Abstract
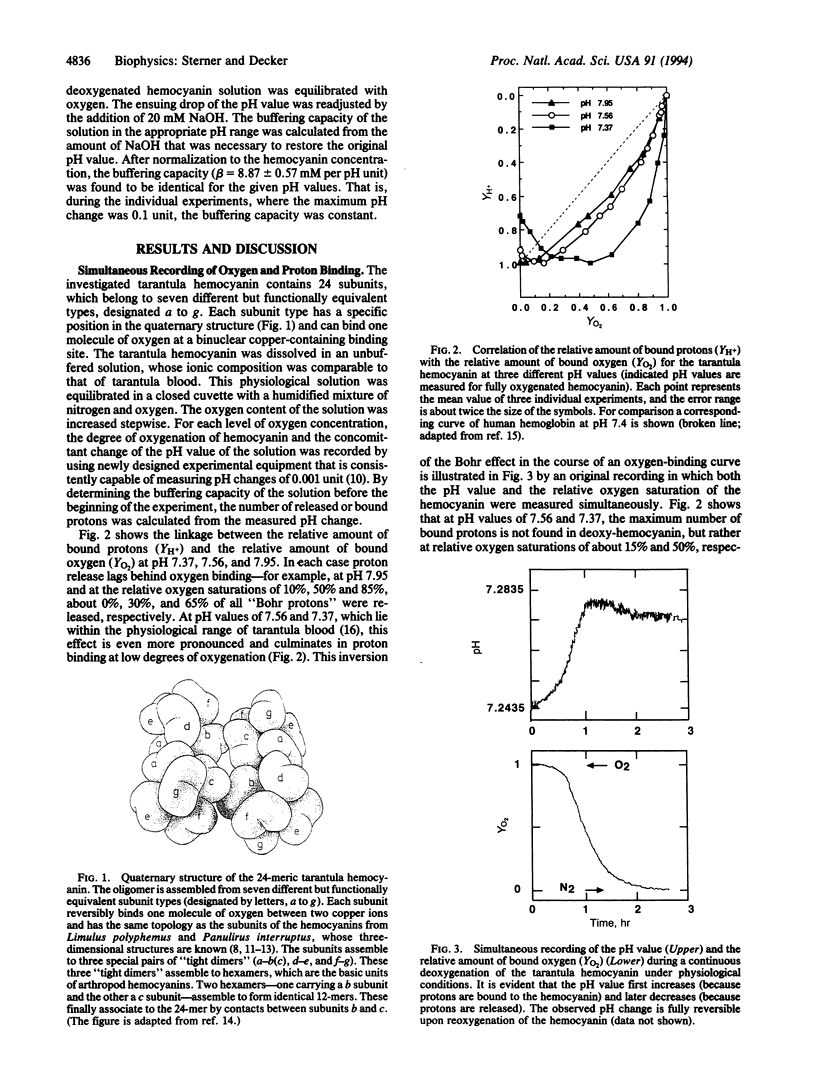
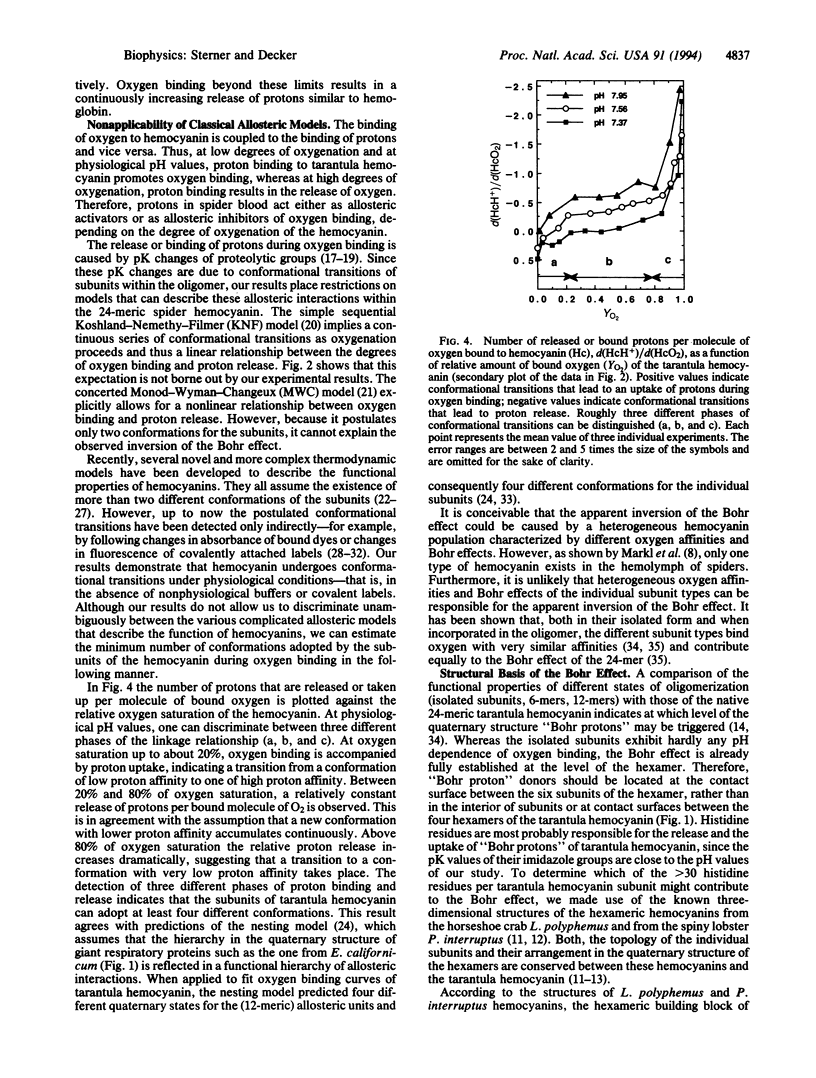
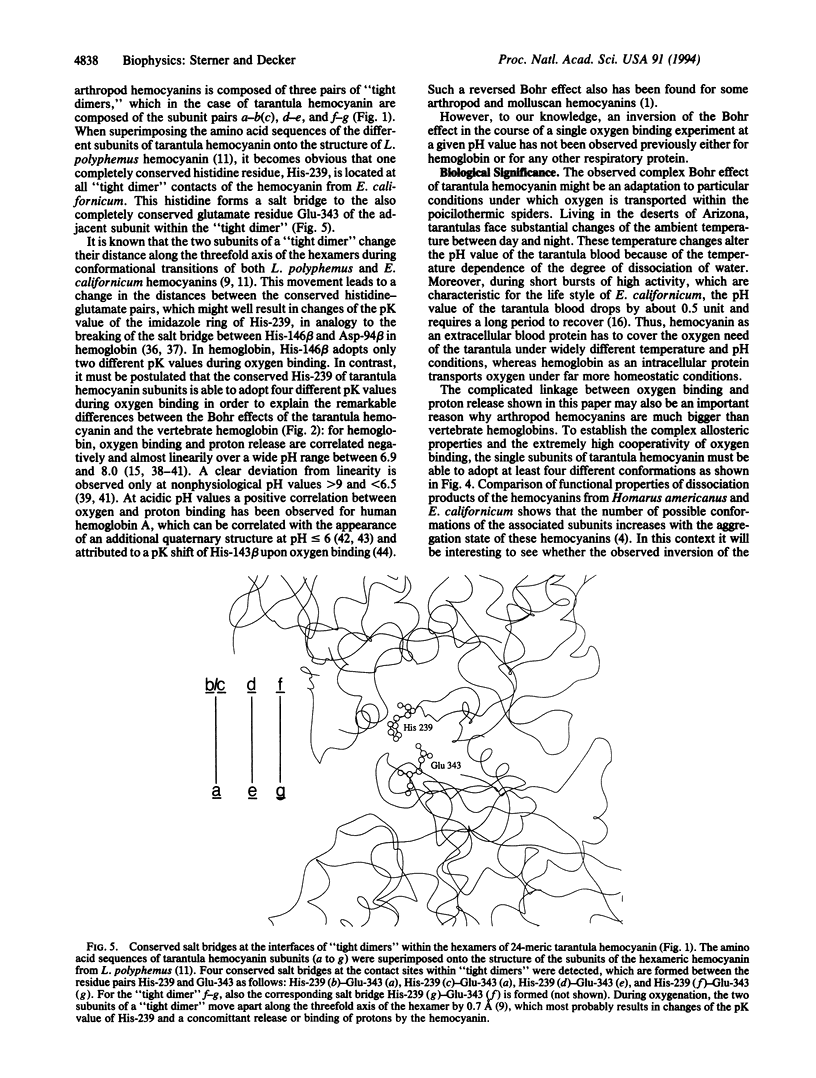
The Bohr effect describes the usually negative coupling between the binding of oxygen and the binding of protons to respiratory proteins. It was first described for hemoglobin and provides for an optimal oxygen supply of the organism under changing physiological conditions. Our measurements of both oxygen and proton binding to the 24-meric tarantula hemocyanin establish the unusual case where a respiratory protein binds protons at low degrees of oxygenation but releases protons at high degrees of oxygenation. In contrast to what is observed with hemoglobin and other respiratory proteins, this phenomenon amounts to the inversion of the Bohr effect in the course of an oxygen-binding curve at a given pH value. Therefore, protons in spider blood can act either as allosteric activators or as allosteric inhibitors of oxygen binding, depending on the degree of oxygenation of hemocyanin. These functional properties of tarantula hemocyanin, which cannot be explained by classical allosteric models, require at least four different conformational states of the subunits. Inspection of the known x-ray structures of closely related hemocyanins suggests that salt bridges between completely conserved histidine and glutamate residues located at particular intersubunit interfaces are responsible for the observed phenomena.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brouwer M., Bonaventura C., Bonaventura J. Analysis of the effect of three different allosteric ligands on oxygen binding by hemocyanin of the shrimp, Penaeus setiferus. Biochemistry. 1978 May 30;17(11):2148–2154. doi: 10.1021/bi00604a019. [DOI] [PubMed] [Google Scholar]
- Brouwer M., Serigstad B. Allosteric control in Limulus polyphemus hemocyanin: functional relevance of interactions between hexamers. Biochemistry. 1989 Oct 31;28(22):8819–8827. doi: 10.1021/bi00448a021. [DOI] [PubMed] [Google Scholar]
- Busch M. R., Mace J. E., Ho N. T., Ho C. Roles of the beta 146 histidyl residue in the molecular basis of the Bohr effect of hemoglobin: a proton nuclear magnetic resonance study. Biochemistry. 1991 Feb 19;30(7):1865–1877. doi: 10.1021/bi00221a020. [DOI] [PubMed] [Google Scholar]
- Chien J. C., Mayo K. H. Carp hemoglobin. II. The alkaline Bohr effect. J Biol Chem. 1980 Oct 25;255(20):9800–9806. [PubMed] [Google Scholar]
- Decker H., Connelly P. R., Robert C. H., Gill S. J. Nested allosteric interaction in tarantula hemocyanin revealed through the binding of oxygen and carbon monoxide. Biochemistry. 1988 Sep 6;27(18):6901–6908. doi: 10.1021/bi00418a036. [DOI] [PubMed] [Google Scholar]
- Decker H., Markl J., Loewe R., Linzen B. Hemocyanins in spiders, VIII. Oxygen affinity of the individual subunits isolated from Eurypelma californicum hemocyanin. Hoppe Seylers Z Physiol Chem. 1979 Oct;360(10):1505–1507. [PubMed] [Google Scholar]
- Decker H., Savel-Niemann A., Körschenhausen D., Eckerskorn E., Markl J. Allosteric oxygen-binding properties of reassembled tarantula (Eurypelma californicum) hemocyanin with incorporated apo- or met-subunits. Biol Chem Hoppe Seyler. 1989 Jun;370(6):511–523. doi: 10.1515/bchm3.1989.370.1.511. [DOI] [PubMed] [Google Scholar]
- Decker H., Sterner R. Hierarchien in der Struktur und Funktion von sauerstoffbindenden Proteinen. Naturwissenschaften. 1990 Dec;77(12):561–568. doi: 10.1007/BF01133724. [DOI] [PubMed] [Google Scholar]
- Decker H., Sterner R. Nested allostery of arthropodan hemocyanin (Eurypelma californicum and Homarus americanus). The role of protons. J Mol Biol. 1990 Jan 5;211(1):281–293. doi: 10.1016/0022-2836(90)90027-J. [DOI] [PubMed] [Google Scholar]
- Di Cera E., Doyle M. L., Gill S. J. Alkaline Bohr effect of human hemoglobin Ao. J Mol Biol. 1988 Apr 5;200(3):593–599. doi: 10.1016/0022-2836(88)90545-1. [DOI] [PubMed] [Google Scholar]
- Hazes B., Magnus K. A., Bonaventura C., Bonaventura J., Dauter Z., Kalk K. H., Hol W. G. Crystal structure of deoxygenated Limulus polyphemus subunit II hemocyanin at 2.18 A resolution: clues for a mechanism for allosteric regulation. Protein Sci. 1993 Apr;2(4):597–619. doi: 10.1002/pro.5560020411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Yonetani T. PH dependence of the Adair constants of human hemoglobin. Nonuniform contribution of successive oxygen bindings to the alkaline Bohr effect. J Biol Chem. 1975 Mar 25;250(6):2227–2231. [PubMed] [Google Scholar]
- Johnson B. A., Bonaventura C., Bonaventura J. Allostery in Callinectes sapidus hemocyanin: cooperative oxygen binding and interactions with L-lactate, calcium, and protons. Biochemistry. 1988 Mar 22;27(6):1995–2001. doi: 10.1021/bi00406a028. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Leidescher T., Decker H. Conformational changes of tarantula (Eurypelma californicum) haemocyanin detected with a fluorescent probe, 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole. Eur J Biochem. 1990 Feb 14;187(3):617–625. doi: 10.1111/j.1432-1033.1990.tb15345.x. [DOI] [PubMed] [Google Scholar]
- Linzen B., Soeter N. M., Riggs A. F., Schneider H. J., Schartau W., Moore M. D., Yokota E., Behrens P. Q., Nakashima H., Takagi T. The structure of arthropod hemocyanins. Science. 1985 Aug 9;229(4713):519–524. doi: 10.1126/science.4023698. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Makino N. An oxygenation-linked dye binding to Limulus polyphemus hemocyanin. Eur J Biochem. 1985 Feb 1;146(3):563–569. doi: 10.1111/j.1432-1033.1985.tb08688.x. [DOI] [PubMed] [Google Scholar]
- Makino N. Analysis of oxygen binding to Panulirus japonicus hemocyanin. The effect of divalent cations on the allosteric transition. Eur J Biochem. 1986 Jan 2;154(1):49–55. doi: 10.1111/j.1432-1033.1986.tb09357.x. [DOI] [PubMed] [Google Scholar]
- Makino N. The oxygenation-linked binding of neutral red to spiny lobster hemocyanin. A structural study of the partially oxygenated protein. Eur J Biochem. 1987 Feb 16;163(1):35–41. doi: 10.1111/j.1432-1033.1987.tb10733.x. [DOI] [PubMed] [Google Scholar]
- Markl J., Savel A., Decker H., Linzen B. Hemocyanins in spiders, IX. Homogeneity, subunit composition and the basic oligomeric structure of Eurypelma californicum hemocyanin. Hoppe Seylers Z Physiol Chem. 1980 May;361(5):649–660. doi: 10.1515/bchm2.1980.361.1.649. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Kilmartin J. V., Nishikura K., Fogg J. H., Butler P. J., Rollema H. S. Identification of residues contributing to the Bohr effect of human haemoglobin. J Mol Biol. 1980 Apr 15;138(3):649–668. doi: 10.1016/s0022-2836(80)80022-2. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Mechanisms of cooperativity and allosteric regulation in proteins. Q Rev Biophys. 1989 May;22(2):139–237. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Robert C. H., Decker H., Richey B., Gill S. J., Wyman J. Nesting: hierarchies of allosteric interactions. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1891–1895. doi: 10.1073/pnas.84.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savel-Niemann A., Markl J., Linzen B. Hemocyanins in spiders. XXII. Range of allosteric interaction in a four-hexamer hemocyanin. Co-operativity and Bohr effect in dissociation intermediates. J Mol Biol. 1988 Nov 20;204(2):385–395. doi: 10.1016/0022-2836(88)90583-9. [DOI] [PubMed] [Google Scholar]
- Shih D. T., Luisi B. F., Miyazaki G., Perutz M. F., Nagai K. A mutagenic study of the allosteric linkage of His(HC3)146 beta in haemoglobin. J Mol Biol. 1993 Apr 20;230(4):1291–1296. doi: 10.1006/jmbi.1993.1242. [DOI] [PubMed] [Google Scholar]
- Silva M. M., Rogers P. H., Arnone A. A third quaternary structure of human hemoglobin A at 1.7-A resolution. J Biol Chem. 1992 Aug 25;267(24):17248–17256. [PubMed] [Google Scholar]
- Sterner R., Bardehle K., Paul R., Decker H. Tris: an allosteric effector of tarantula haemocyanin. FEBS Lett. 1994 Feb 14;339(1-2):37–39. doi: 10.1016/0014-5793(94)80379-x. [DOI] [PubMed] [Google Scholar]
- Tyuma I., Ueda Y. Non-linear relationship between oxygen saturation and proton release, and equivalence of the Bohr and Haldane coefficients in human hemoglobin. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1278–1283. doi: 10.1016/s0006-291x(75)80368-8. [DOI] [PubMed] [Google Scholar]
- Volbeda A., Hol W. G. Crystal structure of hexameric haemocyanin from Panulirus interruptus refined at 3.2 A resolution. J Mol Biol. 1989 Sep 20;209(2):249–279. doi: 10.1016/0022-2836(89)90276-3. [DOI] [PubMed] [Google Scholar]
- Zolla L., Calabrese L., Brunori M. Distribution of copper atoms and binding of carbon monoxide in partially copper-depleted hemocyanin. Biochim Biophys Acta. 1984 Jul 31;788(2):206–213. doi: 10.1016/0167-4838(84)90263-2. [DOI] [PubMed] [Google Scholar]
- Zolla L., Coletta M., di Cera E., Giardina B., Kuiper H., Brunori M. Discrimination of tertiary and quaternary Bohr effect in the O2 binding of Helix pomatia beta-hemocyanin. Biophys Chem. 1986 Aug;24(3):319–325. doi: 10.1016/0301-4622(86)85037-2. [DOI] [PubMed] [Google Scholar]
- van Holde K. E., Miller K. I. Haemocyanins. Q Rev Biophys. 1982 Feb;15(1):1–129. doi: 10.1017/s0033583500002705. [DOI] [PubMed] [Google Scholar]



