Abstract
Neural transplantation is a promising strategy for restoring dopaminergic dysfunction and modifying disease progression in Parkinson's disease (PD). Human embryonic stem cells (hESCs) are a potential resource in this regard because of their ability to provide a virtually limitless supply of homogenous dopaminergic progenitors and neurons of appropriate lineage. The recent advances in developing robust cell culture protocols for directed differentiation of hESCs to near pure populations of ventral mesencephalic (A9-type) dopaminergic neurons has heightened the prospects for PD cell therapy. Here, we focus our review on current state-of-the-art techniques for harnessing hESC-based strategies toward development of a stem cell therapeutic for PD. Importantly, we also briefly describe a novel genetic-programming approach that may address many of the key challenges that remain in the field and that may hasten clinical translation.
Indexing Terms: Parkinson's disease, Cell therapy, Human embryonic stem cell, Transplantation, Stem cell clinical trial, Genetic programming
Parkinson's disease (PD) is the most common motor disorder worldwide, with an overall incidence of 1 in 1,000 among people above the age of 70. The patients suffer from progressive motor dysfunction resulting in resting tremor, bradykinesia, rigidity, postural instability, and motor freezing. Many of the afflicted also exhibit nonmotor symptoms like extended daytime sleepiness, increased fear, and cognitive impairment. Whereas the nonmotor symptoms are accounted for by widespread neuronal damage in the brain, e.g., in the cerebral cortex, the early hallmark motor symptoms that distinguish PD from other neurodegenerative disorders result primarily from loss of A9-type dopaminergic (DA) neurons belonging to the ventrolateral and caudal regions of the substantia nigra pars compacta (SNpc).
Existing therapies for PD only treat symptoms but do not address the underlying cause. Currently the major treatment for PD is l-DOPA, a dopamine precursor (plus carbidopa to prevent systemic effects of the drug). Related drugs, such as DA agonists (e.g., pramipexole and ropinirole) and drugs that slow the catabolism of DA in the brain (e.g., entacapone) are also used. Additionally, a recent, albeit contentious, report suggested that the drug rasagiline, a selective irreversible inhibitor of monoamine oxidase-type B, can slow or delay disease progress and may offer disease modification (Olanow et al., 2009). Taken together, these drugs are considered the gold standard of pharmacological treatment to restore dopaminergic function, although, with time, patients no longer respond to these treatments. Moreover, many patients who are subjected to long-term administration of l-DOPA display on-off fluctuations, off-state dystonia, and dyskinesias (l-DOPA– induced dyskinesia [LID]).
In patients who are nonresponsive to pharmacological interventions, neurosurgical approaches, such as pallidotomy, thalamotomy, or, more recently, deep brain (electrical) stimulation (DBS) are considered. However, some patients do not tolerate these treatments well or suffer serious side effects. Another alternative, neuro-protective or enzymatic enhancement therapy, although promising, suffers from delivery issues pertaining to the short half-life of growth factors in the body, and their inability to diffuse into tissue or across the blood–brain barrier. Clearly, a reliable long-term treatment to halt the progression of the disease and restore motor and cognitive function remains elusive.
At the time of clinical diagnosis of PD, most patients have already incurred a loss of 60–80% of their mid-brain DA neurons. Therefore, replacing these lost neurons by transplantation is a promising strategy for improving dopaminergic function. The rationale behind neural transplantation is that by grafting DA neurons into the dennervated striatum, regulated neurotransmission is reestablished and function restored. In this review we will discuss the potential of human embryonic stem cells (hESC)-based strategies in PD transplantation therapy, and the challenges that remain for clinical translation of this approach.
Human ESCs as a Source of Neural Stem Cells for Neural Transplantation in PD
PD transplantation clinical trials conducted in the 1980s and 1990s were performed by using brain-derived DA neurons obtained from aborted fetuses. These initial open-label studies reported an encouraging outcome and produced great enthusiasm in the field (Barker et al., 2013). However, two National Institutes of Health (NIH)-sponsored double-blind placebo controlled transplantation trials that followed could not meet the primary endpoints and also reported unwanted side effects of the transplanted cells, including increased dyskinesias (Freed et al., 2001; Olanow et al., 2003). However, when some of these patients who received the graft were followed up for 2–4 years after surgery, an improvement in PD motor symptoms was apparent (Ma et al., 2010). Moreover, careful evaluation of the differences between the initial open-label studies and the NIH studies suggested that the reason for the poor outcome in the latter could be attributed, at least in part, to the inadequacies in the transplantation protocol and/or study design (Barker et al., 2013; Petit et al., 2014). However, the findings from this research, which has spanned the past 25 years, have demonstrated the feasibility of cell transplantation approaches in restoring function in human PD patients, as well as provided invaluable information for developing more effective cell therapy strategies (Barker et al., 2013).
Although human fetal tissue transplantation might be beneficial in PD cell therapy, there are limitations associated with this approach, including high tissue variability, lack of scalability, ethical concerns, inability to obtain an epidemiologically meaningful quantity of tissue, and graft-induced dyskinesia (GID) (Hagell et al., 2002). With the discovery of human hESCs and the advent of cell reprograming approaches for human induced pluripotent stem cells (hiPSCs) (Takahashi et al., 2007), human induced neural stem/progenitor cells (hiNSCs) (Ring et al., 2012; Kim et al., 2012; Zhu et al., 2014), and human induced neurons (hiNs) (Ambasudhan et al., 2011; Pang et al., 2011; Caiazzo et al. 2011; Kim et al., 2012), we now have other attractive resources for the supply of appropriate human cells for transplantation.
Cell reprogramming involves the introduction of certain fate-determining “factors” (transcription factors, microRNAs, etc.) into somatic cells, resulting in a stable change in identity to pluripotent (as in hiPSCs), multipotent (as in hiNSCs), or terminally differentiated somatic cells (as in hiNs). Although proof-of-concept studies using hiPSCs (Wernig et al., 2008; Kikuchi et al., 2011) and hiNs (Caiazzo et al., 2011) have suggested great prospects for such approaches in PD cell therapy, these technologies are currently far from clinical translation. Moreover, although these strategies may facilitate personalized medicine in the future, their utility as widely applicable off-the-shelf therapeutics for transplantation for a wide population remains uncertain. Thus, hESCs are a potential alternative in this regard that may be taken to the clinic in a more expeditious fashion.
hESCs are pluripotent cells derived from the inner cell mass of the blastocyst stage of embryos. These cells have numerous virtues that support their candidacy for cell therapy. These features include their self-renewal potential, affording a virtually limitless supply of transplantable cells in terms of scalability, the existence of well-established protocols for good manufacturing practice (GMP)-grade production and cell banking, the availability of prior clinical trials (Tabar and Studer, 2014), and the opportunity for genetic manipulation that allows more robust outcomes. In the following sections we will discuss the current state of the art in developing such an hESC-based PD cell therapy.
Generation of A9 Dopaminergic Neurons from HESCs
Because PD involves selective loss of DA neurons in the SNpc, a key to successful outcomes after transplantation will be the availability of a homogenous population of A9-type DA neurons. Toward this end, numerous tissue culture protocols have been developed based on our understanding of mammalian midbrain development (Ono et al., 2007). Midbrain development is tightly orchestrated by a panoply of transcription factors (e.g., OTX2, LMX1a, FOXa2, LMX1b, MSX1, EN1, NGN2, NURR1, and PITX3) and signaling molecules (e.g., SHH, WNT, and FGF8). Most existing differentiation protocols take advantage of the instructive capacity of these factors in specifying DA neurons (Table 1). Other strategies include co-culture with feeder cells (Perrier et al., 2004; Roy et al., 2006) and genetic programming of ESCs with DA-inducing transcription factors (Kim et al., 2002, 2006; Chung et al., 2005). Co-culture with feeder cells (i.e., stromal cells or astrocytes) has been widely used to improve the yield of cells producing tyrosine hydroxylase (TH), a critical enzyme involved in DA synthesis. However, the non-human origin of many of these feeders and the lack of information regarding the identity of the factors they secrete render poorly defined cultures that are unsuitable for human clinical translation. Similarly, although genetic manipulation of ESCs by overexpression of DA-inducing transcription factors like Nurr1 and Lmx1a can dramatically improve DA neuron differentiation from mouse ESCs (Kim et al., 2002, 2006; Chung et al., 2005), these strategies have not generally worked in hESCs.
Table 1. Prior Publications Utilizing hESCs as a Source for Deriving Neural Progenitors or Dopaminergic Neurons for Transplantation in Parkinson's Disease.
| hESC line | Growth Conditions | Genetic Engineering | Soluble Factors | % DA Cells in Culture | Implanted Cell Survival | References |
|---|---|---|---|---|---|---|
| H1 | Grown as spheres, Rosette isolation | - | bFGF, SHH, FGF8, AA, BDNF, GDNF, TGFβ3, Wnt1, Wnt5a | 17% of total neurons | Many cells grafted, 0% TH+, Vervet monkey | Wakeman et al., 2013 |
| H9, PiPSC | Monolayer | - | SHH, FGF8, BDNF, GDNF, TGFβ3, AA | 40% of sorted cells* | 30,000 TH+ cells/ mm3, Rat | Sunderberg et al., 2013 |
| H9, SA121 | Grown as spheres, Rosette isolation | - | SHH, Noggin, BDNF, GDNF, dbCAMP, DAPT | 81% of cells | 8.4% of grafted TH+ cells, Rat | Kirkeby et al., 2012 |
| H1, H9 | Monolayer | - | SHH, FGF8, BDNF, GDNF, TGFβ3, AA | 80% of all cells | 15,000 TH + grafted cells, Rat | Kriks et al., 2011 |
| HSF-6 | MS5 or PA6 co-culture | FOXA2 and NURR1 Overexpression | bFGF, BDNF, GDNF, dbCAMP, AA | 33% of transduced. Total transduction efficiency 10% | - | Lee, H.S., 2010 |
| H9 | Grown as spheres, Rosette Isolation | - | SHH, FGF8, Wnt3A, BDNF, GDNF, TGFβ3, AA, dbCAMP | 40% of Tuj+ cells | 1273 TH+ cells/ graft, Rat | Yang et al., 2008 |
| H7, H9 | MS5 co-culture | - | Noggin, SHH, FGF8, BDNF, GDNF, TGFβ3, AA | 23% | 160 cells (mixed neuronal sub-types)/graft, Rat | Sonnatag et al., 2007 |
| H1, H9 | hTERT immortalized astrocyte co-culture | - | SHH, FGF8, BDNF, GDNF | 60% of Tuj+ cells (40% of total cells Tuj+) | 20,000 TH+ cells/ mm3, Rat | Roy et al., 2006 |
| SA002 | PA6 co-culture | - | - | 7% of total cells | 10–50 TH+ cells/ graft | Brederlau et al., 2006 |
| HSF-6, SNUhES- 3, Miz-hES-1 | PA6 co-culture | - | bFGF, SHH, FGF8, NT3, BDNF | 41% of 30% Tuj+ cells | Rare TH+ cells | Park et al., 2005 |
| BG01 | PA6 co-culture | - | bFGF | 87% of colonies | 9 cells/section, Rat | Zeng et al., 2004 |
| BG01, BG03 | Grown as spheres | - | bFGF, BDNF, GDNF | 75% of Tuj+ cells | Occasional TH+ cells | Schulz et al., 2004 |
| HES1 | Grown as spheres | - | bFGF, EGF | 1% | 389 TH+ cells/ graft | Ben-Hur et al., 2004 |
Sorted for CD29low NCAM +
For abbreviations, see list.
Preclinical testing with cells generated by using the above strategies have attained only limited success so far. At least part of the reason for this may be the heterogeneity of the cells transplanted. Moreover, the proportion of bona fide A9-type DA neurons (defined by co-expression of key transcription factors like LMX1A/ FOXA2, the expression of inwardly rectifying potassium channels [GIRK2], and the capacity to produce pacemaker activity mediated by Cav1.3 calcium channels) among the total TH+ neurons described in these protocols is unclear. Recently, addressing this aspect of the problem, Lorenz Studer's group described an elegant floor-plate–based strategy involving tight temporal control of key factor exposure of cultures to yield over 80% expression of TH in the differentiated cells, many of which exhibited the A9 phenotype (Kriks et al., 2011). The authors were also able to prove the functionality of these cells by electrophysiology as well as by evaluating DA production, transport, and release. We have recently demonstrated the reproducibility of this protocol to yield mature A9 DA neurons in a relatively short period (∼6 weeks) of neural differentiation from pluripotent cells (Ryan et al., 2013). The overall scheme for A9 type hNSC production and characterizations leading to human transplantation is depicted in Figure 1.
Figure 1.
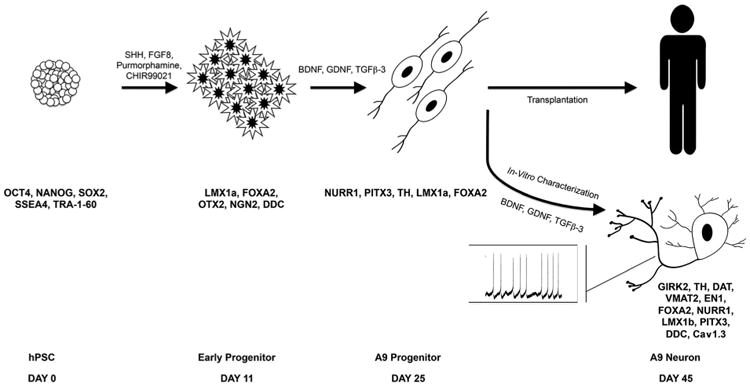
Schematic describing the floor plate–based method (Kirks et al., 2011) for generation of A9-type dopaminergic (DA) neural progenitors from hESCs. Here, hESCs and their progeny are exposed to key signaling molecules (SHH, WNT, and FGF8) in a temporally controlled manner to yield robust generation of A9-type DA neural precursors for transplantation. The cells co-express NURR1, LMX1a, and FOXA2 at approximately day 25, the stage at which they are used for transplantation. When allowed to differentiate in vitro, these cells develop clear A9 DA neuronal phenotypes by day 35–45, expressing key markers (GIRK2, Cav1.3, PITX 3, and TH) and exhibiting hallmark electrophysiological properties, including spontaneous bursting. For abbreviations, see list.
Before embarking on transplantation studies, it is also critical to ensure the scalability of the methodology to yield sufficient quantities of transplantable cell types. Moreover, the long-term karyotypic stability, absence of genomic alterations or gene expression changes, and nontumorogenic nature of the cells (e.g., absence of growth in soft agar) need to be established.
hESC Transplantation in Preclinical Models
The most common PD models used for transplantation studies are rodent and monkeys in which brain lesions are induced by exposure to 6-hydroxydopamine (6-OHDA) or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The pioneering studies of hESC-derived DA neuronal transplantation into 6-OHDA–lesioned rats conducted by Benjamin Reubinoff's group revealed low levels of graft survival and modest recovery of amphetamine/apomorphine-induced rotation behavior in the transplanted rats (Ben-Hur et al., 2004). Other hESC transplantation studies reported similar findings (Zeng et al., 2004; Roy et al., 2006; Sonnatag et al., 2007; Yang et al., 2008). These results were in sharp contrast to the much more encouraging data reported by others in rodents and primates using mouse ESCs and monkey ESCs, respectively (Kawasaki et al., 2000; Kim et al., 2002; Takagi et al., 2005). The transplanted DA neurons or progenitor cells in those models engrafted well and produced good behavioral recovery.
Recent improvements in cell culture protocols in producing a more homogenous population of mesencephalon DA neurons have produced new hope for the success of hESC-based strategies. Two new studies using temporal activation of WNT signaling produced efficient differentiation of hESC to DA neural progenitors, and substantial improvement in animal behavior was noted when these cells were transplanted in vivo (Kriks et al., 2011; Kirkeby et al., 2012). Especially encouraging is the study by Studer and Kordower's groups demonstrating robust, long-term engraftment of such cells and complete restoration of behavior in immunosuppressed rodent models; they also demonstrated scalability of this approach in a primate model (Kriks et al., 2011). This study raises the exciting prospect of clinical translation of these approaches if acceptable levels of efficacy and toxicity can be established. Interestingly, robust outgrowth of fibers from the graft has been touted as an important indication of success, and this is still being optimized in the primate model. However, a legitimate question is whether such outgrowth might also potentially contribute to aberrant innervation and thus increased DA-induced dyskinesias in transplanted subjects. Although increased outgrowth generally correlates with improved function, below we consider the use of genetically programmed A9-type DA neurons, which not only allows robust DA tissue replacement but also limits excessive outgrowth and, thus, aberrant innervation. We hypothesize therefore that such an approach may possibly provide improved transplantation outcomes.
Clinical Translation
Currently, there are no US Food and Drug Administration (FDA)-approved ongoing clinical trials using hESC-derived cells for transplantation in PD. However, the field is approaching the clinical stage, and these studies will receive guidance from the lessons learned from earlier trials using fetal ventral mesencephalon (fvm) tissue (Barker et al., 2013; Petit et al., 2014). Moreover, new insights may also be obtained from the TRANSEURO studies, the recent multicenter European initiative on PD transplantation using fvm tissue (www.transeuro.org.uk).
Trial design
Trial design should aim at producing clear benefits to the patient motoric ability via DA neuron (or precursor) transplantation above a placebo effect. Although fvm-based studies have shown that DA fetal grafts can survive, reinnervate PD brains, release DA, and integrate into host neural circuits, functional improvements in the patients (evaluated by the Unified Parkinson's Disease Rating Scale [UPDRS motor scale]) have been highly variable. This variability is evident when the initial open-label trials are directly compared with the double-blind control studies supported by the NIH, suggesting that the benefits seen in the open-label trials may be attributed to placebo effects (Freed et al., 2001; Olanow et al., 2003; Petit et al., 2014). Indeed, it is known that patient expectations regarding treatment outcome may result in DA release and complicate the interpretation of treatment benefits (de la Fuente-Fernandez et al., 2001). Additionally, drawbacks in the NIH studies may be attributed, at least in part, to inadequacies in cell preparation and storage, patient selection, trial design, immunosuppression, and selected endpoints (Petit et al., 2014). One of the NIH studies did not include immunosuppression, whereas the second study used cyclosporin A–based immunosuppression for only 6 months, but the patients in this second study developed GID after the immunosuppression was stopped. Although it is not clear whether patient complications can be fully attributed to absence of or poor immunosuppression, immunosuppression seems to be a critical requirement in neural transplantations.
This conclusion is further supported by rodent studies in which either genetic suppression of T-cell activation in the host (Rong et al., 2014) or nuclear transfer– mediated therapeutic cloning to confer immune compatibility between donor and recipient (Tabar et al., 2008) produced robust transplantation outcomes. These results, combined with those of Piccini et al. (2005), in which no adverse clinical outcome was noted when immunosuppression was withdrawn 2 years after transplantation, suggest an optimal period of immunosuppression between 6 months and 2 years post transplantation for future trials. Moreover, more prolonged immunosuppression may also have additional side effects, including opportunistic infections precipitating graft rejection (Lopez et al., 2006). In this context, creation of genotyped/HLA-typed hESC banks may be able to supply cells matching the recipient genotype; similar strategies have been proposed for hiPSC-based transplantation in the future (Taylor et al., 2005; Nakajima et al., 2007).
A major goal of PD transplantation approaches is to devise a therapy that provides not only symptomatic relief but also long-term disease-modifying benefits. Clinic trial endpoints need to be set with this in mind. After transplantation, a reasonable indicator for benefit of therapy is improved motor function measured by UPDRS along with clinical improvement on the Hoehn and Yahr scale such that an improvement category is achieved, e.g., from Stage IV to Stage III, with no signs of GID, and reduced dependence on levodopa. On the basis of the fvm-based trials, it also seems reasonable that for both open-label and double-blinded trials, at least 2 years of follow- up is required to yield a meaningful clinical outcome. Moreover, patients should submit subjective self-evaluations and be monitored by functional imaging using positron emission tomography (PET) at regular intervals (e.g., with ligands like 18F-dopa, 23I-FP-SPECT, 11C-raclopride displacement for DA-associated changes, or 11C-DASB for presynaptic serotonin [5-HT] terminals).
Patient selection
Generally patients who no longer respond to pharmacological intervention are selected for cell therapy trials. However, prior trial results suggest a requirement for more careful selection of patients. It is well known that advanced age is often associated with poor clinical outcome in PD. A retrospective analysis of patents recruited in prior studies indicated that subjects who exhibit more benign symptoms and those who are <60 years of age have a better prognosis (Evans et al., 2012; Barker et al., 2013). Moreover, DA neuronal transplantation may be more effective in patients whose clinical symptoms are correlated with restricted nigral pathology rather than those who have a widespread brain pathology associated with mild cognitive impairment (MCI) or even frank dementia. Moreover, because a number of groups have suggested that a history of LID is a risk factor for developing GID post transplantation, this parameter should also be factored into clinical trial design (Lane et al., 2009; Garcia et al., 2011).
Cell preparation and surgical aspects
Although many possible factors have been suggested to explain GIDs observed in a subset of patients, a potential factor is the presence of serotonergic (5-HT– containing) cells in the grafts. Ensuring purity of the cell preparations to maximize DA cells while minimizing serotonergic cells would be beneficial in this regard. For an hESC-based, off-the-shelf cell product to be successful in PD therapy, methods for cryopreservation, storage, and robust recovery of functional cells post thaw need to be thoroughly optimized. Moreover, the optimal stage of DA neural cells (e.g., progenitors vs. mature neurons) at the time of transplantation needs to be determined. A recent study using mouse ESC transgenic reporter lines provided compelling data in this regard, showing that the Nurr1+ neuroblast stage is an ideal stage for grafting (Ganat et al., 2012).
Although there is no consensus on the number of surviving cells that are required to produce clinical benefit, it has been suggested that at least 100,000 surviving DA neurons per putamen are needed for a successful outcome (Hagell and Brundin, 2001). Moreover, various groups have tested both bilateral and unilateral grafts, but there seems as yet to be no consensus on which will work best, although more recent studies have favored bilateral putamenal grafts.
Critical Remaining Issues for hESC-Based Transplantation Therapy
Although hESCs are currently the most reliable and self-renewable source of homogeneous and qualified populations of DA neurons for cell transplantation therapy in PD, several challenges remain for realizing their clinical potential. The major hurdles include devising methods for sustained clinical-grade production of immune-tolerable and genetically stable cells, prevention of hyperproliferation/teratoma formation of the graft, improving graft survival accompanied by appropriate differentiation and integration of new A9-type DA neurons into host circuits, and strategies to avoid untoward side effects like GID, as have been observed in fvm tissue trials. As discussed in the previous sections, development of robust protocols that generate homogenous populations of A9 DA neurons and greatly optimized study design may circumvent many of these issues. Others have also proposed various strategies to avoid tumor formation, including prolonged predifferentiation of hESCs (Doi et al., 2012), engineering cells to block tumorigenic pathways (Parish et al., 2005; Jung et al., 2007), and sorting to select differentiated cells prior to transplantation.
We have recently devised an alternate strategy to address many of the issues outlined above. We have produced stable hESCs cell lines that are genetically engineered to express a constitutively active form of the transcription factor MEF2C (designated MEF2CA). The scientific rationale behind using the myocyte enhancer factor 2 (MEF2) family is that manipulation of this gene alone is sufficient to improve transplantation results in multiple ways. MEF2 is antiapoptotic (Mao et al. 1999; Okamoto et al., 2000; Li Z et al., 2008), a property that will promote graft survival. MEF2 is also a neurogenic factor and activates many genes, including Nurr1, which is critical for A9-type DA neurogenesis (Li H et al., 2008; Cho et al., 2011); this feature promotes A9-type DA neuronal differentiation in vitro and prevents hyperproliferation of cells in vivo, also avoiding the generation of 5-HT–producing cells that would otherwise contribute to dyskinesias. Additionally, in mature neurons MEF2 can downregulate synapse formation (Flavell et al., 2006; Shalizi et al., 2006), a function required for the prevention of aberrant outgrowth and maintenance of optimal connectivity among mature neurons. This aspect of MEF2 activity may prove beneficial in preventing dyskinesias. Finally, MEF2 can also regulate the expression of trophic factors such as brain-derived neurotrophic factor (BDNF) (Lyons et al., 2012), which can augment the repair process by activating host cells.
We initially tested the concept of programming stem cells with this transcription factor by using lentiviral overexpression of MEF2CA in hESC-derived hNSCs. We reported that the transplanted cells significantly improved behavioral deficits in rodent PD models and generated more neurons, particularly of the TH+ DA phenotype (Fig. 2). Interestingly, the transplanted brains also displayed many TH+ fibers in the host striatal parenchyma that were not GFP+, suggesting a non-cell–autonomous or trophic effect of the graft on the host tissue (Fig. 2A,B).
Figure 2.
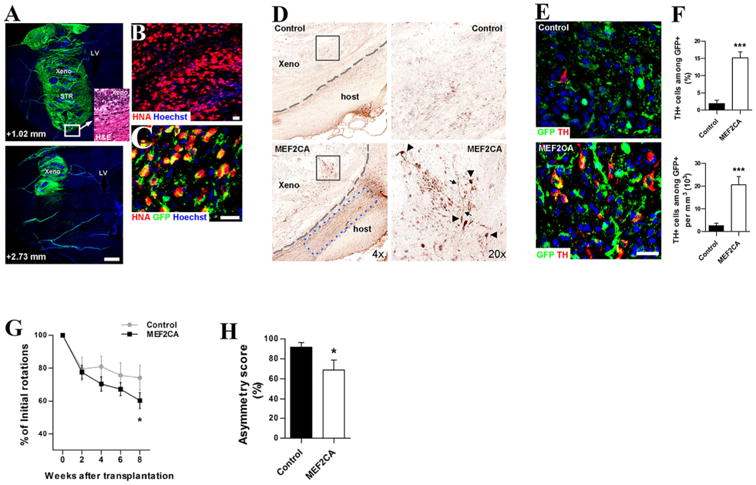
Functional recovery of 6-OHDA-lesioned rats after MEF2CA-programmed hNSC transplantation. A: Transplanted hNSCs (GFP+/ green) shown in sagittal brain sections along the mediolateral axis. Hoechst dye-labeled DNA (blue). Inset: Hematoxylin/eosin (H&E) stain shows xenograft/host boundary (dashed line). B: Human origin of cells verified by human nuclear antigen (HNA). C: Infected cells (GFP+) co-expressed HNA (red), yielding merged yellow stain. D: Density of TH+ neurons in MEF2CA-programmed hNSC xenografts (xeno) was higher than for control-hNSCs (boxed areas enlarged in right panels). Arrowheads, TH+ neuronal cell bodies; arrows, neuronal processes; dashed lines, graft/host boundary; blue dashed box, endogenous host TH+ fibers. E: MEF2CA-hNSC versus control-hNSC DA/TH+ neurons. Transplanted cells (GFP+); TH+ (red). F: Upper: TH+ neurons as percent of GFP+ cells. Lower: density of TH+/GFP+ cells as absolute cell number. Values are mean + SEM, n= 19; ***,P< 0.0001 by t-test. G: Apomorphine-induced rotations in 6-OHDA-lesioned rats after hNSC transplantation. Values are mean ± SEM, n= 14. Rats receiving MEF2CA-programmed hNSCs show increasing improvement versus controls (*, P≤ 0.035 by ANOVA). H: Cylinder asymmetry test 9 weeks post transplant. Values are mean + SEM, n= 11; *, P < 0.03 by t-test. For abbreviations, see list. Figure adapted from Cho et al. (2011). Scale bar= 1 mm in A; 25 μm in B,C.
Encouraged by these findings, we recently developed hESC–MEF2CA stable lines by introducing the constitutively active form of MEF2 (MEF2CA) into an H9 hESC clone (Fig. 3). The generation of such stable lines should allow the scalability needed to perform future human clinical trials. In preliminary studies on the MEF2CA–hESC stable lines using the floor-plate method for differentiation (Kirks et al., 2011), we found that these cells followed a neural differentiation program typical of mesencephalic precursors (Fig. 4A,B) and yielded A9-type DA neurons at >90% purity by day 45 in vitro (Fig. 4C–F). Furthermore, upon transplantation into a 6-OHDA–lesioned rat model, the MEF2CA-programmed hNSCs engrafted well (Fig. 5) and yielded significant behavioral improvement on tests of amphetamine-induced rotation and paw preference. Similarly, good engraftment and improved behavior were noted in parallel transplantation experiments performed in the MPTP-lesioned monkey model (data not shown). Moreover, with MEF2 driving neuronal differentiation of the stable line, there was no trace of hyper-proliferation or tumor formation.
Figure 3.
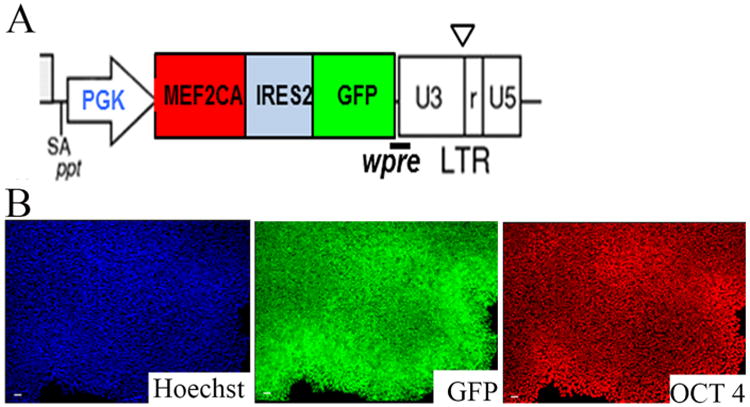
Sustained and homogenous expression of the transgene in MEF2CA-programmed hESC stable lines. A: Schematic depicting the lentiviral construct used for generating the MEF2CA-hESC stable lines. B: Representative image of an MEF2CA-hESC colony displaying stable homogenous expression of GFP (green; middle panel) and OCT3/4 (red; right panel) at passage 5. hESCs (H9 line) were transduced with lentivirus carrying the vector shown in A. Forty-eight hours after viral transduction, the cells were FACS-sorted to select for GFP-positive cells and were seeded as single cells at clonal density on Matrigel-coated plates containing hESC medium supplemented with ROCK inhibitor. Individual colonies were isolated, expanded, and then immunostained with GFP and OCT3/4 antibodies to test their homogeneity and stable expression of the transgene. For abbreviations, see list. Scale bar =50 μm XX in B.
Figure 4.
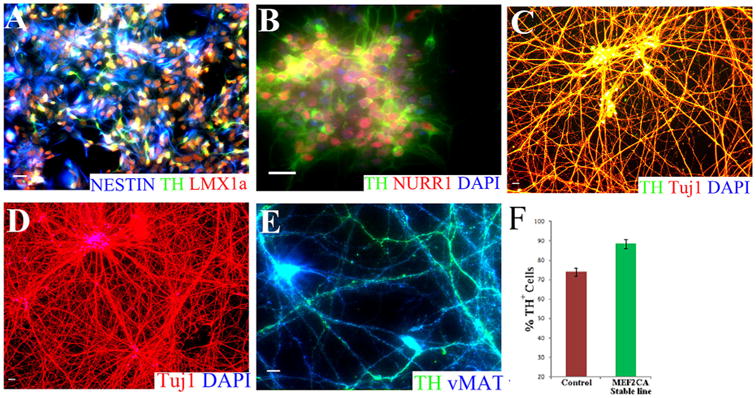
Highly efficient generation of DA neurons from MEF2CA-hESC stable lines. A: Representative image of day 16 hNSCs showing immunostaining for the stem cell marker NESTIN (blue) and DA lineage markers LMX1A (red) and TH (green). B: Immunocytochemical image of day 20 hNSCs displaying homogenous expression of DA lineage markers TH (green) and Nurr1 (red). DAPI-stained nuclei are shown in blue. Note the high homogeneity of the cells expressing DA lineage-specific proteins. C: Representative image of day 60 neuronal cultures displaying TUJ1/TH/DAPI immunoreactivity. D: Representative image of day 60 neuronal cultures displaying TUJ1/DAPI immunoreactivity. Note that 100% of the cells manifest neuronal identity. E: The vast majority of the neurons at day 60 of differentiation exhibit co-expression of the DA neuronal markers vMAT (blue) and TH (green). F: Histogram showing the proportion of TUJ1-positive cells that were also positive for TH after 60 days in culture. Neuronal cultures derived from MEF2CA-hESC stable lines consistently produced significantly more DA neurons compared with control hESCs that were also subjected to Studer's floor plate method for DA neuron differentiation. Images shown in A–E show merged fluorescent channels. For abbreviations, see list. Scale bar = 50 μm in A–E.
Figure 5.
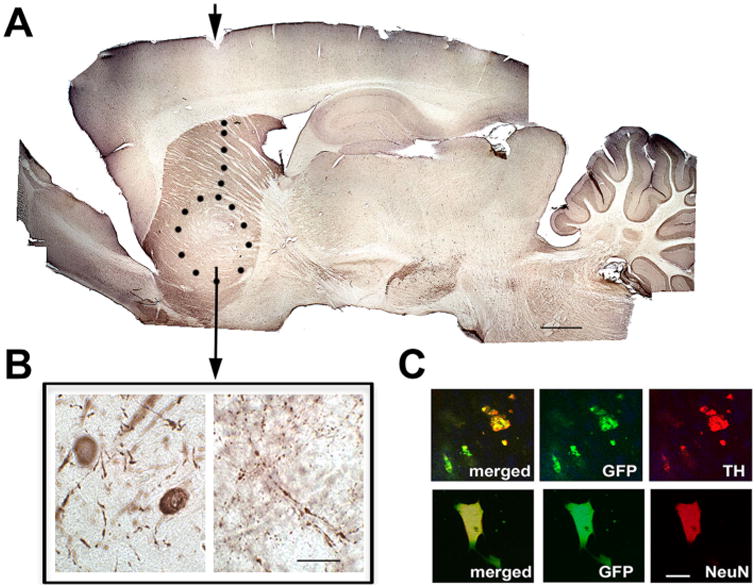
Histology of rat brain 6 months after striatal transplantation of the stable MEF2CA-hESC-derived neural progenitors. These data are representative of six rats that were transplanted, monitored behaviorally, and then sacrificed and perfused for histological analysis. A: Representative image of a whole-mount section of rat brain immunostained with the dopaminergic neuronal marker TH showing the extent of the implant in the striatum (indicated by dotted line with arrowhead showing injection site). B: Higher magnification image from the grafted region, revealing TH+ neuronal cell bodies and neuronal fibers. C: Representative immunofluorescence images reflecting the fact that GFP+ cells (indicating transplanted cells) were also positive for NeuN and TH (neuronal and DA markers, respectively). Over 90% of the GFP+ cells were also TH+. For abbreviations, see list. Scale bar = 250 μm in A; 25 μm in B; 10 μm in C.
Outlook for hESC-Based Transplantation Therapy for PD
For a cell transplantation strategy to be widely acceptable, its benefits must outweigh the various surgical and medical complications involved. More importantly, the procedure should be able to confer disease-modifying benefit that no other existing PD therapy can provide. Ongoing fetal transplantation trials, especially the concerted efforts of TRANSEURO, are likely to provide proof in this regard that will generate confidence in the minds of scientists, physicians, and patients alike in this approach. However, owing to limited tissue availability and other issues surrounding cell therapy, the use of fetal tissue is unlikely to become a routine treatment for PD. Currently, the major focus in this area is on developing pluripotent/reprogrammed cell-based strategies. With interest and support from around the globe, including philanthropic and governmental bodies like the California Institute of Regenerative Medicine (CIRM), and in view of new NIH policies favoring hESC research, major developments in the area of clinical transplantation of hESC-derived cells are expected in the days to come.
Acknowledgments
We apologize to colleagues whose important work could not be discussed here due to space limitations.
Grant sponsor: the California Institute of Regenerative Medicine; Grant numbers: RC1–00125-1 and TR4–06788.
Abbreviations
- AA
ascorbic acid
- bFGF
basic fibroblast growth factor
- BDNF
brain-derived neurotrophic factor
- DA
dopamine
- DAPI
4,6-diamidino-2-phenylindole
- DAPT
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester
- dbCAMP
dibutyryl-cyclic adenosine monophosphate
- EGF
epidermal growth factor
- FGF8
fibroblast growth factor 8
- FOXA2
forkhead box A2
- GDNF
glial cell–derived neurotrophic factor
- GFP
green fluorescent protein
- hESC
human embryonic stem cell
- hNSC
human neural stem/progenitor cell
- hPSC
human pluripotent stem cell
- IRES2
Internal Ribosome Entry Site 2
- LMX1a
LIM homeobox transcription factor 1
- LTR
Long Terminal Repeat
- LV
Lateral Ventricle
- MEF2
myocyte enhancer factor 2
- MS5
mouse bone marrow–derived stromal cell line
- NeuN
Neuronal Nuclei
- NURR1
nuclear receptor–related 1 protein
- OCT3/4
Octamer-binding protein 3/4
- PA6
skull bone marrow–derived stromal cell line
- PGK
Phosphoglycerate Kinase
- SA
Splice Acceptor
- SHH
sonic hedgehog
- STR
Striatum
- TGFβ3
transforming growth factor β3
- TH
tyrosine hydroxylase
- vMAT
Vesicular Monoamine Transporter
- Wnt 1
wingless-type MMTV integration site family, member 1
- Wnt 5A
wingless-type MMTV integration site family, member 5A
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
Literature Cited
- Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RA, Barrett J, Mason SL, Bjorklund A. Fetal dopa-minergic transplantation trials and the future of neural grafting in Parkinson's disease. Lancet Neurol. 2013;12:84–91. doi: 10.1016/S1474-4422(12)70295-8. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, Reubinoff BE. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22:1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U, Carta M, Hanse E, Takahashi J, Sasai Y, Funa K, Brundin P, Eriksson PS, Li JY. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–40. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Cho EG, Zaremba JD, McKercher SR, Talantova M, Tu S, Masliah E, Chan SF, Nakanishi N, Terskikh A, Lipton SA. MEF2C enhances dopaminergic neuron differentiation of human embryonic stem cells in a parkinsonian rat model. PLoS One. 2011;6:e24027. doi: 10.1371/journal.pone.0024027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Hedlund E, Hwang M, Kim DW, Shin BS, Hwang DY, Kang UJ, Isacson O, Kim KS. The homeodomain transcription factor Pitx3 facilitates differentiation of mouse embryonic stem cells into AHD2-expressing dopaminergic neurons. Mol Cell Neurosci. 2005;28:241–252. doi: 10.1016/j.mcn.2004.09.008. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- Doi D, Morizane A, Kikuchi T, Onoe H, Hayashi T, Kawasaki T, Motono M, Sasai Y, Saiki H, Gomi M, Yoshikawa T, Hayashi H, Shinoyama M, Refaat MM, Suemori H, Miyamoto S, Takahashi J. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson's disease. Stem Cells. 2012;30:935–945. doi: 10.1002/stem.1060. [DOI] [PubMed] [Google Scholar]
- Evans JR, Mason SL, Barker RA. Current status of clinical trials of neural transplantation in Parkinson's disease. Prog Brain Res. 2012;200:169–198. doi: 10.1016/B978-0-444-59575-1.00008-9. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Garcia J, Carlsson T, Dobrossy M, Nikkhah G, Winkler C. Extent of pre-operative L-DOPA-induced dyskinesia predicts the severity of graft-induced dyskinesia after fetal dopamine cell transplantation. Exp Neurol. 2011;232:270–279. doi: 10.1016/j.expneurol.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Ganat YM, Calder EL, Kriks S, Nelander J, Tu EY, Jia F, Battista D, Harrison N, Parmar M, Tomishima MJ, Rutishauser U, Studer L. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J Clin Invest. 2012;122:2928–2939. doi: 10.1172/JCI58767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagell P, Brundin P. Cell survival and clinical outcome following intrastriatal transplantation in Parkinson disease. J Neuropathol Exp Neurol. 2001;60:741–752. doi: 10.1093/jnen/60.8.741. [DOI] [PubMed] [Google Scholar]
- Hagell P, Piccini P, Bjorklund A, Brundin P, Rehncrona S, Widner H, Crabb L, Pavese N, Oertel WH, Quinn N, Brooks DJ, Lindvall O. Dyskinesias following neural transplantation in Parkinson's disease. Nat Neurosci. 2002;5:627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- Jung J, Hackett NR, Pergolizzi RG, Pierre-Destine L, Krause A, Crystal RG. Ablation of tumor-derived stem cells transplanted to the central nervous system by genetic modification of embryonic stem cells with a suicide gene. Hum Gene Ther. 2007;18:1182–1192. doi: 10.1089/hum.2007.078. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, Saiki H, Miyamoto S, Takahashi J. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson's disease. J Parkinsons Dis. 2011;1:395–412. doi: 10.3233/JPD-2011-11070. [DOI] [PubMed] [Google Scholar]
- Kim DW, Chung S, Hwang M, Ferree A, Tsai HC, Park JJ, Nam TS, Kang UJ, Isacson O, Kim KS. Stromal cell-derived inducing activity, Nurr1, and signaling molecules synergistically induce dopaminergic neurons from mouse embryonic stem cells. Stem Cells. 2006;24:557–567. doi: 10.1634/stemcells.2005-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ambasudhan R, Ding S. Direct lineage reprogramming to neural cells. Curr Opin Neurobiol. 2012;22:778–784. doi: 10.1016/j.conb.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane EL, Vercammen L, Cenci MA, Brundin P. Priming for L-DOPA-induced abnormal involuntary movements increases the severity of amphetamine-induced dyskine-sia in grafted rats. Exp Neurol. 2009;219:355–358. doi: 10.1016/j.expneurol.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Lee HS, Bae EJ, Yi SH, Shim JW, Jo AY, Kang JS, Yoon EH, Rhee YH, Park CH, Koh HC, Kim HJ, Choi HS, Han JW, Lee YS, Kim J, Li JY, Brundin P, Lee SH. Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival. Stem Cells. 2010;28:501–512. doi: 10.1002/stem.294. [DOI] [PubMed] [Google Scholar]
- Li H, Radford JC, Ragusa MJ, Shea KL, McKercher SR, Zaremba JD, Soussou W, Nie Z, Kang YJ, Nakanishi N, Okamoto S, Roberts AJ, Schwarz JJ, Lipton SA. Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci U S A. 2008;105:9397–9402. doi: 10.1073/pnas.0802876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, McKercher SR, Cui J, Nie Z, Soussou W, Roberts AJ, Sallmen T, Lipton JH, Talantova M, Okamoto S, Lipton SA. Myocyte enhancer factor 2C as a neurogenic and antiapoptotic transcription factor in murine embryonic stem cells. J Neurosci. 2008;28:6557–6568. doi: 10.1523/JNEUROSCI.0134-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MM, Valenzuela JE, Alvarez FC, Lopez-Alvarez MR, Cecilia GS, Paricio PP. Long-term problems related to immunosuppression. Transplant Immunol. 2006;17:31–35. doi: 10.1016/j.trim.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Lyons MR, Schwarz CM, West AE. Members of the myocyte enhancer factor 2 transcription factor family differentially regulate Bdnf transcription in response to neuronal depolarization. J Neurosci. 2012;32:12780–12785. doi: 10.1523/JNEUROSCI.0534-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Tang C, Chaly T, Greene P, Breeze R, Fahn S, Freed C, Dhawan V, Eidelberg D. Dopamine cell implantation in Parkinson's disease: long-term clinical and (18)F-FDOPA PET outcomes. J Nucl Med. 2010;51:7–15. doi: 10.2967/jnumed.109.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science. 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- Nakajima F, Tokunaga K, Nakatsuji N. Human leukocyte antigen matching estimations in a hypothetical bank of human embryonic stem cell lines in the Japanese population for use in cell transplantation therapy. Stem Cells. 2007;25:983–985. doi: 10.1634/stemcells.2006-0566. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Krainc D, Sherman K, Lipton SA. Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation. Proc Natl Acad Sci U S A. 2000;97:7561–7566. doi: 10.1073/pnas.130502697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, Langston W, Melamed E, Poewe W, Stocchi F, Tolosa E. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N Engl J Med. 2009;361:1268–1278. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- Ono Y, Nakatani T, Sakamoto Y, Mizuhara E, Minaki Y, Kumai M, Hamaguchi A, Nishimura M, Inoue Y, Hayashi H, Takahashi J, Imai T. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134:3213–3225. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CL, Parisi S, Persico MG, Arenas E, Minchiotti G. Cripto as a target for improving embryonic stem cell-based therapy in Parkinson's disease. Stem Cells. 2005;23:471–476. doi: 10.1634/stemcells.2004-0294. [DOI] [PubMed] [Google Scholar]
- Park CH, Minn YK, Lee JY, Choi DH, Chang MY, Shim JW, Ko JY, Koh HC, Kang MJ, Kang JS, Rhie DJ, Lee YS, Son H, Moon SY, Kim KS, Lee SH. In vitro and in vivo analyses of human embryonic stem cell-derived dopa-mine neurons. J Neurochem. 2005;92:1265–76. doi: 10.1111/j.1471-4159.2004.03006.x. [DOI] [PubMed] [Google Scholar]
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit GH, Olsson TT, Brundin P. The future of cell therapies and brain repair: Parkinson's disease leads the way. Neuropathol Appl Neurobiol. 2014;40:60–70. doi: 10.1111/nan.12110. [DOI] [PubMed] [Google Scholar]
- Piccini P, Pavese N, Hagell P, Reimer J, Bjorklund A, Oertel WH, Quinn NP, Brooks DJ, Lindvall O. Factors affecting the clinical outcome after neural transplantation in Parkinson's disease. Brain. 2005;128:2977–2986. doi: 10.1093/brain/awh649. [DOI] [PubMed] [Google Scholar]
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Z, Wang M, Hu Z, Stradner M, Zhu S, Kong H, Yi H, Goldrath A, Yang YG, Xu Y, Fu X. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell. 2014;14:121–130. doi: 10.1016/j.stem.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopa-minergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Ryan SD, Dolatabadi N, Chan SF, Zhang X, Akhtar MW, Parker J, Soldner F, Sunico CR, Nagar S, Talantova M, Lee B, Lopez K, Nutter A, Shan B, Molokanova E, Zhang Y, Han X, Nakamura T, Masliah E, Yates JR, 3rd, Nakanishi N, Andreyev AY, Okamoto S, Jaenisch R, Ambasudhan R, Lipton SA. Isogenic human iPSC Parkinson's model shows nitrosative stress-induced dysfunction in MEF2-PGC1alpha transcription. Cell. 2013;155:1351–1364. doi: 10.1016/j.cell.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- Schulz TC, Noggle SA, Palmarini GM, Weiler DA, Lyons IG, Pensa KA, Meedeniya AC, Davidson BP, Lambert NA, Condie BG. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22:1218–38. doi: 10.1634/stemcells.2004-0114. [DOI] [PubMed] [Google Scholar]
- Sonnatag KC, Pruszak J, Yoshizaki T, Arensbergen J, Sanches-Pernaute R, Isacson O. Enhanced yield of neuroepi-thelial precursors and midbrain-like dopaminergic neurons from embryonic stem cells using the bone morphogenic protein antagonist Noggin. Stem Cells. 2007;25:411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg M, Bogetofte H, Lawson T, Jansson J, Smith G, Astradsson A, Moore M, Osborn T, Cooper O, Spealman R, Hallett P, Isacson O. Improved cell therapy protocols for Parkinson's disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells. 2013;31:1548–1562. doi: 10.1002/stem.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet. 2014;15:82–92. doi: 10.1038/nrg3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar V, Tomishima M, Panagiotakos G, Wakayama S, Menon J, Chan B, Mizutani E, Al-Shamy G, Ohta H, Wakayama T, Studer L. Therapeutic cloning in individual parkinsonian mice. Nat Med. 2008;14:379–381. doi: 10.1038/nm1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115:102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- Wakeman DR, Weiss S, Sladek JR, Elsworth JD, Bauereis B, Leranth C, Hurley PJ, Roth RH, Redmond E. Survival and integration of neurons derived from human embryonic stem cells in MPTP lesioned primates. Cell Transplant. 2013 doi: 10.3727/096368913X664865. [DOI] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang ZJ, Oldenburg M, Ayala M, Zhang SC. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008;26:55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Cai J, Chen J, Luo Y, You ZB, Fotter E, Wang Y, Harvey B, Miura T, Backman C, Chen GJ, Rao MS, Freed WJ. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22:925–940. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]
- Zhu S, Ambasudhan R, Sun W, Kim HJ, Talantova M, Wang X, Zhang M, Zhang Y, Laurent T, Parker J, Kim HS, Zaremba JD, Saleem S, Sanz-Blasco S, Masliah E, McKercher SR, Cho YS, Lipton SA, Kim J, Ding S. Small molecules enable OCT4-mediated direct reprogramming into expandable human neural stem cells. Cell Res. 2014;24:126–129. doi: 10.1038/cr.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]


