Ser473; 1/1000) and anti-Akt (1/1000) Abs (Cell Signaling), respectively. Results are presented as ratio of phospho-Akt to total Akt with actin normalization. *, p > 0.05 vs wild-type neutrophils by Student’s t test. E and F, fMLP-induced calcium signaling in neutrophils. Wild-type and FAK−/− neutrophils were loaded with fura 2-AM for 45 min at 37°C. E, fura 2-loaded wild-type and FAK−/− neutrophils were allowed to loosely adhere on a fibronectin (10 μg/ml)-coated MatTek dish for 1.5 min. Cells were uniformly stimulated with 1 μM fMLP, and changes of [Ca2+]i were monitored every 2 s for 10 min using a ×40 objective. Results are a single cell from wild-type and FAK−/− neutrophils representative of at least 40 cells from two independent experiments. Red line, wild type; blue line, FAK−/−. Two videos of the experiment described in this figure are included in supplement data (Movie 10). F, Neutrophils in suspension were stimulated with 1 μM fMLP. The changes in intracellular calcium ([Ca2+]i) were monitored for 3 min after stimulation. Results are means ± SD from two independent experiments. Red line, wild type; blue line, FAK−/−. Arrow, addition of fMLP. G, Peaks of changes in [Ca2+]i of wild-type and FAK−/− neutrophils after fMLP stimulation. Results are means ± SD from two independent experiments. ■, Wild type; □, FAK−/−.
The increase of intracellular calcium concentration was measured in wild-type and FAK−/− neutrophils using fura-2 AM ratiometric fluorescence indicator. When neutrophils loosely adhered on a 10 μg/ml fibronectin-coated dish, fMLP stimulation-elicited inside-out signal initiated further integrin engagement, resulting in activation of downstream signals such as calcium. FAK−/− neutrophils displayed a reduced response to fMLP compared with wild-type neutrophils, indicating a crucial role of FAK in this signaling process (Fig. 6E, supplemental Fig. 8, and supplemental Movie 10). Different from what was observed in adherent neutrophils, the level of fMLP-induced increase in intracellular Ca2+ was comparable in wild-type and FAK−/− neutrophils in suspension with continuously stirring (Fig. 6, F and G). The maximum increases of intracellular calcium in these cells were comparable (Fig. 6G). These results suggest that FAK plays a role in chemoattractant-elicited calcium signaling in adherent neutrophils, but not in suspended neutrophils. It is likely that the engagement of integrin receptors on the cell surface is necessary for the involvement of FAK in chemoattractant-elicited calcium signaling.
The bacterial killing capability was reduced in FAK null neutrophils
Both phagocytosis and NADPH oxidase-mediated superoxide production play an important role in bacterial killing by neutrophils. In chronic granulomatous disease, impairment of NADPH oxidase function leads to elevated susceptibility of patients to microbial infection (49). Similarly, the defects of phagocytosis and superoxide production in FAK null neutrophils may lead to impaired antimicrobial mechanism in FAK knockout mice. To test this, we first conducted an in vitro bacterial killing assay. Serum-opsonized E. coli particles were incubated with either wild-type or FAK−/− neutrophils at a ratio of 5 E. coli to 1 neutrophil at 37°C for 30 and 120 min. The numbers of surviving bacteria were quantified using a colony-forming assay. Although the bacterial killing ability of wild-type and FAK−/− neutrophils was still comparable at 30 min, FAK−/− neutrophils showed a significant defect in killing E. coli (~60% reduction compared with wild-type) at 2 h (Fig. 7A). We also investigated the function of FAK in in vivo bacterial killing using the peritonitis model. Live E. coli cells were injected into the peritoneum of wild-type and FAK−/− mice. Peritoneal lavage was collected from these mice 4 h after the injection. Serial dilutions of this lavage (1/500, 1/1000, and 1/5000) were plated onto LB agar to measure the number of surviving bacteria (Fig. 7B). Three times more live E. coli cells were detected in the inflamed peritoneal cavity of FAK−/− mice than in the wild-type littermates (Fig. 7C). Because the number of neutrophils accumulated in the peritoneal cavity after E. coli injection was comparable in the wild-type and FAK−/− mice (Fig. 3E), the elevated bacteria number in FAK knockout mice is most likely due to a bacterial killing defect in neutrophils.
FIGURE 7.
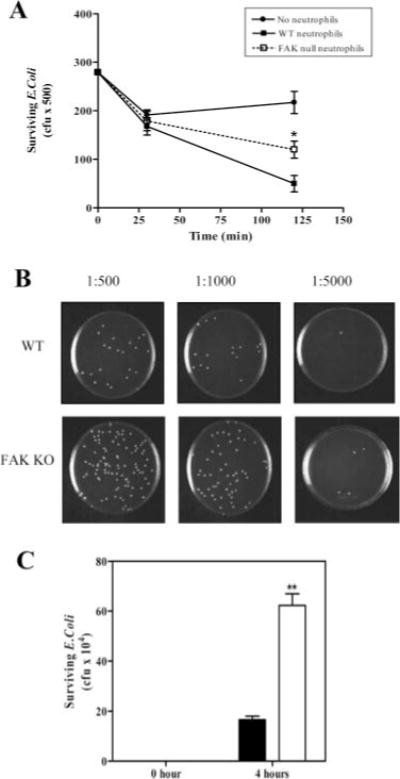
FAK−/− neutrophils are defective in killing of E. coli in vitro and in vivo. A, Wild-type (WT) and FAK−/− (KO) neutrophils were incubated with serum-opsonized E. coli at 37°C for 30 and 120 min, and the numbers of surviving bacteria were determined and presented as CFUs. B and C, Wild-type and FAK−/− mice were injected i.p. with 2 × 106 E. coli in 0.9% NaCl. After 4 h, mice were killed, and the peritoneal lavage fluid was collected. Surviving bacteria were enumerated by plating several dilutions (1/500, 1/1000, and 1/5000) onto LB agar (B) and total numbers of surviving bacteria per animal were calculated (C). Results are means ± SD of four mice. *, p < 0.05; **, p < 0.01 vs wild-type neutrophils by Student’s t test. ■, Wild type; □, FAK−/−.
The spontaneous death was accelerated in FAK null neutrophils
Neutrophils are terminally differentiated cells. They normally have a very short life span (6–7 h in blood and 1–4 days in tissue) and readily undergo spontaneous programmed cell death (apoptosis; Ref. 50). Neutrophil death shares many features of classical apoptosis, such as cell body shrinkage, exteriorization of phosphatidylserine (PS) from the inner to the outer leaflet of plasma membrane, mitochondria depolarization, nuclear condensation, and DNA fragmentation (51). Recently, we established PtdIns(3,4,5)P3/Akt deactivation as one of the causal mediators of apoptosis in neutrophils. PtdIns(3,4,5)P3/Akt signal is strongly deactivated during neutrophil death. Inhibition of this signal pathway promotes neutrophil spontaneous death, whereas augmentation of this signal prevents neutrophil death (27). Because the PtdIns(3,4,5)P3/Akt pathway was significantly suppressed in both adherent and suspended FAK null neutrophils, we next explored whether the programmed death of these neutrophils was altered. The number of neutrophil undergoing spontaneous death was quantified using FACS analysis. In the FACS assays, we use Annexin V, an anticoagulant protein that has high affinity and selectivity for PS, to detect PS exteriorization and 7-aminoactinomycin D, a nucleic acid dye which intercalates into the double-stranded nucleic acid and penetrates cell membranes of dying or dead cells (Fig. 8A). The PS exposure was evident in neutrophils by 16 h, and the level increased to 35 ± 4% at 24 h. We detected a concomitant increase in the apoptotic (R2, lower right quadrant) and necrotic (R3 plus R4) populations at 24 h, suggesting that at later time points many of the apoptotic cells have proceeded into secondary necrosis (Fig. 8A). As expected, FAK null neutrophils displayed a much higher level of cell death than did wild-type neutrophils, and this effect was observed at all time points examined. Only 34% of FAK null neutrophils could live longer than 24 h in the culture, whereas ~64% wild-type neutrophils were detected healthy under the same condition (Fig. 8B). These results demonstrated that FAK also plays an essential role in modulating the life span of neutrophils, probably through the Akt/PIP3 pathway.
FIGURE 8.
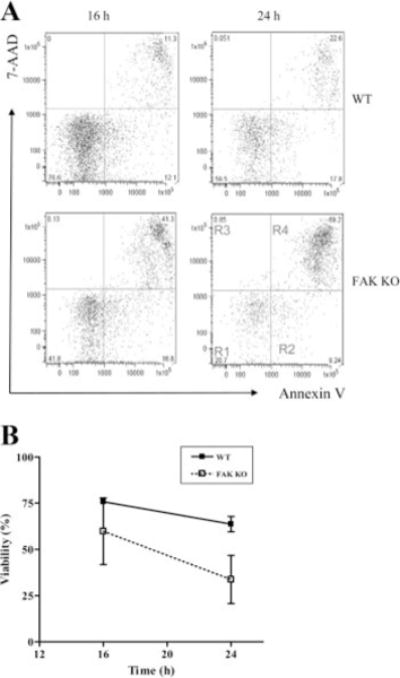
FAK deletion enhances spontaneous neutrophil death. A, Bone marrow-derived wild-type (WT) and FAK−/− (KO) neutrophils were cultured in RPMI 1640 containing 10% heat-inactivated FBS at a density of 5 × 105 cells/ml. Apoptotic cells were detected by annexin V-FITC staining and 7-aminoactinomycin D (7-AAD) staining. Ten thousand cells were collected at the indicated time points and analyzed by FlowJo software. Region R1, viable cell; Region R2, early apoptotic cells; Regions R3 and R4, late apoptotic cells and necrotic cells. B, Time course of neutrophil spontaneous death. Results are means ± SD of two separate experiments.
Discussion
In current study, using myeloid-specific conditional FAK knockout mice, we demonstrated that FAK, a non-receptor protein tyrosine kinase which is mostly known as a regulator of cell adhesion and migration, plays a crucial role in regulating various neutrophil functions. These results revealed previously unrecognized roles of FAK in neutrophil function and provided a potential target for treatment of a variety of infectious and inflammatory diseases.
FAK is a ubiquitously expressed protein tyrosine kinase, the function of which has been well studied in fibroblasts, where it plays an important regulatory function in cell matrix/integrin-dependent adhesion and motility (24, 25). Following integrin-mediated binding to extracellular matrix proteins such as fibronectin, FAK is catalytically activated and undergoes autophosphorylation at Tyr397, which serves as a binding site for Src homology 2 (SH2) domain containing Src family kinases. The FAK-Src kinase complex leads to further phosphorylation at additional FAK sites and to the recruitment and activation of multiple downstream signaling proteins, including PI3K and PLCγ. It has been hypothesized that FAK signaling is associated with the disassembly of integrin-based adhesion sites and thus coordinates lamellipodial formation and regulates adhesion turnover in migrating cells. FAK null fibroblasts show excess focal contact formation and defect in cell movement (33). FAK is also expressed in hemopoietic cells. Owen et al. (38) recently reported that FAK null macrophages exhibited elevated protrusive activity at the cell periphery, reduced adhesion turnover, and a marked inability to form the stable lamellipodia necessary for directional movement. As a result, these macrophages failed to migrate to sites of infection and inflammation in vivo. FAK also plays a role in megakaryopoiesis and platelet function. FAK null platelets exhibit diminished spreading on immobilized fibrinogen (52). Stimuli-induced FAK phosphorylation was also observed in neutrophils. Ryu et al. reported that pituitary growth hormone (GH) triggered tyrosine phosphorylation of FAK and focal localization of phosphorylated FAK into the membrane rufflings and uropods of human neutrophils (53). FAK phosphorylation was also detected in neutrophils stimulated with disintegrin peptides (54) and after integrin activation (55). Consistently, in current study, we demonstrated that FAK is required for regulation of focal adhesion dynamics when neutrophils adhere to fibronectin or ICAM-1. Interestingly and unexpectedly, FAK−/− neutrophils showed an adhesion efficiency similar to that of wild-type cells when the surface was coated with VCAM-1, suggesting that the effect of FAK on cell adhesion might be ligand specific. In addition, FAK−/− neutrophils also exhibited normal chemoattractant-elicited chemotaxis and chemokinesis, indicating that FAK is also not essential for neutrophil migration. An alternative explanation involves the FAK homolog pyk2 which is highly expressed in neutrophils. It is possible that pyk2 is able to compensate in the absence of FAK to maintain normal neutrophil migration, despite the fact that pyk2 protein expression and phosphorylation were not altered in the FAK null neutrophils (supplemental Fig. 9). It will be intriguing to examine whether any neutrophil migration defect can be detected in FAK/pyk2 double-knockout mice.
We also examined neutrophil trafficking in live animals. We detected similar rolling influx, rolling velocity, adhesion, and emigration in MIP-2-challenged FAK−/− and wild-type mice in a cremaster muscle model. Consistently, in a mouse peritonitis inflammation model, we observed a similar degree of recruitment of neutrophils to an inflamed peritoneal cavity in FAK−/− and wild-type mice, suggesting that FAK is not essential for neutrophil transendothelial migration in vivo. These results are somewhat surprising, considering that FAK is essential for fibronectin and ICAM-1-mediated adhesion. In the myeloid specific FAK knockout mice, FAK expression is also ablated in monocytes and macrophages, and maybe some dendritic cells. However, in the peritonitis model and the cremaster muscle model described above, macrophages migrated to the sites of inflammation after neutrophil recruitment. Thus, it is unlikely that macrophages are involved in the recruitment of neutrophils. Neutrophil recruitment to the peritoneum in response to bacteria has previously been shown not to require β2 integrins (56). However, it seems that other types of integrins (e.g., β1 and β7) are involved in this model, given that it was recently reported that extravasation of integrin−/− leukocytes (all integrins were depleted in these mice) from the blood stream into the inflamed sites was completely abolished, suggesting that integrins are essential mediators in neutrophil transmigration (57). In addition, it was well known that in the cremaster muscle model used above, β2 integrins are critical for chemokine-induced neutrophil transmigration (58). Thus, the most logical explanation for the observed normal neutrophil adhesion/trafficking in the FAK knockout mice is that neutrophil adhesion on endothelial cells in these models might be mainly mediated by VCAM-1 or other factors that are not regulated by FAK.
The pathogen-killing capability of neutrophils is mediated by a network of intracellular signaling pathways. Our data have defined FAK as a key regulator in neutrophil phagocytosis and NADPH oxidase-mediated superoxide production. Its involvement in phagocytosis is not surprising because it is well known that integrin plays an essential role in phagocytosis of pathogens by neutrophils (15, 16). Phagocytosis is mediated by either FcγR or CR3 (17). CR3 (CD11b/CD18, αMβ2, Mac-1) is one of the members of the β2 integrin subfamily and is necessary for productive phagocytic signaling. Impairment of CR3 receptor leads to defect in engulfment of complement-opsonized bacteria by neutrophils (18). Additionally, β1 integrin has been implicated in the phagocytosis of certain bacteria via regulating phagosome maturation through Rac expression (19). Our findings underscored the essential role of FAK in mediating integrin signaling during complement-mediated phagocytosis. In FAK null neutrophils, no abnormality was detected in integrin-independent IgG-mediated phagocytosis. It was previously reported that complement-mediated phagocytosis is dependent on Rho activation, whereas IgG-mediated is dependent on Cdc42 and Rac (59). We investigated whether Rho activation is diminished in FAK−/− neutrophils during complement-mediated phagocytosis. No significant difference in the cellular level of activate form of Rho (Rho-GTP) was detected between the wild-type and FAK knockout neutrophils (supplemental Fig. 10). This suggests that phagocytosis-induced Rho activation might not be a downstream event of phosphatidylinositol 4,5-trisphosphate signaling, which was significantly reduced in the FAK knockout neutrophils. Or at least phosphatidylinositol 4,5-trisphosphate is not the only signal that controls Rho activation.
Neutrophil adhesion to extracellular matrix or the surface of endothelial cells elicits NADPH oxidase-mediated superoxide production. We have found that adhesion-induced superoxide production and fMLP-induced superoxide production in adherent neutrophils are much reduced in FAK-deficient neutrophils. This is consistent with the involvement of integrin in these adhesion-mediated cellular events. FAK is localized within integrin-enriched focal adhesion contact sites, and the FAK-mediated signaling pathway leads to NADPH oxidase activation and subsequent release of superoxide. However, the fact that FAK is also involved in fMLP-induced superoxide production in suspended neutrophils is somewhat surprising. This suggests that FAK is also regulated by G protein-coupled chemoattractant receptor, independent of cell adhesion-elicited integrin activation. A similar effect was recently observed in chemokine CXCL12-stimulated REH pro-B cells (60) and hemopoietic progenitor cells (61). Upon CXCL12 stimulation, activated CXCR4 receptors form clusters in lipid raft domains and subsequently trigger signaling molecules, such as Gi protein, Src family proteins, and FAK. This event can happen in suspension without cell adhesion. It provides an inside-out signaling that activates progenitor B cell surface integrins, such as VLA-4, and subsequently promotes cell adhesion (60).
To delineate the mechanisms underlying the cellular defects observed in FAK-deficient neutrophils, we examined the involvement of several downstream targets in FAK-mediated neutrophil functions. Phosphorylation and activation of FAK provides a binding site for SH2 domain-containing proteins such as Src, p85 subunit of PI3K, and PLCy (24, 25, 62). The FAK-Src kinase complex leads to further phosphorylation at additional FAK sites and to the recruitment and activation of other downstream signaling proteins. Associations of FAK with p85 leads to activation of PI3K and the subsequent generation of signal molecule PtdIns(3,4,5)P3. As another important signaling molecule, Ins(1,4,5)P3, is formed from the hydrolysis of phosphatidylinositol 4,5-bisphosphate by PLC (63). In mammals, multiple PLC genes were identified and divided into four major types, β, γ, δ, and ε. They differ in their domain structure, regulation, and tissue distribution. PLCγ contains an SH2 domain and can be activated by FAK and responsible for cell adhesion-mediated calcium signaling. In agreement with these previous reports, we revealed that in adherent cells, disruption of FAK suppressed both PtdIns(3,4,5)P3 signaling and chemoattractantelicited calcium signaling. Interestingly, in suspended neutrophils, only PtdIns(3,4,5)P3 signaling was reduced upon FAK disruption, whereas the fMLP-elicited calcium signal was not altered, indicating that engagement of fMLP receptor can initiate calcium signaling required for NADPH oxidase activation independent of FAK (Fig. 9). The PLCβ family, which consists of four isoforms, β1–β4, can be directly regulated by G protein. It was reported that PLC/β is activated by fMLP in neutrophils and that the activation is mediated by the G protein βγ subunits that are released from the Gi protein upon chemoattractant receptor activation (64, 65). The exact pathway leading to G protein-coupled chemoattractant receptor-induced FAK activation in the absence of cell adhesion is still largely unknown. It appears that Src plays a role in adhesioninduced FAK activation, because NADPH oxidase activity was inhibited by Src inhibitors (data not shown). In contrast, Src inhibitors had no effect on fMLP-induced FAK activation (Fig. 9). A recent study demonstrated that RhoG is important in superoxide release in neutrophils (66). However, because the level of activated Rho is the same in the FAK null neutrophils (supplemental Fig. 10), it is unlikely that the NADPH oxidase defect seen in FAK−/− neutrophils is caused by impaired Rho activation.
FIGURE 9.
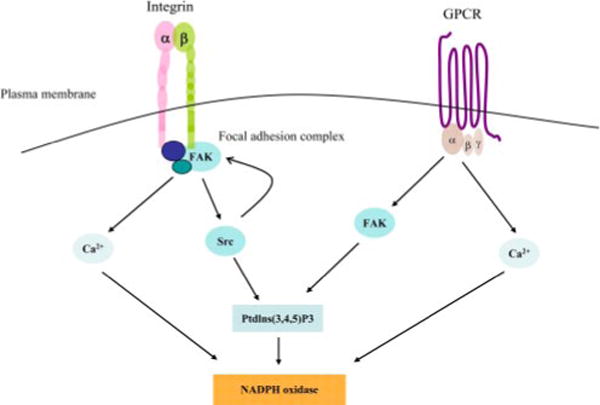
FAK mediates NADPH oxidase activation in mouse neutrophils. Cell adhesion-mediated integrin aggregation leads to activation of FAK and its downstream signaling molecules including Src family kinases, PtdIns(3,4,5)P3, and Ca2+. Elevation of PtdIns(3,4,5)P3 and Ca2+ results in activation of NADPH oxidase. In nonadherent neutrophils, binding of fMLP to its receptor(s) triggers activation of FAK and downstream PtdIns(3,4,5)P3 signaling. Ca2+ signaling is directly regulated by fMLP receptor independent of FAK. GPCR, G protein-coupled chemoattractant receptor.
The reduced PtdIns(3,4,5)P3/Akt signaling in FAK null neutrophils parallels and may account for the accelerated spontaneous death observed in these cells. PtdIns(3,4,5)P3/Akt signaling pathway possesses prosurvival and antiapoptotic activities in a variety of cell types. Akt contains a pleckstrin homology domain which specifically binds PtdIns(3,4,5)P3. The PtdIns(3,4,5)P3-mediated membrane translocation of Akt is essential for its phosphorylation and activation. Activated Akt, in turn, phosphorylates a variety of proteins, including several associated with cell survival/death pathways such as BAD, Forkhead, ASK1, and NF-κB, leading to diminished apoptotic cell death (67, 68). We recently established Akt deactivation as a causal mediator in neutrophil spontaneous death. Augmentation of PtdIns(3,4,5)P3/Akt signal prevents neutrophil spontaneous death, whereas inhibition of PtdIns(3,4,5)P3/Akt signal further promotes neutrophil death. Thus, the elevated death in FAK null neutrophils is likely due to the reduced PtdIns(3,4,5)P3/Akt signaling in these cells, although we cannot completely rule out the involvement of other death/survival pathways downstream of FAK. It has been reported that activated FAK can augment the activity of Ras-MAPK pathway (25, 62), resulting in elevated cell survival (24, 25). FAK also binds to the death domain kinase receptor-interacting protein (RIP), a major component of the death receptor complex that has been shown to interact with Fas and TNFR1 through its binding to adapter proteins. The proapoptotic activity of RIP is suppressed by its binding to FAK (69). Nevertheless, the involvement of RIP and Ras in FAK-mediated neutrophil survival/death remains unknown and should be further investigated. Neutrophil spontaneous death might play a role in neutrophil homeostasis. We examined whether disruption of FAK can increase peripheral blood neutrophil count due to delayed spontaneous death. However, we did not detect any alteration in the peripheral blood neutrophil count (supplemental Fig. 3) or the percentage of apoptotic neutrophils in the peripheral blood (supplemental Fig. 11). The peripheral blood neutrophil count is decided by multiple cellular processes, such as cytokineelicited mobilization from bone marrow, spontaneous death, transmigration from blood to tissues, as well as clearance by phagocytic cells. Conceivably, depletion of FAK can affect processes other than spontaneous death and these effects are able to overcome the effect elicited by delayed neutrophil death, leading to unaltered peripheral blood neutrophil count. Alternatively, neutrophil spontaneous death might not be a deciding factor for regulating peripheral blood neutrophil count in mouse.
Supplementary Material
Acknowledgments
We thank John Manis, Li Chai, and people in Joint Program in Transfusion Medicine for helpful discussions.
Footnotes
B.S. is supported by National Institutes of Health Training Grant HL066987, and H.L. is supported by National Institutes of Health Grants HL085100, AI076471, and GM076084 and a Research Scholar Grant from American Cancer Society.
Abbreviations used in this paper: ROS, reactive oxygen species; CR3, complement receptor 3; FAK, focal adhesion kinase; PtdIns(3,4,5)P3, phosphatidylinositol (3,4,5)-trisphosphate; LB, Luria-Bertani; PLC, phospholipase C; PS, phosphatidylserine; SH2, Src homology 2; RIP, receptor-interacting protein
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996;32:733–742. [PubMed] [Google Scholar]
- 2.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 3.Burg ND, Pillinger MH. The neutrophil: function and regulation in innate and humoral immunity. Clin Immunol. 2001;99:7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- 4.Lowell CA, Berton G. Integrin signal transduction in myeloid leukocytes. J Leukocyte Biol. 1999;65:313–320. doi: 10.1002/jlb.65.3.313. [DOI] [PubMed] [Google Scholar]
- 5.Cox EA, Huttenlocher A. Regulation of integrin-mediated adhesion during cell migration. Microsc Res Tech. 1998;43:412–419. doi: 10.1002/(SICI)1097-0029(19981201)43:5<412::AID-JEMT7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Williams MA, Solomkin JS. Integrin-mediated signaling in human neutrophil functioning. J Leukocyte Biol. 1999;65:725–736. doi: 10.1002/jlb.65.6.725. [DOI] [PubMed] [Google Scholar]
- 7.Berton G, Lowell CA. Integrin signalling in neutrophils and macrophages. Cell Signal. 1999;11:621–635. doi: 10.1016/s0898-6568(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Hilden TJ, Nurmi SM, Fagerholm SC, Gahmberg CG. Interfering with leukocyte integrin activation: a novel concept in the development of anti-inflammatory drugs. Ann Med. 2006;38:503–511. doi: 10.1080/07853890600969130. [DOI] [PubMed] [Google Scholar]
- 9.Liles WC, Ledbetter JA, Waltersdorph AW, Klebanoff SJ. Cross-linking of CD18 primes human neutrophils for activation of the respiratory burst in response to specific stimuli: implications for adhesion-dependent physiological responses in neutrophils. J Leukocyte Biol. 1995;58:690–697. doi: 10.1002/jlb.58.6.690. [DOI] [PubMed] [Google Scholar]
- 10.Garnotel R, Monboisse JC, Randoux A, Haye B, Borel JP. The binding of type I collagen to lymphocyte function-associated antigen (LFA) 1 integrin triggers the respiratory burst of human polymorphonuclear neutrophils: role of calcium signaling and tyrosine phosphorylation of LFA 1. J Biol Chem. 1995;270:27495–27503. doi: 10.1074/jbc.270.46.27495. [DOI] [PubMed] [Google Scholar]
- 11.Berton G, Yan SR, Fumagalli L, Lowell CA. Neutrophil activation by adhesion: mechanisms and pathophysiological implications. Int J Clin Lab Res. 1996;26:160–177. doi: 10.1007/BF02592978. [DOI] [PubMed] [Google Scholar]
- 12.Nathan CF. Neutrophil activation on biological surfaces: massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987;80:1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehr J, Moser R, Leppert D, Groscurth P. Antiadhesive properties of biological surfaces are protective against stimulated granulocytes. J Clin Invest. 1985;76:535–542. doi: 10.1172/JCI112003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao T, Benard V, Bohl BP, Bokoch GM. The molecular basis for adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J Clin Invest. 2003;112:1732–1740. doi: 10.1172/JCI19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee WL, Harrison RE, Grinstein S. Phagocytosis by neutrophils. Microbes Infect. 2003;5:1299–1306. doi: 10.1016/j.micinf.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Cougoule C, Wiedemann A, Lim J, Caron E. Phagocytosis, an alternative model system for the study of cell adhesion. Semin Cell Dev Biol. 2004;15:679–689. doi: 10.1016/j.semcdb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Jutras I, Desjardins M. Phagocytosis: at the crossroads of innate and adaptive immunity. Annu Rev Cell Dev Biol. 2005;21:511–527. doi: 10.1146/annurev.cellbio.20.010403.102755. [DOI] [PubMed] [Google Scholar]
- 18.Edwards MS, Wessels MR, Baker CJ. Capsular polysaccharide regulates neutrophil complement receptor interactions with type III group B streptococci. Infect Immun. 1993;61:2866–2871. doi: 10.1128/iai.61.7.2866-2871.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang QQ, Li H, Oliver T, Glogauer M, Guo J, He YW. Integrin β1 regulates phagosome maturation in macrophages through Rac expression. J Immunol. 2008;180:2419–2428. doi: 10.4049/jimmunol.180.4.2419. [DOI] [PubMed] [Google Scholar]
- 20.Guan JL, Trevithick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- 23.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 26.Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu D, Hattori H, Jo H, Jia Y, Subramanian KK, Loison F, You J, Le Y, Honczarenko M, Silberstein L, Luo HR. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc Natl Acad Sci USA. 2006;103:14836–14841. doi: 10.1073/pnas.0605722103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim YC, Henault L, Wagers AJ, Kansas GS, Luscinskas FW, Lichtman AH. Expression of functional selectin ligands on Th cells is differentially regulated by IL-12 and IL-4. J Immunol. 1999;162:3193–3201. [PubMed] [Google Scholar]
- 29.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638–4648. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 30.Forlow SB, Ley K. Selectin-independent leukocyte rolling and adhesion in mice deficient in E-, P-, and L-selectin and ICAM-1. Am J Physiol Heart Circ Physiol. 2001;280:H634–H641. doi: 10.1152/ajpheart.2001.280.2.H634. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian KK, Jia Y, Zhu D, Simms BT, Jo H, Hattori H, You J, Mizgerd JP, Luo HR. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109:4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizgerd JP, Quinlan WM, LeBlanc BW, Kutkoski GJ, Bullard DC, Beaudet AL, Doerschuk CM. Combinatorial requirements for adhesion molecules in mediating neutrophil emigration during bacterial peritonitis in mice. J Leukocyte Biol. 1998;64:291–297. doi: 10.1002/jlb.64.3.291. [DOI] [PubMed] [Google Scholar]
- 33.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 34.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 35.Owen JD, Ruest PJ, Fry DW, Hanks SK. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol Cell Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112(Pt 16):2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 37.Prossnitz ER, Ye RD. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol Ther. 1997;74:73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- 38.Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, Parsons JT, Beggs HE, Stanley ER, Bouton AH. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol. 2007;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 40.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- 41.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 42.Metheniti A, Paraskevopoulou N, Lambropoulou M, Marmaras VJ. Involvement of FAK/Src complex in the processes of Escherichia coli phagocytosis by insect hemocytes. FEBS Lett. 2001;496:55–59. doi: 10.1016/s0014-5793(01)02405-x. [DOI] [PubMed] [Google Scholar]
- 43.Bengtsson T, Orselius K, Wettero J. Role of the actin cytoskeleton during respiratory burst in chemoattractant-stimulated neutrophils. Cell Biol Int. 2006;30:154–163. doi: 10.1016/j.cellbi.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Akagi T, Murata K, Shishido T, Hanafusa H. v-Crk activates the phosphoinositide 3-kinase/AKT pathway by utilizing focal adhesion kinase and H-Ras. Mol Cell Biol. 2002;22:7015–7023. doi: 10.1128/MCB.22.20.7015-7023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 46.Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 47.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 48.Milburn CC, Deak M, Kelly SM, Price NC, Alessi DR, Van Aalten DM. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem J. 2003;375:531–538. doi: 10.1042/BJ20031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 50.Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol. 2008;83:288–295. doi: 10.1002/ajh.21078. [DOI] [PubMed] [Google Scholar]
- 51.Hengartner MO, Horvitz HR. Programmed cell death in Caenorhabditis elegans. Curr Opin Genet Dev. 1994;4:581–586. doi: 10.1016/0959-437x(94)90076-f. [DOI] [PubMed] [Google Scholar]
- 52.Hitchcock IS, Fox NE, Prevost N, Sear K, Shattil SJ, Kaushansky K. Roles of focal adhesion kinase (FAK) in megakaryopoiesis and platelet function: studies using a megakaryocyte lineage specific FAK knockout. Blood. 2008;111:596–604. doi: 10.1182/blood-2007-05-089680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryu H, Lee JH, Kim KS, Jeong SM, Kim PH, Chung HT. Regulation of neutrophil adhesion by pituitary growth hormone accompanies tyrosine phosphorylation of Jak2, p125FAK, and paxillin. J Immunol. 2000;165:2116–2123. doi: 10.4049/jimmunol.165.4.2116. [DOI] [PubMed] [Google Scholar]
- 54.Coelho AL, De Freitas MS, Mariano-Oliveira A, Oliveira-Carvalho AL, Zingali RB, Barja-Fidalgo C. Interaction of disintegrins with human neutrophils induces cytoskeleton reorganization, focal adhesion kinase activation, and extracellular-regulated kinase-2 nuclear translocation, interfering with the chemotactic function. FASEB J. 2001;15:1643–1645. doi: 10.1096/fj.00-0812fje. [DOI] [PubMed] [Google Scholar]
- 55.Umanskiy K, Robinson C, Cave C, Williams MA, Lentsch AB, Cuschieri J, Solomkin JS. NADPH oxidase activation in fibronectin adherent human neutrophils: a potential role for β1 integrin ligation. Surgery. 2003;134:378–383. doi: 10.1067/msy.2003.253. [DOI] [PubMed] [Google Scholar]
- 56.Mizgerd JP, Kubo H, Kutkoski GJ, Bhagwan SD, Scharffetter-Kochanek K, Beaudet AL, Doerschuk CM. Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice. J Exp Med. 1997;186:1357–1364. doi: 10.1084/jem.186.8.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 58.Forlow SB, Foley PL, Ley K. Severely reduced neutrophil adhesion and impaired host defense against fecal and commensal bacteria in CD18−/−P-selectin−/− double null mice. FASEB J. 2002;16:1488–1496. doi: 10.1096/fj.02-0230com. [DOI] [PubMed] [Google Scholar]
- 59.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 60.Le Y, Honczarenko M, Glodek AM, Ho DK, Silberstein LE. CXC chemokine ligand 12-induced focal adhesion kinase activation and segregation into membrane domains is modulated by regulator of G protein signaling 1 in pro-B cells. J Immunol. 2005;174:2582–2590. doi: 10.4049/jimmunol.174.5.2582. [DOI] [PubMed] [Google Scholar]
- 61.Wang JF, I, Park W, Groopman JE. Stromal cell-derived factor-1a stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide-3 kinase and protein kinase C. Blood. 2000;95:2505–2513. [PubMed] [Google Scholar]
- 62.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer: a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 63.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu D, Huang CK, Jiang H. Roles of phospholipid signaling in chemoattractant-induced responses. J Cell Sci. 2000;113(Pt 17):2935–2940. doi: 10.1242/jcs.113.17.2935. [DOI] [PubMed] [Google Scholar]
- 65.Cicchetti G, Allen PG, Glogauer M. Chemotactic signaling pathways in neutrophils: from receptor to actin assembly. Crit Rev Oral Biol Med. 2002;13:220–228. doi: 10.1177/154411130201300302. [DOI] [PubMed] [Google Scholar]
- 66.Condliffe AM, Webb LM, Ferguson GJ, Davidson K, Turner M, Vigorito E, Manifava M, Chilvers ER, Stephens LR, Hawkins PT. RhoG regulates the neutrophil NADPH oxidase. J Immunol. 2006;176:5314–5320. doi: 10.4049/jimmunol.176.9.5314. [DOI] [PubMed] [Google Scholar]
- 67.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 68.Brazil DP, Park J, Hemmings BA. PKB binding proteins: getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 69.Kurenova E, Xu LH, Yang X, Baldwin AS, Jr, Craven RJ, Hanks SK, Liu ZG, Cance WG. Focal adhesion kinase suppresses apoptosis by binding to the death domain of receptor-interacting protein. Mol Cell Biol. 2004;24:4361–4371. doi: 10.1128/MCB.24.10.4361-4371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


