Abstract
Mosquito eggs laid on water surfaces typically hatch spontaneously soon after the embryos within them become fully formed first-instar larvae. However, we have found that Anopheles gambiae Giles, an important vector of malaria in Africa, exhibits delayed hatching until the water surface is agitated, a feature overlooked in most laboratory colonies. Agitation within 24 h postoviposition, before embryonation was complete, failed to stimulate delayed postembryonic hatching of isolated eggs on the following day (day 2), when <1% had hatched spontaneously. However, 5 min of water agitation of these dormant pharate first-instar larvae on day 2 resulted in an almost immediate hatch of 63.3 versus 0% of nonagitated controls, plus another 3.9 versus 0.3%, respectively, during the following 24 h. With daily agitation, installment hatching occurred mainly during 2– 6 d postoviposition. The mean cumulative hatch after 7 d of daily agitation was 83.1 versus 1.1% of nonagitated eggs. Experiments with eggs in groups demonstrated that egg density and activity of already-hatched larvae had no stimulatory effect. Eggs stored 1–4 wk at 25.5 or at 15.5°C, and then agitated daily for 6 d at 25.5°C, showed a gradual decline in viability. Viability was sustained longer at the lower temperature. Implications of agitation-induced egg hatching for rainy-season and dry-season ecology of An. gambiae are discussed. Suspended hatching and cool storage already are proving convenient for efficient mass rearing and accurate modeling of weather-based population dynamics.
Keywords: Anopheles gambiae, egg, hatch, agitation, temperature
Anopheles gambiae Giles sensu stricto is a major vector of human malaria in equatorial Africa (Coetzee 2004), where ∼ 175.5 million cases and 91% of worldwide deaths by the disease were reported in 2009 (WHO 2010). Epidemics are correlated with wet years (Craig et al. 1999). Rainfall provides this species both with developmental sites and with high humidity that probably favors adult survival and flight activity. About 2–3 d after each bloodmeal, a female lays ∼50 –200 boat-shaped eggs bearing the distinctive anopheline floats and protruding tubercles. The eggs are laid singly on the surfaces of open bodies of fresh water or on hy-poosmotic damp soil at their periphery (Valencia et al. 1996, Minakawa et al. 2001, Huang et al. 2005, Munga et al. 2005, Miller et al. 2006). Eggs laid on damp or moist substrates have been reported to hatch spontaneously even at a level of dampness in which the first-instar larvae wait inside the cap-opened chorions, with only the head protruding, until water arrives (Huang et al. 2006a). If eggs are kept in damp soil, they remain viable for ∼ 14 –18 d, depending on the soil type (Deane and Causey 1943, Beier et al. 1990, Shililu et al. 2004). Eggs of An. gambiae are incapable of hatching until ∼2 d postoviposition; most hatch (∼90%) 2–3 d postoviposition (Beier et al. 1990, Yaro et al. 2006). Environmental factors such as atmospheric humidity, type of water (Yaro et al. 2006), and temperature (Valencia et al. 1996, Bayoh and Lindsay 2003, Huang et al. 2006b, Impoinvil et al. 2007) all affect the proportion that hatch. The eggs’ wide tolerable temperature range, 4–40°C (Beier et al. 1990, Huang et al. 2006b, Impoinvil et al. 2007), makes them the most cold- and heat-tolerant of An. gambiae’s developmental stages. Successful completion of development, from egg to adult, occurs only between ∼18 and 34°C (Bayoh and Lindsay 2003).
Most research on egg hatching of mosquitoes has been conducted on aedine eggs. Because aedine eggs typically are laid out of water, on container walls, plant debris, or soil, hatching generally requires submergence in water containing organic matter and microorganisms, which reduces oxygen tension (Gjullin et al. 1941, Borg and Horsfall 1953, Judson et al. 1965, Ponnusamy et al. 2011). In addition, bacteria themselves may promote egg hatching (Ponnusamy et al. 2011), and previous exposure to high humidity, warm temperature, and long photoperiod also may be required (Shroyer and Craig 1980).
The first record of egg hatching stimulated by water agitation was reported for the aedine Psorophora ferox (von Humboldt) (Dupree and Morgan 1902). Two decades later, Young (1922) found that more hatching occurred when Aedes aegypti (L.) eggs were agitated in water or left in rain for 5 min. Borg and Horsfall (1953) referred to studies on agitation-stimulated hatching in other aedines, but they concluded that chemical stimuli outweighed the physical ones. In later studies, water agitation has been suggested as a stimulus for egg hatching of Aedes caspius (Pallas), Aedes vittatus (Bigot) (Roberts 2001), and Aedes atropalpus (Coquillett) (G. F. O’Meara, personal communication).
Among anophelines, James and Liston (1904) found that An. gambiae eggs laid on water hatched in 2 d, and those lying dormant on mud would hatch if they were stimulated by adding water. However, there was no mention of any effect of water-induced agitation or disturbance until Muirhead Thomson (1946) suggested that a film on the water surface, produced by iron bacteria, was responsible for keeping dormant eggs unhatched until the thin film was broken-up by rain. Other studies have demonstrated that an abrupt increase in water temperature or a drastic change in water salinity stimulates hatching in Anopheles melas Theobald (Giglioli 1965). Water agitation was first suggested as a natural anopheline hatching stimulus for Anopheles squamifemur Antunes (Boreham and Baerg 1974). In An. gambiae sensu lato, most studies have concluded that flooding is the hatching stimulus for dry or semidry eggs (Deane and Causey 1943, Beier et al. 1990, Shililu et al. 2004, Impoinvil et al. 2007), and it is generally assumed that eggs laid on water will hatch as soon as embryonation is complete (Minakawa et al. 2001). However, the impact of agitation on An. gambiae eggs had gone unnoticed, except for a recent examination of the genetic connection between staggered time to hatch and insecticide resistance (Kaiser et al. 2010).
The following study was prompted by a 2006 observation in our laboratory that a large proportion of An. gambiae eggs, left undisturbed in oviposition cups, did not hatch until they were rinsed in a jet of tap water >1 wk after being laid. We surmised that the hatching stimulus was mechanical agitation of water and that heavy rainfall might provide a similar stimulus under natural conditions. In this paper, we describe the effect of agitation on hatching of eggs that have been laid and maintained on open water, where natural agitation might occur and available moisture is not a limiting factor. To provide details of this effect and explore its ecological and practical implications, we measured 1) the effect of water agitation on both daily hatch proportion and total proportion of eggs hatching after 1wk of daily agitations, 2) the effect of other eggs and of previously hatched first-instar larvae on hatching, and 3) the effect of cool storage for up to 4 wk on the proportion hatching.
Materials and Methods
Rearing and Maintenance
The Mbita strain of An. gambiae s.s. (S form) originally was colonized and identified from locally collected material in 2001 by the staff of the International Centre of Insect Physiology and Ecology at Mbita Point, Suba District, Nyanza, Kenya. A colony of this strain, maintained at The Ohio State University for several years, provided the eggs used in the current study, which was conducted during 2008–2011. All stages were held in a rearing and colony maintenance room at 25.5 ± 1°C, 70 ± 5% RH, and a photoperiod of ∼12:12 (L:D) h. Total darkness occurred between 2000 and 0730 hours, preceded and followed, respectively, by 45-min crepuscular periods of gradual dimming and brightening. Larvae were reared in shallow pans in aged tap water, as described by Gary and Foster (2001). Adults were held in 30 by 30 by 50-cm clear acrylic cages, with cotton wicks providing water and 10% sucrose.
Egg Collection and Preparation
To obtain eggs, one of the authors (B. E.) allowed 50 –70 females to blood-feed on his hand between 1600 and 1800 hours (Biomedical IRB protocol No. 200440193, Institutional Biosafety Protocol No. 2005R0020). The hand was removed after ∼15 min, when ∼70–80% of mosquitoes had engorged and ceased feeding. At 3 d post bloodmeal, when the mosquitoes would be gravid, a shallow plastic oviposition cup (9 cm in diameter) that was half-filled with 50 ml aged tap water was placed in the cage. The cup containing the eggs laid that night was removed slowly in the morning, and the eggs were transferred gently to experimental containers (see later text) at 1400–1600 hours, ∼18–20 h postoviposition (referred to as “1 d postoviposition” in this study), assuming that most oviposition occurred in early night under these conditions (Sumbaetal. 2004). At this age and temperature, embryonic development would not yet be complete (Valle et al. 1999).
Floating eggs were transferred from the oviposition cup to 3.5-ml wells in 24-well Linbro tissue-culture plates (McLean, VA) that each held 1.5 ml water. Each well was 1.7 cm in diameter by 1.6 cm in depth. Each egg was transferred individually by a clean glass Pasteur pipette tip, which was inserted into the water near the egg and moved toward it until the pipette touched the egg. The egg adhered to the side of the pipette tip, which was then slowly lifted out of the water and reinserted into one of the plate wells, where it detached and floated free when the pipette tip was drawn slowly down and away. When all wells contained eggs, the plate was covered with a fitted lid to prevent evaporation and maintain atmospheric humidity at almost 100% RH. Total time to prepare each plate was ∼10 min.
Hatching terminology
For the purposes of the current study, the repeated hatching of a portion of all eggs present, following each application of a triggering stimulus, will be referred to as “installment hatching,” sensu Wilson and Horsfall (1970), typical of aedine mosquitoes. This is a distinct subset of “delayed hatching” or “late hatching,” described by other authors in anophelines, when the eggs hatch at various intervals of time after an environmental condition has been applied. Experimental results reported here include the daily hatch (DH), total hatch (TH), and total hatch by agitation (THA).
Experiment 1: Effect of Daily Agitation on Isolated Eggs
Three batches of eggs were used, each from a different mosquito generation. From each batch, ten 24-well plates were prepared, each with one egg per well. The plates were randomly assigned to treatment and control groups, five plates each, and stored together on an undisturbed shelf. The treatment plates were agitated daily for 7 d, starting 1 h after plate preparation. Both control and treatment plates were inspected by naked eye, both immediately before and ∼10 min after each agitation, and hatched first-instar larvae were counted. The final inspection was 24 h after the seventh agitation. For analysis, DHi, where 0 ≤ i ≤ 6, was defined as the number of eggs that hatched within 24 h after each agitation, divided by the total number of eggs in each plate. For example, DH0 was the proportion hatching before the first agitation of pharate first-instar (fully developed) larvae, examined 2 d postoviposition. The DH on day 1 (DH1) was the sum of hatched eggs after the first agitation but before the second agitation of pharate first-instar larvae, divided by the number of eggs. Numbers hatched in nonagitated controls were counted at the same times. The TH for each plate was calculated by dividing the total number of hatched eggs after 7 d by the number of eggs.
At each agitation, each plate in the treatment group was uncovered, placed on a bench surface, and swirled by hand in a 10-cm-diameter circle at ∼100 revolutions per min for 5 min. The swirling was slow enough to prevent the water and eggs from spilling out of the wells. If an egg became lodged on the side of a well, it was washed by pipette back onto the water surface by one or two drops of water from its respective well. Five min of agitation was chosen, because preliminary experiments demonstrated that egg hatch did not increase significantly between 4 and 10 min of agitation. Food was not provided in the wells, so hatched larvae died within 3 d.
Experiment 2: Effect of the Presence of Other Eggs and of First-Instar Larvae
To determine whether the presence of other eggs increases the hatching rate in the absence of water agitation, multiple eggs were added to wells in plates, as described earlier for single eggs. On the day after oviposition, from each of the three replicate batches of eggs, five wells of a plate (n = 5) were supplied with 10 eggs per well and four wells (n = 4) were supplied with 30–40 eggs per well. Results were compared with isolated nonagitated eggs in the previous experiment, which served as controls. The plate was held undisturbed on a shelf for 7 d postoviposition without agitation, after which the wells were inspected. TH was calculated for each well, and the two treatments were compared with each other and with the controls.
In a separate experiment involving a single batch of eggs, to determine whether the activity of newly hatched larvae increases the hatching rate, 10 wells of a plate were supplied with 10 eggs each. Five of these wells (n = 5) received one newly hatched larva from another egg batch, and the other five received (n = 5) two larvae. The plate was held for 7 d postoviposition without agitation, after which the wells were inspected, and TH of the two treatments were compared with each other and with the nonagitated isolated-egg controls of Experiment 1.
Experiment 3: Effect of Long-term Storage at Two Temperatures
To determine whether prolonged egg storage (i.e., egg age) affects agitation-induced hatching and whether low temperature extends egg viability during dormancy, eggs were stored at either 15.5 or 25.5°C for up to 4 wk postoviposition, then agitated after acclimation at the higher temperature. In this study, 15.5°C was chosen, because it is the approximate mean minimum temperature in malarious equatorial Africa (Bayoh and Lindsay 2003). Eggs were prepared and tested as follows: 10 eggs were transferred 1 d postoviposition (i.e., embryos <24-h-old) by pipette (see Experiment 1) to each of fifty 10-ml glass test tubes (10 cm by 12 mm in diameter) containing 5 ml of aged tap water. Each tube was sealed with Parafilm M, placed in one of two tube racks, then the racks were placed on their sides so that the tubes were horizontal. If an egg adhered to the wall of the tube, the tube was rotated gently within the rack until the egg floated free. One rack of 25 tubes was stored in the rearing room at 25.5 ± 1°C. The other rack immediately was placed in a 15.5 ± 0.5°C refrigerator, with 12:12 (L:D) h fluorescent lighting but no crepuscular period. The relative humidity inside the sealed tubes was virtually 100%. Storage commenced on day 1 postoviposition, when the embryos were incompletely developed, so that mechanical disturbance during transfer of the eggs to tubes would not induce hatching.
The eggs were not agitated until the assigned day. On days 2, 8, 15, 22, and 29 postoviposition, five randomly selected tubes from each rack were transferred to a tray, while remaining horizontal, and were agitated by swirling the tray for 5 min at 100 revolutions per min. The samples stored in the warm room were agitated immediately, whereas those stored in the refrigerator were allowed to acclimate for ∼30 min in the warm room before receiving the first agitation, because a preliminary experiment showed that egg agitation at the low temperature did not stimulate hatching (B. E., unpublished data). Tubes from each storage temperature then were kept in the warm room for the remainder of the experiment, where they were agitated daily for another 5 d. The number of hatched larvae were counted by naked eye before, and 10 min after, each agitation. The last count occurred on day 6 after removal from storage, 10 min after the sixth agitation. For each tube, the DHi and TH were calculated to assess the effect of egg storage at each temperature on hatching. DHi (where 0 ≤ i ≤ 6) was defined as the number of eggs that hatched within 24 h after each agitation, divided by the total number of eggs in each test tube. Similar to Experiment 1, DH0 was the proportion hatching before the first agitation of pharate first-instar larvae (i.e., 2 d postoviposition). Because TH included a few hatched eggs before the first agitation, THA also was calculated, by deducting the small number of eggs hatched before the first agitation from TH for each tube.
A separate study evaluated the long-term viability of eggs stored at 25.5°C for 1, 3, 6, and 12 mo postoviposition. Eggs collected 1 d after oviposition were placed, as previously described, on aged tap water in four small half-filled lidded cups (5.3 cm in diameter, 3.5 cm in height), 100 –150 eggs per container. The containers were inspected daily for hatched larvae during the first week, then monthly. The water level was checked weekly, and more water was added by pipette as needed to compensate for evaporation from the loose-fitting lids. To avoid agitation, the tip of the pipette was first submerged, then water was expelled into the container. No eggs hatched immediately as a result of these replenishments. At the end of each storage duration, the lids were tightened and the cups vigorously agitated for 10 min on each of three consecutive days.
Experiment 4: Effect of Vigorous Agitation During Embryonic Development
To confirm the effect of egg agitation during embryonic development on the likelihood of hatching after it has been completed, eggs were treated as follows: vigorous and prolonged shaking was applied, rather than swirling. Ten eggs from day 1 (i.e., <24 h postoviposition) were placed in each of five tubes as earlier (Experiment 3) at 25.5°C. After 10 min, they were shaken by hand for 15 min. Tubes were examined for the presence of larvae 10 min after agitation, and again 24 h later, when they would be pharate first-instar larvae, capable of hatching spontaneously.
Statistical Analysis
For analysis, proportions hatching at various times were analyzed as mathematical ratios between 0 and 1, but they were converted to percentages for reporting results. In Experiment 1, hatching data did not differ significantly among generations and were combined for analysis. TH for each plate was the cumulative hatch over 7 d, divided by the 24 eggs in the plate. Each plate provided a single unit of measure, so in this case, n = 15 (five plates × three generations) for 360 agitated eggs and 360 control eggs. Data from the other experiments were treated similarly.
The pattern of DHi after each agitation (i.e., installment hatching) was described by a model (model 1) employing the following independent variables: agitation day (0 ≤ i ≤ 6) and its log- and square-transformed data. A stepwise method eliminated insignificant independent variables from the equation. Independent variables of the best equation were described by their coefficients and their standardized partial regression coefficients (β) and are reported in the results. The greater the absolute value of β of a variable, the larger was its impact (Sokal and Rohlf 1995).
When data were not normally distributed, as determined by Kolmogorov–Smirnov test, they were transformed (e.g., log [100 DHi + 0.1]), and their normality was retested. Student’s t-test and one-way analysis of variance (ANOVA) were applied to normalized data. If normality was not achieved, differences between two groups or among multiple groups were evaluated by Mann–Whitney U or Kruskal–Wallis tests, respectively (Sokal and Rohlf 1995). For all tests, P = 0.05 was the criterion for statistical significance.
Two types of tests were applied to compare long-term hatch patterns at two temperatures (Experiment 3). One-way ANOVA was used to compare proportions hatching after storage at each temperature, before the first agitation and again after it. Two stepwise linear regression models (models 2 and 3) were run to predict log-transformed DH on day i (log [100 DHi + 0.1]) at the two temperatures. In model 4, temperature (temp) was included. The following independent variables and their log- and square-transformed data were used in the models: agitation day (0 ≤ i ≤ 6), egg storage duration (2 ≤ age ≤ 29), storage temperature (temp = 15.5 or 25.5), and agitation day by egg storage duration (i • age). A stepwise linear regression line was estimated for THA. SPSS (ver. 19.0; IBM, Armonk, NY) software package was used for all tests and to create 3-D graphics.
Results
Experiment 1: Effect of Daily Agitation on Isolated Eggs
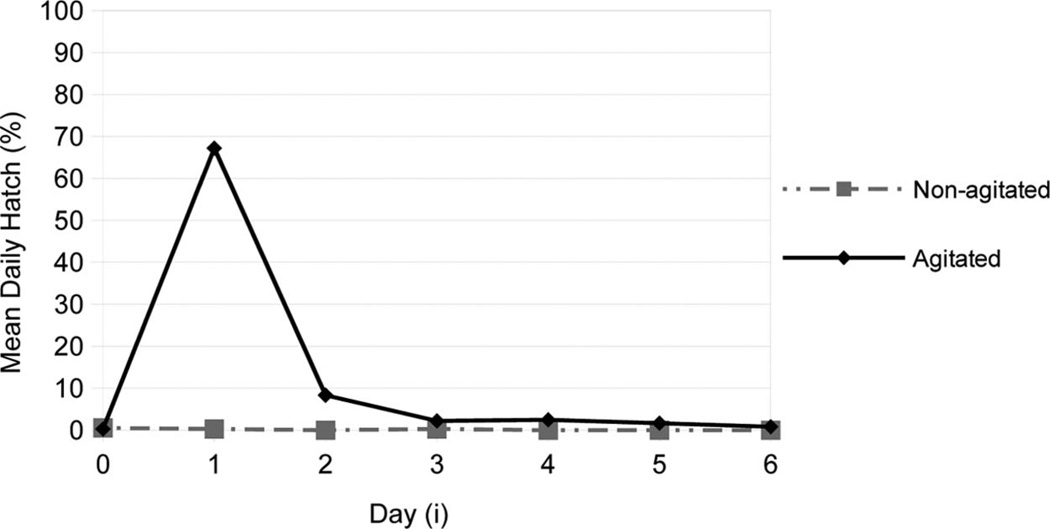
No eggs hatched among agitated or control groups after agitation of the treated group on day 1 (Fig. 1). Furthermore, before the second agitation (2 d postoviposition, i = 1), the proportion hatching spontaneously was <1%. But immediately after the second agitation (i = 1), 63% of the treated eggs hatched. Most eggs hatched immediately after each agitation, and negligible proportions hatched between agitations (Table 1). This pattern of declining DHi after two or more applications of a hatching stimulus is the distinguishing feature of installment hatching. A linear regression model (model 1) of the DHi decline that fits the data is as follows: r = 0.955 and adjusted R2 = 90.9% (F = 298.45; df = 3, 86; P < 0.0001), where (model 1):
DHi = 1.12(i) − 4.92 log(i) − 0.07(i)2 − 0.38
Fig. 1.
Effect of seven consecutive daily agitations on mean daily hatch (% DHi). Comparison of DHi of nonagitated and agitated (5 min/d) Anopheles gambiae eggs (1 egg/1.5 ml in well) from i= 0 (1 d postoviposition) until i= 6 (7 d postoviposition). Temperature 25.5°C. Details presented in Table 1.
Table 1.
Details of the effect of seven consecutive daily agitations on mean proportion (%) hatching of Anopheles gambiae eggs (1 egg/1.5 ml in well)
| ia | Agitation dayb |
Hatch count before/after agitation |
Group | Mean ± SE | Mann–Whitney U | P valuec |
|---|---|---|---|---|---|---|
| 0 | 1 | After | Control | 0 | 112.5 | 1.00 |
| Treatment | 0 | |||||
| 2 | Before | Control | 0.6 ± 0.4 | 105 | 0.775 | |
| Treatment | 0.3 ± 0.3 | |||||
| 1 | 2 | After | Control | 0 | 0 | < 0.0001 |
| Treatment | 63.3 ± 4.4 | |||||
| 3 | Before | Control | 0.3 ± 0.3 | 50.5 | 0.009 | |
| Treatment | 3.9 ±1.0 | |||||
| 2 | 3 | After | Control | 0 | 45 | 0.004 |
| Treatment | 5.8 ±1.8 | |||||
| 4 | Before | Control | 0 | 60 | 0.029 | |
| Treatment | 2.5 ± 0.8 | |||||
| 3 | 4 | After | Control | 0 | 82.5 | 0.217 |
| Treatment | 1.4 ± 0.7 | |||||
| 5 | Before | Control | 0.3 ± 0.3 | 97.5 | 0.539 | |
| Treatment | 0.8 ± 0.5 | |||||
| 4 | 5 | After | Control | 0 | 90 | 0.367 |
| 6 | Before | Control | 0 | 75 | 0.126 | |
| Treatment | 1.7 ± 0.7 | |||||
| 5 | 6 | After | Control | 0 | 97 | 0.539 |
| Treatment | 1.4 ± 1.1 | |||||
| 7 | Before | Control | 0 | 105 | 0.775 | |
| Treatment | 0.3 ± 0.3 | |||||
| 6 | 8 | After | Control | 0 | 90 | 0.367 |
| Treatment | 0.8 ± 0.5 |
Three data sets are compared: 1) daily spontaneous hatch rates of control (nonagitated) groups on successive days at the same times as the treatment (5 min/d agitation) group counts, and 2) daily stimulated hatch rates of treatment (agitated) groups a) immediately after (<10 min) each agitation, and b) during the 24-h period before the next agitation.
i, period after each agitation and before next one, that was used to define daily hatch on day i (DHi)
In this exp (Experiment 1), agitation day = day postoviposition.
P, probability of no difference between control (nonagitated) and treatment (agitated) groups, by Mann–Whitney U test, before and after each agitation time.
The β for (i), log(i), and (i)2 were 7.62, −5.15, and − 3.39, respectively. This means that DH i was mainly affected by agitation day (i) and its log-transform. The mean TH by day 8, after seven periods of agitation, was 83.1% (SE = 1.7). By contrast, the mean TH of non-agitated controls was 1.1% (SE = 0.5) (U = 0; n1, n2 = 15; P < 0.0001). Daily differences in hatching proportions between treated and control groups were statistically significant after agitations on days 2 and 3 and before agitations on days 3 and 4 (Table 1).
Experiment 2: Effect of the Presence of Other Eggs and of First-Instar Larvae
After 1 wk, groups of 10 eggs per well had a TH of 6.7% (SE = 2.4). In groups of 30–40 per well, the TH was 3.5% (SE = 0.5). These values were close to the spontaneous TH of isolated eggs in Experiment 1 (1.1%, SE = 0.5) and were not significantly different. In an ancillary analysis, hatch rates of isolated eggs of Experiment 1 were compared with groups of 10 eggs per tube (see Experiment 3), both before the first agitation of pharate first-instar larvae (DH0, 0.3 vs 0%) and also after 1 wk of daily agitations (THA, 82.8 vs 84.0%). There was no difference between the results, confirming that egg density did not affect hatching. In the experiment to detect the effect of larvae among wells containing one larva added to 10 eggs, none had hatched by the end of the week. Among those with two larvae, 4.7% (SE = 1.9) had hatched. Again, the differences among these treatments, including the single-egg spontaneous-hatch rate (Experiment 1), were not significant.
Experiment 3: Effect of Long-term Storage at Two Temperatures
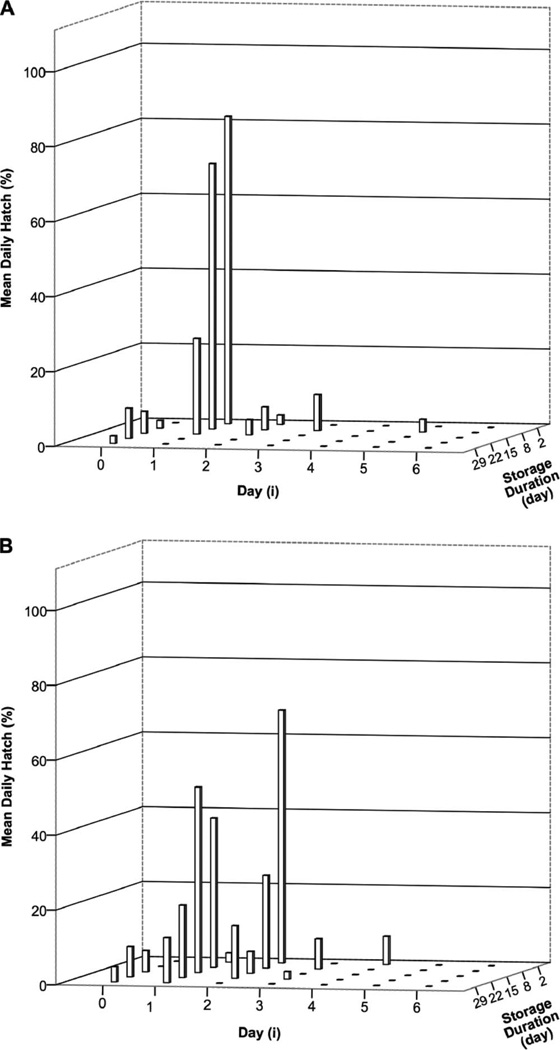
The proportion of eggs hatching before the first agitation (i.e., spontaneously) was very low and did not differ significantly among the five age-groups stored at the two temperatures (Table 2). Among the 2-d-old eggs stored at 25.5°C, 82% hatched after the first agitation, whereas those stored at 15.5°C first hatched between the first and second agitations (2.5%), followed by substantial numbers after the second agitation (67.5%) (Fig. 2). Among 8-d-old samples held at 25.5°C, a few eggs hatched before the first agitation (2.0%) but many more afterward (70.7%), whereas among those stored at 15.5°C, only 39.8% hatched after the first agitation and fewer after subsequent agitations. Installment hatching was particularly common among eggs stored for 8 d and was more common among those stored at 15.5°C. At both temperatures, after progressively longer storage durations, fewer eggs hatched after the initial agitation, and fewer hatched at each subsequent agitation, i.e., both the initial-hatch numbers and the installment-hatch numbers diminished. By 29 d of storage, those at 15.5°C exhibited no installment hatching, that is, they hatched in response to day-1 agitation only. No eggs hatched on day 6 of egg agitation, regardless of storage temperature or storage duration.
Table 2.
Details of effect of storage duration and temperature on proportion of Anopheles gambiae eggs hatching (10 eggs/5 ml in test tube)
| Temperature | Hatch count | Storage duration (day) |
F | df | P valuea | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 8 | 15 | 22 | 29 | |||||
| 15.5°C | DH0 | 0 | 0 | 5.6 ± 3.7 | 8.0 ± 3.7 | 4.0 ± 2.5 | 1.66 | 4, 19 | 0.201 |
| <10 min after | 0 | 20 ± 6.3 | 41.5 ± 8.8 | 7.6 ±4.7 | 0 | 10.06 | 4, 19 | < 0.0001 | |
| TH | 77.5 ± 9.5 | 72.6 ±1.9 | 60.9 ± 12.1 | 43.3 ± 6.9 | 16.0 ± 6.8 | ||||
| 25.5°C | DH0 | 0 | 2.0 ± 2.0 | 5.8 ± 2.4 | 8.0 ± 4.9 | 2.0 ± 2.0 | 1.41 | 4, 20 | 0.268 |
| <10 min after | 82 ± 5.8 | 59.5 ± 2.5 | 23.6 ± 5.2 | 0 | 0 | 99.96 | 4, 20 | < 0.0001 | |
| TH | 84.0 ±5.1 | 88.4 ± 5.7 | 35.3 ± 2.3 | 8.0 ± 4.9 | 2.0 ± 2.0 | ||||
n= 5 except for 2-d-old eggs at 15.5°C, where n= 4.
Comparison of mean hatch rates (± SE) after storage for five durations (2–29 d) at 15.5 and 25.5°C, before the first agitation (DH0) and again <10 min after the first agitation of pharate first-instar larvae, and of total hatch (TH) after 6 consecutive days of agitation. Poststorage (6-d agitation period) temperature 25.5°C.
P, probability of no difference among storage durations, by one-way ANOVA, before and immediately after the first agitation at each temperature.
Fig. 2.
Effect of storage duration and temperature on mean daily hatch (% DHi) of Anopheles gambiae eggs (10 eggs/5 ml in test tube) agitated 5 min/d on 6 consecutive days, from i= 0 (before the first agitation) until i=6 (after the sixth agitation). Comparisons after each of five different storage durations (2–29 d) at either (A) 25.5°C or (B) 15.5°C. Poststorage (6-d agitation period) temperature 25.5°C.
To predict DHi after egg storage, two stepwise linear regression models (2 and 3) were estimated separately for 15.5°C and 25.5°C, respectively (Table 3). In the 15.5°C model, the greatest absolute value of β belonged to log-transformed agitation day (log [i]), and then secondarily to the square of the storage duration (age2). This means that the DHi at the lower temperature was affected only by these two variables. By contrast, in the 25.5°C model, age was more important than the log-transformed agitation day. When temp was included as an independent variable in the DHi model (model 4), log-transformed agitation day and storage duration were found to be more important than the other variables (Table 3).
Table 3.
Regression models describing relationship between daily hatch (DHi) of Anopheles gambiae eggs (10 eggs/5 ml in test tube) after 5-min agitation per day (0 ≤i ≤ 6), storage duration (2 ≤ age ≤ 29), and temperature (temp = 15.5 or 25.5)
| Modela | r | Adjusted R2 | F*** | df | log(i) a |
(i)-(age) b |
(age) c |
(age)2 d |
log(age) e |
(temp) f |
g |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 0.702 | 48.5 | 68.33 | 2, 141 | −2.742 | 0 | 0 | −0.001 | 0 | — | 1.198 |
| (β) | (−0.674) | (0) | (0) | (−0.194) | (0) | — | |||||
| 3 | 0.806 | 63.7 | 49.03 | 4, 132 | −4.074 | 0.022 | −0.38 | 0.005 | 3.19 | — | 1.152 |
| (β) | (−1.154) | (1.085) | (−3.849) | (1.517) | (1.37) | — | |||||
| 4 | 0.724 | 51.5 | 60.64 | 5, 276 | −3.679 | 0.013 | −0.101 | 0 | 0.710 | −0.028 | 1.934 |
| (β) | (−0.959) | (0.611) | (−0.943) | (0) | (0.279) | (−0.136) |
DHi ranged between 0 and 1. log(100 DHi+ 0.1) =a log(i) + b (i)·(age) + c (age) + d (age)2 + e log(age) + f (temp) + g.
Model 2: 15.5°C; model 3: 25.5°C; model 4: both temperatures were included.
standardized partial regression coefficient.
P value <0.0001 for all three models.
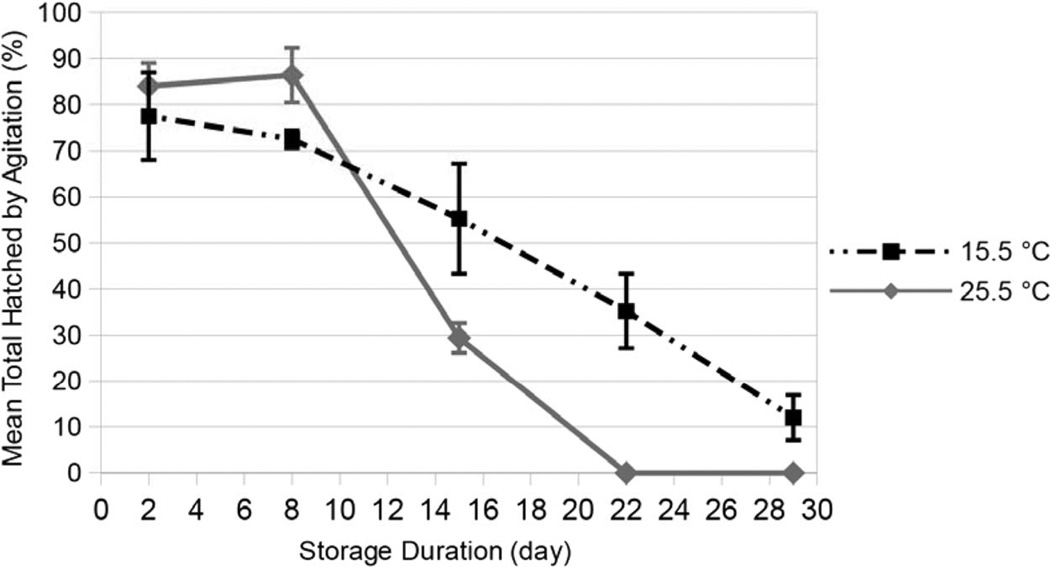
Eggs at both temperatures reached ∼66% THA at around day 10 (Fig. 3). After that day, the slope downward at 25.5°C was almost twice as steep as at 15.5°C. The following model (model 5) estimates THA, with storage duration (age) and temperature (temp) as independent variables:
THA = 1.165 − 0.032 age − 0.011 temp
Fig. 3.
Effect of storage duration and temperature on mean (±SE) total hatch by agitation (%THA) of Anopheles gambiae eggs (10 eggs/5 ml in test tube) after 6 consecutive days of agitation (5 min/d) following removal from storage. Comparisons of THA after each of five different storage durations (2–29 d) at either 15.5° or 25.5°C. Poststorage (6-d agitation period) temperature 25.5°C.
The regression line observed was r = 0.877, with adjusted R2 = 75.9% (F = 76.57; df = 2, 46; P < 0.0001). In the aforementioned model, the β value of age (− 0.867) was larger than temp (− 0.161), indicating that age (storage duration) had the greater (and negative) effect on THA.
After storage of batches of eggs for 1, 3, 6, and 12 mo postoviposition at 25.5°C, then agitation for 10 min each day for 3 d, no eggs hatched. Reagitating these eggs 18 mo postoviposition did not stimulate hatch. Microscopic examination of these eggs, prepared according to Shililu et al. (2004), demonstrated that the embryos within them were dead but had developed fully. These pharate first-instar larvae probably would have hatched, had they been agitated at an earlier age.
Experiment 4: Effect of Vigorous Agitation During Embryonic Development
After vigorous and prolonged shaking of eggs laid <24 h earlier, no eggs hatched immediately after the agitation, and only 1 of 50 eggs (2.0%, SE = 2.0) hatched without further agitation the following day. This is not statistically different from the 1.1% rate of its comparable group in Experiment 1. This result confirmed the absence of a delayed effect of agitation, if applied during embryonic development.
Discussion
The results demonstrated that the eggs of An. gambiae, when laid on the surface of open bodies of clean water where they undergo embryonic development, rarely hatch without the stimulus of mechanical agitation. Within minutes of agitation, the pharate first-instar larvae dehisce from their chorions. Embryonated but unstimulated eggs almost always remain unhatched, and the larvae within them can survive in this dormant condition for up to several weeks. Most eggs hatch right after the initial stimulus, but small numbers will hatch after a second agitation 1 d later, and still smaller numbers on successive agitations. This is the installment-hatching pattern commonly observed in eggs of aedine mosquitoes after each series of successive inundations (Wilson and Horsfall 1970). How the pattern might change if anopheline agitations were more frequent than once a day, or if the water temperature were higher or lower, remains to be investigated. TH averaged only 1.1% without an immediately preceding agitation and 83.1% when eggs were agitated. The decline in the proportions hatching in response to this stimulus, after various periods of storage, was similar to that reported by Shililu et al. (2004), when eggs were held up to 20 d in a high-moisture environment, then inundated, although our hatch rates were ∼30% higher than theirs at each duration of storage. Tests of the effect of the presence of other eggs and of hatched first-instar larvae on the spontaneous hatching of nonagitated eggs showed that these were not factors that affect hatching. It appears that the browsing action of the small larvae was insufficient to act as a substitute for agitation of the water. Furthermore, application of the agitation stimulus before the completion of embryonic development demonstrated that agitation before the larvae were fully developed or capable of dehiscence had no effect on the spontaneous hatching rate after that period had been completed. We tentatively conclude that the mechanism for triggering hatching has no delayed-action component.
Munga et al. (2005) and Yaro et al. (2006) suggested that chemicals in the water may stimulate hatching. That could explain the observation of Huang et al. (2006a) that eggs laid on an agarose gel, a substitute for damp soil or mud surrounding rain puddles, and apparently left undisturbed, nonetheless hatched as soon as sufficient moisture or water became available. We have supported this, in part, by finding that, when left meticulously undisturbed, 2–3% hatched spontaneously on water containing suspended powdered fish food, whereas none hatched on clean-water controls (W.A.F., unpublished data). This suggests that chemicals and sufficient water do indeed induce a very small amount of spontaneous hatching in the absence of all agitation.
The precise mechanism by which agitation causes egg hatching remains obscure. We have considered the possibility that the stimulus is actually physicochemical, just as reduced oxygen tension has been demonstrated to be a hatching trigger in aedines (Gjullin et al. 1941, Judson et al. 1965). However, our observations on An. gambiae suggest otherwise: the water we used was clean, aged tap water. The eggs floated on the surface of that water, where the upper chorion was in contact with the atmosphere throughout development and during undisturbed storage. Falling droplets of water, which contain more, not less, dissolved oxygen (Graedel and Weschler 1981), provided a reliable egg-hatch stimulus (B. E., unpublished data). The mechanism cannot involve removal of the exochorion, other structural changes, or enhanced water permeability. Otherwise, eggs shaken on day 1 would have hatched in high numbers in the absence of further agitation after embryonation was complete on day 2. Furthermore, a fully tanned chorion would seem unlikely to be susceptible to changes in permeability long after oviposition (Schreuders et al. 1996, Valencia et al. 1996), when our eggs nonetheless hatched in large numbers immediately after a single agitation. Possibly hatching is stimulated by any increase in wetting of the chorion, including strong agitation of the water on which the egg floats. But we observed that floating eggs hatched immediately after they were prodded by the tip of a pipette (B. E., unpublished data). Furthermore, inundation by itself almost inevitably has an agitation component. Therefore, until other ideas are tested, we conclude that the required agitation for egg-hatching in this study was a mechanical effect on the pharate larva itself.
Previous workers have reported total hatching rates of An. gambiae that ranged from 73 to 93% under various conditions of temperature and water quality (Yaro et al. 2006, Impoinvil et al. 2007), in the same range as our average of ∼83% after agitation. Yet the role of agitation has gone unnoticed. This suggests the possibility that delayed-hatching eggs might have been agitated inadvertently during their handling after oviposition, as was the case in our laboratory. Embryonated eggs can be disturbed very easily when the oviposition container is moved from a cage to another site, when poured from the container into a larval pan, or when rinsed with water to remove them from filter-paper cones within oviposition cups. The last method of collecting anopheline eggs is common in laboratories. This interpretation is supported by the genetic analysis of delayed (“late”) hatching by Kaiser et al. (2010), who noticed that the frequency of “early” hatching was increased by egg disturbance.
When stored at a cool temperature (15.5°C), dormant eggs remained viable for a longer period than when stored undisturbed at room temperature (25.5°C). Spontaneous hatching remained uncommon, regardless of storage temperature or duration. These results suggest a method for retaining un-hatched eggs for extended periods in the laboratory. Yet, under the conditions we used, viability declined at both temperatures by the second and third weeks of storage, even at the lower temperature. None was capable of stimulated hatching beyond 1 mo of storage. Similar viability results, without intentional agitation, have been reported by Deane and Causey (1943), Beier et al. (1990), and Shililu et al. (2004). Storage under different conditions, including lower temperatures, followed by agitations, needs to be explored.
The statistical shape of installment hatching was affected by storage time (egg age), storage temperature, and agitation day (Fig. 2). Among eggs stored warm, maximum hatch occurred at the youngest age, having been stored the least amount of time (i.e., 2 d postoviposition), and after the first agitation. Among those stored cool, maximum hatch occurred in the youngest group, but only after the second agitation, i.e., fewer cool-stored eggs were immediately capable of agitation-induced hatching when returned to the warmer temperature, almost certainly a result of chilling while they were still embryonic. The fact that eggs did become competent to respond to agitation after a further period of cold storage demonstrates that embryonic development continued at 15.5°C, but more slowly. Another notable result of our storage test was that among the eggs stored for 1–3 wk, the installment hatchings of cool-stored eggs tended to be more spread out over 2–3 daily agitations. This difference from eggs stored at room temperature suggests lingering effects of the cold treatment, retarding readiness to hatch. The model showed that a 50% THA was obtained around day 12 at 25.5°C, similar to that obtained by Deane and Causey (1943). When we applied 15.5°C and 25.5°C to the Impoinvil et al. (2007) model, with target hatches of 10 and 50%, shorter periods of incubation resulted, suggesting a shorter survival than we obtained, probably due to a difference in temperature and our use of agitation. Our THA of embryos <1-wk-old at 25.5°C supports the results of Shililu et al. (2004), indicating that there is a time window of 1 wk for embryos to hatch in maximum numbers in response to the stimulus, and after 1 wk the embryos begin to die. At 15.5°C, however, the slower but steady decline in egg viability reflected lower metabolism and slower embryonic development.
Both agitation-induced installment hatching by a series of agitations and delayed hatching after a single stimulus may have ecological significance. Many studies have focused on predicting mosquito populations based on environmental variables (Craig et al. 1999). The models of this study take into account agitation and dormancy at two temperatures to estimate future populations of first-instar larvae. The adaptive value of agitation-induced hatching appears similar to that of inundation for aedine mosquitoes. In anophelines, the impact of raindrops on eggs laid in moist soil or on open water surfaces can signal an influx of water and nutrients to rain pools, ditches, and borrow pits, which are commonly used by An. gambiae. When these pools provide nutrients to a finite number of healthy larvae and also are at risk of drying out, a pool that is increasing in size offers a larva better prospects for survival. The installment-hatching feature, as in aedine eggs, appears to be a form of bet-hedging strategy against the possibility that the rain is insufficient to sustain the pool or enhance its nutrient base. Likewise, Minakawa et al. (2001) have suggested that delayed hatching has advantageous consequences for predator avoidance and eventual adult body size. If a larval developmental pool should become entirely dry, unhatched eggs of An. gambiae survive in drying mud longer than small larvae (Deane and Causey 1943, Beier et al. 1990, Koenraadt et al. 2003, Shililu et al. 2004), although they cannot resist the long periods of drought that aedine eggs can (Gjullin et al. 1950). Because evidence exists that mechanical agitation also promotes egg hatching in aedines (Dupree and Morgan 1902, Young 1922, Borg and Horsfall 1953, Roberts 2001), for those species as well, rain may contribute a mechanical signal indicating an influx of water and nutrients in container and floodwater habitats. Our observation that the eggs of An. gambiae failed to hatch after several months and up to 1 yr of storage at high humidity supports the speculation of Koenraadt et al. (2003) that they cannot survive several months of a dry season.
These observations on agitation-dependent hatching have practical value. Planning and preparations for both small-scale experiments and mass rearings of An. gambiae are more efficient and convenient when eggs of several ages can be accumulated and hatched at a designated time. Furthermore, cage densities of adults can be kept constant, and fewer bloodmeals are required. The eggs that are collected 10 –20 h postoviposition can be divided into small sealed containers, in contact with water, and stored where they will remain completely undisturbed until needed. Then the egg containers can be shaken 2–29 d postoviposition, depending on the storage temperature, to obtain a predictable number of mosquitoes on demand. The very few larvae that hatch spontaneously are negligible and die within 2 d in the absence of food.
The hatching behavior of the Mbita strain of An. gambiae, from western Kenya, is described here, but we found similar characteristics of egg dormancy and agitation-induced hatching in the Suakoko strain from Liberia (unpublished data). Furthermore, Kaiser et al. (2010) described a similar phenomenon in the GAH strain from Ghana, although with a higher rate of spontaneous hatching. All three examples are S-form An. gambiae. The presence of this characteristic in M-form An. gambiae (= Anopheles coluzzii Coetzee and Wilkerson) (Coetzee et al. 2013) and in other species of the An. gambiae complex, particularly Anopheles arabiensis Patton, is worth investigating.
Acknowledgments
We extend thanks to Christopher M. Stone, Adrea C Rodriguez-Lovejoy, and David L. Denlinger, Department of Entomology, The Ohio State University (OSU), for their suggestions on experimental design; to Leila Farivar, Department of Economics, OSU, for statistical consulting; and to Robert L. Aldridge for maintaining mosquito colonies and providing experimental eggs in our vector behavior laboratory. This work was supported, in part, by National Institutes of Health grant R01-AI077722 from the National Institute of Allergy & Infectious Diseases (NIAID) to W. A. F. Its content is solely the responsibility of the authors and does not represent the official views of the NIAID or the National Institutes of Health.
References Cited
- Bayoh MN, Lindsay SW. Effect of temperature on the development of the aquatic stages of Anopheles gambiae sensu stricto (Diptera: Culicidae) Bull. Entomol. Res. 2003;93:375–381. doi: 10.1079/ber2003259. [DOI] [PubMed] [Google Scholar]
- Beier JC, Copeland R, Oyaro C, Masinya A, Odago WO, Oduor S, Koech DK, Roberts CR. Anopheles gambiae complex egg-stage survival in dry soil from larval development sites in western Kenya. J. Am. Mosq. Control Assoc. 1990;6:105–109. [PubMed] [Google Scholar]
- Boreham MM, Baerg DC. Description of the larva, pupa and egg of Anopheles (Lophopodomyia) squamifemur Antunes with notes on development (Diptera: Culicidae) J. Med. Entomol. 1974;11:564–569. [PubMed] [Google Scholar]
- Borg AF, Horsfall WR. Eggs of floodwater mosquitoes II. Hatching stimulus. Ann. Entomol. Soc. Am. 1953;46:472–478. [Google Scholar]
- Coetzee M. Distribution of the African malaria vectors of the Anopheles gambiae complex. Am. J. Trop. Med. Hyg. 2004;70:103–104. [PubMed] [Google Scholar]
- Coetzee M, Hunt RH, Wilkerson R, Torre A Della, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–274. [PubMed] [Google Scholar]
- Craig MH, Snow RW, Sueur Dle. A Climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol. Today. 1999;15:105–111. doi: 10.1016/s0169-4758(99)01396-4. [DOI] [PubMed] [Google Scholar]
- Deane MP, Causey OR. Viability of Anopheles gambiae eggs and morphology of unusual types found in Brazil. Am. J. Trop. Med. 1943;23:95–103. [Google Scholar]
- Dupree JW, Morgan HA. Mosquito development and hibernation. Science. 1902;16:1036. doi: 10.1126/science.16.417.1036. [DOI] [PubMed] [Google Scholar]
- Gary RE, Foster WA. Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera: Culicidae) J. Med. Entomol. 2001;38:22–28. doi: 10.1603/0022-2585-38.1.22. [DOI] [PubMed] [Google Scholar]
- Giglioli MEC. Oviposition by Anopheles melas and its effect on egg survival during the dry season in the Gambia, West Africa. Ann. Entomol. Soc. Am. 1965;58:885–891. doi: 10.1093/aesa/58.6.885. [DOI] [PubMed] [Google Scholar]
- Gjullin CM, Hegarty CP, Bollen WB. The necessity of a low oxygen concentration for the hatching of Aedes mosquito eggs. J. Cell Com. Physiol. 1941;17:193–202. [Google Scholar]
- Gjullin CM, Yates WW, Stage HH. Studies on Aedes vexans (Meig.) and Aedes sticticus (Meig.), flood-water mosquitoes, in the lower Columbia River Valley. Ann. Entomol. Soc. Am. 1950;43:262–275. [Google Scholar]
- Graedel TE, Weschler CJ. Chemistry within aqueous atmospheric aerosols and raindrops. Rev. Geo-phys. 1981;19:505–539. [Google Scholar]
- Huang J, Walker ED, Giroux PY, Vulule J, Miller JR. Ovipositional site selection by Anopheles gambiae: influences of substrate moisture and texture. Med. Vet. Entomol. 2005;19:442–450. doi: 10.1111/j.1365-2915.2005.00588.x. [DOI] [PubMed] [Google Scholar]
- Huang J, Miller JR, Chen S, Vulule JM, Walker ED. Anopheles gambiae (Diptera: Culicidae) oviposition in response to agarose media and cultured bacterial volatiles. J. Med. Entomol. 2006a;43:498–504. doi: 10.1603/0022-2585(2006)43[498:agdcoi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Huang J, Walker ED, Vulule J, Miller J. Daily temperature profiles in and around Western Kenyan larval habitats of Anopheles gambiae as related to egg mortality. Malar. J. 2006b;5:87. doi: 10.1186/1475-2875-5-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impoinvil DE, Cardenas GA, Githure JI, Mbogo CM, Beier JC. Constant temperature and time period effects on Anopheles gambiae egg hatching . J. Am. Mosq. Control Assoc. 2007;23:124–130. doi: 10.2987/8756-971x(2007)23[124:ctatpe]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SP, Liston WG. A monograph of the Anopheles mosquitoes of India. 1st ed. Calcutta, India: Thacker Spink; 1904. [Google Scholar]
- Judson CL, Hokama Y, Haydock I. The physiology of hatching of aedine mosquito eggs: Some larval responses to the hatching stimulus. J. Insect Physiol. 1965;11:1169–1177. doi: 10.1016/0022-1910(65)90109-5. [DOI] [PubMed] [Google Scholar]
- Kaiser ML, Koekemoer LL, Coetzee M, Hunt RH, Brooke BD. Staggered larval time-to-hatch and insecticide resistance in the major malaria vector Anopheles gambiae S form. Malar. J. 2010;9:360. doi: 10.1186/1475-2875-9-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenraadt C, Paaijmans KP, Githeko AK, Knols BG, Takken W. Egg hatching, larval movement and larval survival of the malaria vector Anopheles gambiae in desiccating habitats. Malar. J. 2003;2:20. doi: 10.1186/1475-2875-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Huang J, Vulule J, Walker ED. Life on the edge: African malaria mosquito (Anopheles gambiae s. l.) larvae are amphibious. Naturwissenschaften. 2006;94:195–199. doi: 10.1007/s00114-006-0178-y. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Githure JI, Beier JC, Yan G. Anopheline mosquito survival strategies during the dry period in western Kenya. J. Med. Entomol. 2001;38:388–392. doi: 10.1603/0022-2585-38.3.388. [DOI] [PubMed] [Google Scholar]
- Muirhead Thomson RC. Studies on the breeding places and control of Anopheles gambiae and A. gambiae var. melas in coastal districts of Sierra Leone. Bull. Entomol. Res. 1946;36:185–252. [Google Scholar]
- Munga S, Minakawa N, Zhou G, Barrack OJ, Githeko AK, Yan G. Oviposition site preference and egg hatchability of Anopheles gambiae: effects of land cover types. J. Med. Entomol. 2005;42:993–997. doi: 10.1093/jmedent/42.6.993. [DOI] [PubMed] [Google Scholar]
- Ponnusamy L, Böröczky K, Wesson DM, Schal C, Apperson CS. Bacteria stimulate hatching of yellow fever mosquito eggs. PLoS ONE. 2011;6:e24409. doi: 10.1371/journal.pone.0024409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM. Egg hatching of mosquitoes Aedes caspius and Ae. vittatus stimulated by water vibrations. Med. Vet. Entomol. 2001;15:215–218. doi: 10.1046/j.0269-283x.2001.00303.x. [DOI] [PubMed] [Google Scholar]
- Schreuders PD, Smith ED, Cole KW, Mazur P. Characterization of intraembryonic freezing in Anopheles gambiae embryos. Cryobiology. 1996;33:487–501. doi: 10.1006/cryo.1996.9999. [DOI] [PubMed] [Google Scholar]
- Shililu JI, Grueber WB, Mbogo CM, Githure JI, Riddiford LM, Beier JC. Development and survival of Anopheles gambiae eggs in drying soil: influence of the rate of drying, egg age, and soil type. J. Am. Mosq. Control Assoc. 2004;20:243–247. [PubMed] [Google Scholar]
- Shroyer DA, Craig GB. Egg hatchability and diapause in Aedes triseriatus (Diptera: Culicidae): temperature and photoperiod-induced latencies. Ann. Entomol. Soc. Am. 1980;73:39–43. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. New York, NY: W.H. Freeman & Company; 1995. [Google Scholar]
- Sumba LA, Okoth K, Deng AL, Githure J, Knols BG, Beier JC, Hassanali A. Daily oviposition patterns of the African malaria mosquito Anopheles gambiae Giles (Diptera: Culicidae) on different types of aqueous substrates. J. Circadian Rhythms. 2004;2:6. doi: 10.1186/1740-3391-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia M, Miller LH, Mazur P. Permeability of intact and dechorionated eggs of the Anopheles mosquito to water vapor and liquid water: a comparison with Drosophila. Cryobiology. 1996;33:142–148. doi: 10.1006/cryo.1996.0014. [DOI] [PubMed] [Google Scholar]
- Valle D, Monnerat AT, Soares MJ, Rosa-Freitas MG, Pelajo-Machado M, Vale BS, Lenzi HL, Galler R, Lima JBP. Mosquito embryos and eggs: polarity and terminology of chorionic layers. J. Insect Physiol. 1999;45:701–708. doi: 10.1016/s0022-1910(98)00154-1. [DOI] [PubMed] [Google Scholar]
- WHO. World malaria report. Geneva, Switzerland: World Health Organization; 2010. 2010. [Google Scholar]
- Wilson GR, Horsfall WR. Eggs of floodwater mosquitoes XII. Installment hatching of Aedes vexans (Diptera: Culicidae) Ann. Entomol. Soc. Am. 1970;63:1644–1647. [Google Scholar]
- Yaro AS, Dao A, Adamou A, Crawford JE, Ribeiro JM, Gwadz R, Traoré SF, Lehmann T. The distribution of hatching time in Anopheles gambiae. Malar. J. 2006;5:19. doi: 10.1186/1475-2875-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CJ. Notes on the bionomics of Stegomyia calopus Meigen, in Brazil, Part I. Ann. Trop. Med. Parasitol. 1922;16:389–406. [Google Scholar]