Abstract
A strong gender bias is seen in many autoimmune diseases including systemic lupus erythematosus (SLE). To investigate the basis for the female preponderance in SLE, we have been studying BALB/c mice in which B cells express the R4A heavy chain of an anti-DNA antibody in association with an endogenous light chain repertoire (R4Atg mice). In unmanipulated mice, approximately 5% of B cells express the R4A transgene. R4Atg mice do not spontaneously develop elevated serum titers of anti-DNA antibodies. Administration of either estradiol (E2) or prolactin (Pr) results in escape from tolerance of autoreactive B cells, expressed as an increase in transgene-expressing B cells and elevated serum titers of anti-DNA antibodies. We previously demonstrated that autoreactive B cells maturing in an estrogenic milieu develop as marginal zone (MZ) B cells; when these same B cells mature in the presence of increased prolactin, they develop as follicular (Fo) B cells. To determine the long term consequence of this differential maturation of DNA-reactive B cells, we treated R4Atg BALB/c mice with E2 or Pr for 6 weeks until serum titers of anti-DNA antibody were high, at which time hormonal exposure was discontinued. In E2-treated mice, the anti-DNA titers remained high even 3 months after discontinuation of hormone exposure. Nascent B cells underwent normal tolerance induction, but existing autoreactive MZ B cells persisted and continued to secrete autoantibody. In contrast, Pr caused only a short-term increase in anti-DNA antibody titers. By 3 months after cessation of hormone treatment, serum anti-DNA antibody titers and B cell subsets were indistinguishable from those in placebo (P) treated mice. These findings suggest that autoantibody responses are sustained for variable lengths of time depending on the B cell subset producing the autoantibodies. This observation may be relevant to understanding the heterogeneous presentation of patients with SLE and to the design of therapies targeting speci?c B-cell populations in autoimmune disease.
Keywords: B cells, anti-DNA antibody, Autoantibodies, Autoreactive
1. Introduction
Immature B cells leave the bone marrow and migrate to the spleen as transitional B cells. If they escape negative selection, they mature into marginal zone (MZ) or follicular (Fo) B cells. In mice MZ B cells have an IgMhighIgDlowCD21highCD23− phenotype, express high levels of Toll-like receptors (TLRs), and participate in both T cell-independent and T cell-dependant immune responses [1–7]. MZ B cells express immunoglobulin variable genes that are, in general, unmutated and many express polyreactive B cell receptors (BCRs) [1,5,8]. In contrast to MZ B cells, Fo B cells have an IgMint IgDhighCD21lowCD23high phenotype, express more oligospeci?c BCRs, participate in T cell-dependent responses and undergo affinity maturation in a germinal center response [9,10]. MZ and Fo B cells also differ with respect to the kinetics of antibody production with MZ B cells displaying faster kinetics of antibody secretion [7]. Both MZ and Fo B cells have been shown to participate in the anti-DNA response in murine models of systemic lupus erythematosus (SLE) [11,12].
In an effort to understand the selection and maturation of DNA-reactive B cells in SLE, we have been studying B cell maturation in R4Atg BALB/c mice, which harbor a transgene (Tg) encoding the IgG2b heavy chain of an anti-DNA antibody [13]. The R4A heavy chain pairs with endogenous light chains, generating a spectrum of antibodies, some with no binding to DNA, others with low, and some with high af?nity binding to DNA [14–16] (Table 1). Despite expression of an IgG2b heavy chain, B cells mature normally and tolerance is maintained in these mice by negative selection of high af?nity anti-DNA B cells [13].
Table 1.
Relative affinities of DNA-reactive B cells from R4Atg BALB/c mice.
| -Vκ-Jκ usage | Relative Affinitya | Reference |
|---|---|---|
| Vκ1A-Jκ1 | 9.1 × 10−8 | [15, 16] |
| Vκ1-Jκ5 | 4.5 × 10−9 | [16] |
| Vκ1A-Jκ4 | 4.2 × 10−6 | [15] |
| Vκ21-Jκ1 | 2.2 ×10−6 | [16] |
| Vκ21-Jκ2 | 9 × 10−6 | [16] |
The relative affinity of the R4A H chain paired with each L chain was determined in previous studies by inhibition ELISA.
Both E2 and Pr have been reported and to contribute to autoimmunity in general and to SLE pathogenesis in particular [17–29]. Sex hormones have been implicated in risk for developing SLE and in disease activity although the data are controversial [30]. Estrogen, in particular, has been extensively studied as the ratio of females to male with onset of SLE increasing from 3 to 1 before puberty to 9 to 1 after puberty [31]. Clinical epidemiologic studies have suggested that the use of oral contraception increases the risk of SLE [32]. In contrast, the SELENA study of the safety of estrogen in women with SLE failed to demonstrate that the use of oral contraception increased the frequency of disease flares [33]. The data are further complicated by the demonstration that the use of hormone replacement therapy did increase the number of flares [34].
Animal models of SLE-like disease also provide divergent data. Some, like NZB/W mice, display a clear female bias. Moreover, studies in NZB/W mice have demonstrated a dramatic delay in disease onset and reduction in disease severity in female mice following ovariectomy or administration of estrogen receptor antagonists [35,36]. In contrast, others, such as MRL/lpr mice, have a less profound female bias and estrogen exacerbates the autoantibody-mediated glomerulonephritis of MRL/lpr mice while it diminishes vasculitis [37].
Prolactin levels have also been reported to be elevated in a high percentage of patients with a variety of autoimmune diseases [38]. In particular, they are elevated in approximately 25% of SLE patients. While a relationship between disease activity and prolactin level has not been clearly established in human SLE, antibodies to prolactin appear to correlate with lower disease activity [39]. Small clinical trials in SLE patients suggest a therapeutic effect of bromocriptine, but large scale, definitive trials have not been performed [40–42]. Administration of prolactin to NZB/W mice accelerates disease while treatment with bromocriptine, a drug that inhibits prolactin secretion, can retard disease progression [43].
Both estrogen and prolactin affect numerous cells within the immune system [38,44]. To understand their impact on disease expression, it is important to isolate effects on particular cell lineages. We have been studying the impact of estrogen and prolactin on B cells using the R4Atg BALB/c mouse. We use this model as it allows us to isolate the effects of estrogen and prolactin on B cell biology in the absence of a large number of inflammatory factors that can exacerbate or diminish the effects of these hormones. This model also allowed us to remove hormone and observe the impact of hormone withdrawal without the confounding effects of the inflammatory cytokines present in spontaneous models of SLE. When R4Atg BALB/c mice are treated with E2 sufficient to sustain serum levels at the concentration achieved during the luteal phase of the estrus cycle, B cells making high affinity anti-DNA antibodies survive negative selection and mature as MZ B cells [45]. In contrast, when R4Atg BALB/c mice are treated with Pr to cause a doubling of serum Pr concentration, high affinity autoreactive B cells mature as Fo B cells [46]. Previously we demonstrated that both the MZ and Fo B cells express identical BCRs showing the differentiation pathway for a specific B cell can be determined by hormonal milieu [47]. This observation provided an opportunity to study the long-term survival and activation of B cells with identical BCRs having either an MZ or Fo phenotype.
In this study, we report that the antibody response of MZ B cells is of longer duration than that of Fo B cells. Autoreactive B cells are present in Pr-treated mice only while Pr levels are high. In contrast, the persistence of autoreactive MZ B cells is not dependent on the continued presence of E2. These findings have potential implications for understanding clinical SLE and the response to therapy as the time frame for falls in autoantibody titers are likely to depend on the B cell subset responsible for production of the autoantibody.
2. Materials and methods
2.1. Mice and hormone treatment
R4Atg BALB/c mice, which have been described previously [13], were bred in a specific pathogen-free barrier facility. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC). Mice were implanted subcutaneously with 60-day time-release pellets containing placebo (P), E2 (17 β-estradiol; 0.18 mg) or Pr (6 mg) (Innovative Research of America). The E2 pellets lead to a constant serum level of 75–100 pg/ml over a 60-day period [16]. The Pr pellet leads to a two-fold increase in serum Pr levels to 20–35 ng/ml [46]. Prior to implanting hormone pellets, all mice were ovariectomized to eliminate fluctuations in endogenous E2 levels. After 6 weeks of treatment, the E2, Pr or P pellets were removed and the mice were followed for an additional 12 weeks. Serum was obtained before the start of treatment and at biweekly intervals thereafter. To examine the level of proteinuria, urine was also collected at the beginning of treatment and every two weeks thereafter. Kidneys were examined at the end of the study as described below.
2.2. Autoreconstitution
R4Atg mice were implanted with E2 pellets for 6 weeks and subsequently underwent sublethal irradiation with 600 rads of gamma irradiation [48]. Mice were allowed to reconstitute for 12 weeks after irradiation and were then analyzed for B cell subsets. Urine was collected to assess proteinuria. IgG deposition in the kidneys was examined as described.
2.3. Cell surface staining and flow cytometry
Splenocytes from P- and hormone-treated R4Atg mice were isolated and stained with antibodies to cell surface markers. B cell subsets were analyzed by flow cytometry using an LSRII instrument (Becton Dickinson). The monoclonal antibodies employed were B220 (clone 7G6), CD21 (clone 7G6), CD23 (clone B3B4), AA4.1 and γ2b (clone R12-3); all were obtained from BD Pharmingen except for AA4.1 (eBioscience). B cell subsets were identified as transitional T1 (B220+AA4.1hiCD21lowCD23−), T2 (B220+AA4.1hiCD 21hiCD23hi), MZ (B220+AA4.1−CD21hiCD23−) and Fo (B220+AA4.1−CD21lowCD23hi). In reconstitution experiments, CD24 staining was used to identify transitional B cell subsets.
2.4. Single cell RT-PCR and repertoire analysis
Immature (B220+IgG2b+AA4.1+) and mature (B220+IgG2b+AA 4.1−) tg-expressing B cells were sorted from P- and hormone-treated R4Atg mice as single cells into 96-well PCR plates using a FACS Aria cell sorter (BD Biosciences). cDNA was prepared as described previously [49]. Single cell PCR of Ig kappa light chain variable region genes of tg-expressing B cells was accomplished in two rounds as described [50]. The first round was performed using the universal Vκ 5′ primer (5′GGCTGCAGSTTCAGTGGCAGTGGRTCWGGRAC3′) and the constant region primer Cκ (Cκ1), (5′TGGATGGGTGGGAAGATG3′) and the second, nested round with Vκ and Cκ (Cκ2) (5′AAGATGGATACAGTTGGT3′) primers. The PCR products were subjected to exo-SAP treatment (USB Biochemicals) and the kappa light chains were sequenced using the Cκ2 primer (Genewiz Inc.). The samples were analyzed for Vκ-Jκ family usage using the NCBI BLAST program (www.ncbi.nlm.nih.gov/IgBlast).
2.5. ELISAs
Half-well plates (Costar) were dry-coated with calf thymus DNA (100 mg/ml, Sigma), blocked with 2% bovine serum albumin (BSA) and subsequently incubated with dilutions of serum obtained from P- or hormone-treated R4Atg mice. Anti-dsDNA antibody titer was measured with an IgG2b-specific secondary antibody coupled to alkaline phosphatase (AP) and phosphate substrate tablets, as previously described [51].
2.6. ELISpot assay
The frequency of anti-dsDNA antibody secreting B cells was evaluated by ELISpot analysis [16]. Briefly, Immulon 2HB 96-well plates (Thermo LabSystems) were coated with 100 μl/ml sonicated calf thymus DNA. Splenocytes from P- or hormone-treated R4Atg mice were added to the plates in serial dilution and incubated for 12 h at 37 °C followed by biotin-conjugated anti-mouse IgG (1:600 dilution, Southern Biotechnology) and alkaline phosphatase (AP)-conjugated streptavidin (Southern Biotechnology). The plates were developed with 5-bromo-4-chloro-3-indolyl phosphate substrate (Sigma Aldrich) and spots were counted under a dissecting microscope.
2.7. Glomerular IgG deposition
IgG deposition in the glomeruli of R4Atg mice was evaluated as described previously [51]. Kidneys were formalin fixed and paraffin embedded. 10 uM sections were stained with biotinylated anti-mouse IgG antibody and developed with an AP ABC detection kit (Vector Laboratories). IgG deposition in the glomeruli was visualized under a Zeiss microscope at X5 and X20 magnifications. The total number of glomeruli and glomeruli with IgG deposition in three different microscopic fields was determined for each kidney section. At least three mice were analyzed in each group.
2.8. Analysis of proteinuria
Proteinuria was measured using Bayer reagent strips (Bayer) according to the manufacturer’s specifications and expressed as mg/dL. Five mice in each group were examined for these studies.
2.9. Statistical analysis
All results are expressed as mean ± SD. Student’s t test (two-tailed) was used to compare differences between groups and Fisher’s exact test was used to analyze kappa chain repertoire.
3. Results
3.1. Long-term activation of MZ B cells
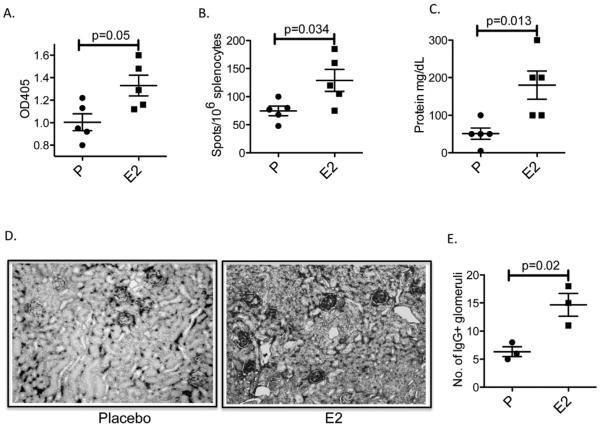
We have shown previously that exposure to E2 or Pr leads to the protection from negative selection of high affinity anti-DNA B cells in R4Atg BALB/c mice and to their subsequent activation as MZ [45] or Fo B cells [46], respectively. Interestingly, the same B cells can mature to either subset, depending on hormone exposure demonstrating that hormonal milieu as well as antigenic specificity, contributes to B cell differentiation [47]. Because MZ B cells are reported to be more long-lived than Fo B cells [52], we assessed the duration of antibody response after hormone levels were no longer elevated. We exposed ovariectomized R4Atg mice to E2 for 6 weeks until serum titers of anti-DNA antibody were high, and then removed hormone pellets to eliminate the source of hormonal stimulation and determined the duration of the anti-DNA response. Surprisingly, as long as 3 months after removal of the E2 pellet, anti-DNA titers remained high and DNA-reactive B cells were present in high number in the spleen (Fig. 1A and B).
Fig. 1.
Persistence of anti-DNA reactivity and IgG deposition in R4Atg mice following discontinuation of E2 exposure. R4Atg mice that had been exposed to E2 for 6 weeks and then followed for 12 weeks displayed (A) elevated levels of anti-DNA antibody, (B) an increase in DNA-reactive splenic B cells, (C) proteinuria and (D and E) glomerular IgG deposition. P values were determined by Student’s t test.
In previous studies using single cell analysis of light chains expressed in association with the R4A heavy chain, we have identified the light chains that confer high or low affinity DNA binding (Table 1). Moreover, we have shown that E2 treatment leads to a light chain repertoire with an increased frequency of VJ sequences that confer high affinity to DNA, especially Vκ1-Jκ1 and Vκ9/10-Jκ5 light chains, in tg-expressing B cells. In contrast, DNA-reactive B cells of P-treated mice exhibit a predominance of Vκ4/5-Jκ5, Vκ1-Jκ5 and Vκ21-Jκ1 light chains that confer low affinity DNA-reactivity [47,50]. We were interested to see whether the tg-expressing B cells of E2-treated R4Atg mice continued to express VJ genes encoding high-affinity DNA-reactive antibodies even 3 months after cessation of exposure to E2. Hence, we performed repertoire analysis of the kappa light chains from the γ2b+ mature B cells in R4Atg mice that received E2 for 6 weeks and were subsequently without hormone exposure for 12 weeks. We observed a persistent increase in the frequency of light chains that confer high affinity DNA-binding in these mice compared to mice treated with P for 6 weeks and subsequently followed for 12 additional weeks (Table 2).
Table 2.
Frequency of high affinity DNA-reactive B cells in R4Atg mice treated with P or E2 following which time, treatment was discontinued for 12 weeks.
| P | E2 | |
|---|---|---|
| Transitional | 11/125 (8.8%) | 8/107 (14.9%) |
| Mature | 9/112 (8%) | 29/131 (23%)* |
Fisher’s exact test was used to analyze the significance between the P- and the E2- treated groups.
denotes p < 0.05.
To confirm that the antibodies present in the serum of R4Atg mice after cessation of E2 exposure were potentially pathogenic, we examined mice for the presence of proteinuria. E2-treated mice continued to exhibit increased proteinuria (Fig. 1C). We also examined the kidneys of these mice and found glomerular IgG deposition (Fig. 1D and E). Thus, renal IgG deposition was present even 3 months subsequent to discontinuation of E2 exposure.
3.2. Continued expansion of MZ B cells subsequent to cessation of E2
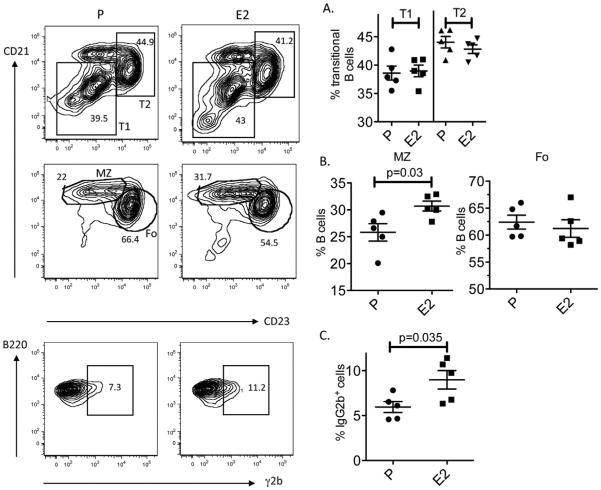
In previous studies, a sustained exposure of R4Atg mice to E2 resulted in a 2-fold increase in tg-expressing γ2b B cells as well as a doubling of MZ B cells in the spleen [30]. We wanted to determine whether the alteration in B cell subsets persisted even 3 months after discontinuation of E2. In R4Atg mice that were implanted with either E2 or P pellets for 6 weeks followed by removal of pellets, the total numbers of B cells were similar (29.06–38.12 × 106 vs. 33.27–39.68 × 106, respectively). R4Atg mice with E2 implanted pellets exhibited no difference in transitional B cell populations but displayed a sustained increase in MZ B cells (Fig. 2A and B). Because we have previously shown that all γ2b-expressing B cells express the tg [13], we analyzed γ2b-expressing B cells and observed a sustained increase in their number (Fig. 2C). These observations demonstrate that E2-induced activation of MZ B cells persisted for at least as long as 12 weeks in a milieu devoid of E2.
Fig. 2.
Alterations in E2-induced B cell subsets persist following withdrawal of E2. The transitional (T1 and T2), mature (MZ and Fo) and the transgenic B cell populations were analyzed by flow cytometry. On left is a representative set of plots. (A) The transitional B cell subsets of E2-treated mice 3 months after removal of the E2 pellet were indistinguishable from those of P-treated mice. (B) There was continued expansion of MZ B cells and a persistent increase in the percentage of tg-expressing B cells. MZ B cells were identified as B220posCD21highCD23negAA4.1low/neg and transitional B cells were identified as B220posCD21lowAA4.1high (T1) and B220posCD21highAA4.1hgh (T2). The results shown represent mean ± SD of 5 mice in each group. Student’s t test was used to determine the significance between the groups.
3.3. Persistence of autoreactive MZ B cells induced by E2
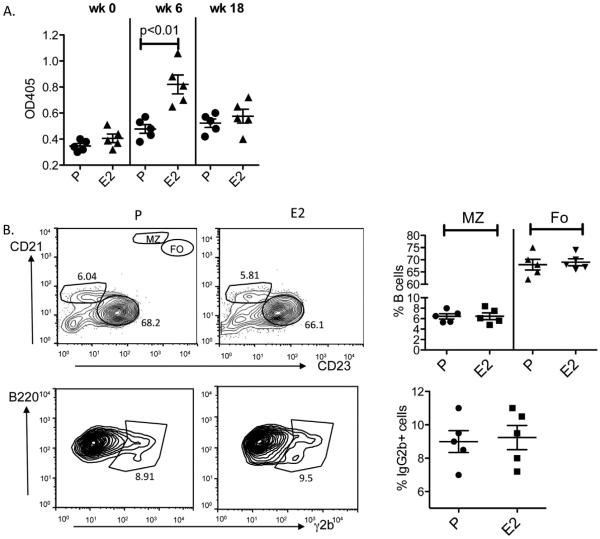
To distinguish whether immature or transitional tg-expressing B cells were continuing to differentiate to MZ B cells, due perhaps to a persisting change in the bone marrow or in splenic stromal cells, we subjected R4Atg mice that had been treated for 4 weeks with E2 to irradiation and analyzed the reconstituted B cell populations. The B cell reconstituted mice displayed no elevation in anti-DNA antibody titers (Fig. 3A). B cell subsets were similar to those observed in P-treated mice; there was no expansion of tg-expressing or MZ B cells (Fig. 3B). Thus, there was no evidence for a long-term E2-induced alteration in B cell microenvironment to support the continued maturation of autoreactive MZ B cells. Rather, prolonged survival of the autoreactive MZ cells led to a persistent elevation in autoantibody titers in E2-treated mice.
Fig. 3.
Anti-DNA antibodies and altered B cell subsets after cessation of E2 and B cell reconstitution. R4Atg mice were treated for 6 weeks with E2 and subsequently irradiated with a sublethal dose of 600 rads of gamma irradiation. 12 weeks after irradiation and lymphoid reconstitution, serum anti-DNA antibody response was determined by ELISA. (A) The anti-DNA antibody levels were no longer elevated in these mice (week 18). (B) All B cell subsets were present at a normal frequency: transitional T1 (B220posCD21lowAA4.1high) and T2 (B220posCD21hiAA4.1high), MZ (B220posCD21highCD23negAA4.1low/neg) and Fo (B220posCD21negCD23hi AA4.1low/neg). An expansion of tg+ B cells was not observed. The results are expressed mean ± SD of 5 mice in each group.
3.4. Short-term activation of autoreactive follicular B cells
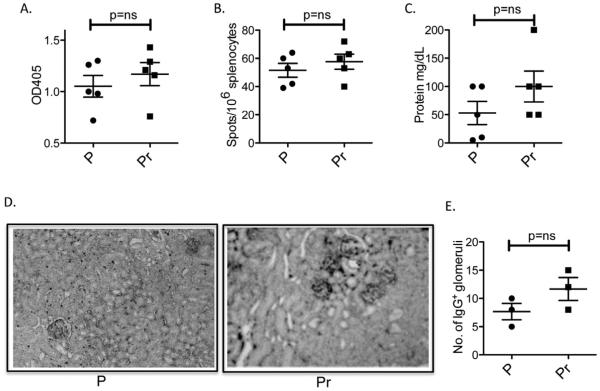
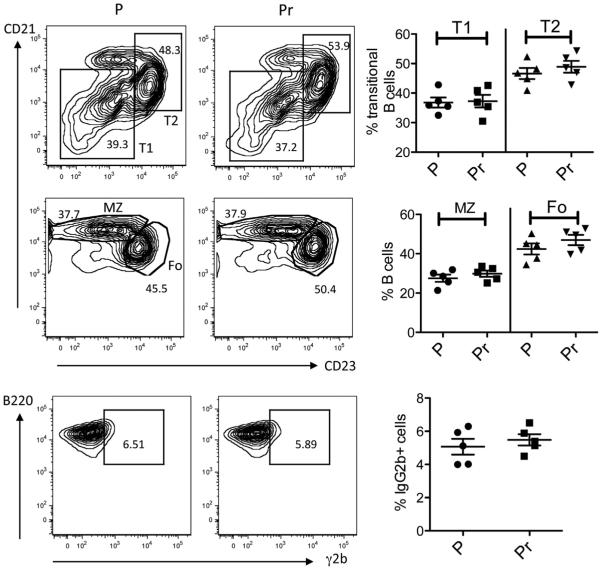
Unlike E2, Pr induces anti-DNA B cells to become Fo B cells in R4Atg mice [46]. We asked whether autoantibody titers were sustained even after cessation of Pr exposure. R4Atg mice were implanted for 6 weeks with Pr or P pellets; the pellets were removed and mice were followed an additional 12 weeks. In contrast to E2, Pr caused only a short-term increase in anti-DNA antibody titer. Anti-DNA titers declined by 6 weeks after removal of the Pr pellet to levels observed in R4Atg mice given P pellets (Fig. 4A). At 12 weeks after cessation of Pr exposure, there was no detectable increase in DNA-reactive B cells (Fig. 4B). Pr- and P-treated mice displayed no difference in the level of proteinuria or glomerular Ig deposition (Fig. 4C–E). Repertoire analysis displayed no increase in the frequency of light chains conferring high-affinity DNA-binding in mature B cells of Pr-treated mice at 12 weeks following discontinuation of Pr treatment (Table 3), demonstrating that the high affinity DNA-reactive B cells were no longer present. Consistent with this observation, by three months after cessation of Pr treatment, B cell subsets and the number of γ2b-expressing B cells were the same as in P-treated mice (Fig. 5). These observations are consistent with other studies showing that the Fo B cell compartment is not long lived and needs constant replenishment from an immature B cell population [52].
Fig. 4.
DNA reactivity in R4Atg mice subsequent to withdrawal of Pr. Six to 8 week-old R4Atg mice were implanted with Pr or P pellets for 6 weeks following which the pellets were removed. After 12 weeks, the mice were analyzed for (A) serum anti-DNA reactivity; (B) DNA-reactive B cells; (C) proteinuria and (D and E) glomerular Ig deposition. At least 5 mice were used in each group for the experiments.
Table 3.
Frequency of high affinity DNA-reactive B cells in R4Atg mice treated with P or Pr for 6 weeks following which time treatment was discontinued for 12 weeks.
| P | Pr | |
|---|---|---|
| Transitional | 3/56 (5.4%) | 4/63 (6.4%) |
| Mature | 4/60 (6.7%) | 7/71 (9.9%) |
No significant differences were observed between the groups.
Fig. 5.
B cell subsets in R4Atg mice following cessation of Pr exposure. In R4Atg mice were treated with Pr or P for 6 weeks followed by removal of the pellet. Transitional T1 and T2 B cells, MZ and Fo B cell populations were similar. No differences in the numbers of tg-expressing B cells were observed, as determined by the Student’s t test, in 5 mice per group.
4. Discussion
Elegant molecular studies have demonstrated the presence of both estrogen and prolactin receptors on numerous lineages of immune cells [38,53]. The effects of these hormones on gene expression and cell function are both pro- and anti-inflammatory [44]. We have been interested in understanding how to improve B cell-targeted therapy in SLE. While autoantibodies are critical to clinical manifestations of disease, we have yet to develop clinically proven effective therapies that target B cell survival, selection or activation [54,55]. This may reflect, in part, an inadequate understanding how to identify the B cell subset that harbors the autoreactive population in individual patients and how to target a specific B cell subset. Indeed, focusing on the subset that harbors the autoreactive B cells might permit us to design therapies that are less broadly immunosuppressive.
We have previously shown that the same DNA-reactive B cell can mature as either MZ or Fo cells depending on hormonal milieu. In this study, we demonstrate that the duration of autoantibody response depends on the differentiation phenotype of the autoreactive B cells.
We performed these studies in a non-spontaneously autoimmune mouse harboring a heavy chain tg for an anti-DNA antibody. This model allows us to identify the effects of hormones on B cell function, independent of the numerous immune perturbations that are present in spontaneous mouse models of SLE. The effects we observe, however, may nonetheless represent either direct effects of estrogen or prolactin on B cells or indirect effects, downstream of hormonal effects on other cell lineages. We observed a persistent effect of E2 exposure on serum anti-DNA antibody titers in R4Atg mice due to the long-term survival of MZ DNA-reactive B cells. Thus, the persistence of high titers of autoantibodies does not necessarily imply the presence of long lived plasma cells arising from the Fo B cell subset. We have previously shown that E2 has direct effects on maturing B cells, protecting them from BCR-mediated apoptosis [56]. E2 has also been shown to enhance the level of BAFF production by myeloid cells [47,50,57], which promotes B cell maturation to a MZ phenotype [58]. E2 also reduces T cell production of IL-2, potentially leading to a reduced population of T regulatory cells [59]. Finally, E2 enhances BCR-mediated apoptosis and augments p202b expression, which protects the B cell from TLR-mediated activation [60]. Therefore, both direct and indirect effects of E2 on B cells can lead to the survival of autoreactive B cells, their differentiation to an MZ phenotype and their activation. Once high E2 levels were no longer present there were no apparent residual effects of E2 exposure on B cell maturation, but the survival of DNA-reactive MZ B cells did not require persistently high levels of E2. Thus, E2 can cause a sustained autoantibody response. It is of interest that some lupus patients have been shown to metabolize E2 to its highly estrogenic forms [61]. These individuals may be those in whom DNA-reactivity resides within the MZ B cell subset.
Pr exposure also alters B cell maturation through direct and indirect effects on B cells. It has been demonstrated that transitional B cells have highest expression of Pr receptor and that levels of receptor expression associate with autoreactivity [62]. Pr has also been shown to upregulate CD40 ligand on T cells, a T cell phenotype that has been reported in SLE patients [63]. Blockade of the CD40:CD40 ligand interaction can reduce SLE disease progression dramatically in NZB/W mice, confirming the importance of CD40 ligand in SLE pathogenesis [64,65]. While a clinical trial in SLE patients was terminated prematurely due to an unanticipated increase in thrombotic events, early evidence suggested therapeutic efficacy [66]. Interestingly, elevated levels of Pr are found in approximately 25% of lupus patients [23–29], although it has not been investigated whether these patients express high levels of CD40 ligand. The current study demonstrates that the B cells that develop in a high prolactin environment become short-lived plasma cells and there must be continuous maturation of transitional DNA-reactive B cells to Fo B cells to maintain a population of autoantibody secreting B cells. It is tempting to speculate that individuals with elevated Pr levels may be those who exhibit fluctuating levels of anti-DNA antibodies.
Here we highlight the importance of knowing the B cell subset responsible for autoantibody production, and targeting it appropriately. It has been demonstrated that type 1 interferon as well as Pr will cause B cells to become short-lived plasma cells [67]. In contrast, BAFF induces selective differentiation of MZ B cells [55]. In mice, a CD20 targeted antibody is more effective at depleting Fo B cells than MZ B cells, although it is not clear the same is true in humans [68,69]. BAFF blockade prevents the maturation of immature B cells, and impairs survival of short-lived plasma cells before affecting survival of MZ B cells [70,71]. Thus, effects of BAFF blockade on autoantibody titers would be expected to occur over a longer period of time if MZ B cells are responsible for the autoreactivity and over a shorter period of time if autoantibodies derive from short-lived plasma cells. Blockade of CD40:CD40 ligand interaction might affect activation of both Fo B cells and MZ B cells but would not affect already activated MZ B cells [72].
Thus, it is apparent that therapy must be tailored to address the underlying B cell subset responsible for autoreactivity and the mechanism involved in B cell differentiation to that subset. Models such as R4Atg mice will allow an exploration of the effects of therapy on specific B cell subsets. Since hormone levels can clearly affect B cell differentiation pathways, it would be instructive to determine hormone levels in patients participating in clinical trials to determine if there are patient cohorts more likely to benefit from a particular therapy and if there are hormonal modulations that might enhance therapeutic efficacy.
5. Conclusion
Our study reveals a sharp contrast in autoantibody responses expressed by MZ and Fo B cells. This finding may be pertinent in human SLE, in which patients display heterogeneity in their autoantibody responses as well as in their response to therapy. An understanding of the B cell subsets that contribute to autoreactivity in patients will greatly facilitate targeted therapies.
Acknowledgments
This study was supported by grants from the ALR (Betty Diamond), a NIH NIAID RO3 AR052041 grant and a Career Development Award from the S.L.E. Lupus Foundation, NY (Venkatesh Jeganathan).
We thank Sylvia Jones for expert secretarial assistance and Jonothan Logan for valuable discussions and critical reading of the manuscript.
Abbreviations
- SLE
systemic lupus erythematosus
- T
transitional
- Tg
transgenic
- κ
kappa
- MZ
marginal zone
- Fo
follicular
- E2
estradiol
- Pr
prolactin
- P
placebo
References
- [1].Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–35. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- [2].Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9:767–77. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- [3].Tarkowski A, Lue C, Moldoveanu Z, Kiyono H, McGhee JR, Mestecky J. Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric IgGA2-subclass antibody responses. J Immunol. 1990;144:3770–8. [PubMed] [Google Scholar]
- [4].Carson PJ, Schut RL, Simpson ML, O’Brien J, Janoff EN. Antibody class and subclass responses to pneumococcal polysaccharides following immunization of human immunodeficiency virus-infected patients. J Infect Dis. 1995;172:340–5. doi: 10.1093/infdis/172.2.340. [DOI] [PubMed] [Google Scholar]
- [5].Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- [6].Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178:7779–86. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- [7].Rubtsov AV, Swanson CL, Troy S, Strauch P, Pelanda R, Torres RM. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J Immunol. 2008;180:3882–8. doi: 10.4049/jimmunol.180.6.3882. [DOI] [PubMed] [Google Scholar]
- [8].Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–86. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- [9].Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–8. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- [10].MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- [11].Zheng D, Lee MK, Tung J, Brendolan A, Strober S. Cutting edge: a role for CD1 in the pathogenesis of lupus in NZB/NZW mice. J Immunol. 2000;164:5000–4. doi: 10.4049/jimmunol.164.10.5000. [DOI] [PubMed] [Google Scholar]
- [12].Marion TN, Krishnan MR, Steeves MA, Desai DD. Affinity maturation and autoimmunity to DNA. Curr Dir Autoimmun. 2003;6:123–53. doi: 10.1159/000066859. [DOI] [PubMed] [Google Scholar]
- [13].Offen D, Spatz L, Escowitz H, Factor S, Diamond B. Induction of tolerance to an IgG autoantibody. Proc Natl Acad Sci U S A. 1992;89:8332–6. doi: 10.1073/pnas.89.17.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Spatz L, Saenko V, Iliev A, Jones L, Geskin L, Diamond B. Light chain usage in anti-double-stranded DNA B cell subsets: role in cell fate determination. J Exp Med. 1997;185:1317–26. doi: 10.1084/jem.185.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bynoe MS, Spatz L, Diamond B. Eur J Immunol. 1999;29:1304–13. doi: 10.1002/(SICI)1521-4141(199904)29:04<1304::AID-IMMU1304>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [16].Bynoe MS, Grimaldi CM, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc Natl Acad Sci U S A. 2000;97:2703–8. doi: 10.1073/pnas.040577497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chapel TA, Burns RE. Oral contraceptives and exacerbation of lupus erythematosus. Am J Obstet Gynecol. 1971;110:366–9. doi: 10.1016/0002-9378(71)90730-7. [DOI] [PubMed] [Google Scholar]
- [18].Travers RL, Hughes GR. Oral contraceptive therapy and systemic lupus erythematosus. J Rheumatol. 1978;5:448–51. [PubMed] [Google Scholar]
- [19].Garovich M, Agudelo C, Pisko E. Oral contraceptives and systemic lupus erythematosus. Arthritis Rheum. 1980;23:1396–8. doi: 10.1002/art.1780231213. [DOI] [PubMed] [Google Scholar]
- [20].Barrett C, Neylon N, Snaith ML. Oestrogen-induced systemic lupus erythematosus. Br J Rheumatol. 1986;25:300–1. doi: 10.1093/rheumatology/25.3.300. [DOI] [PubMed] [Google Scholar]
- [21].Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978;147:1568–83. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lucas JA, Ahmed SA, Casey ML, MacDonald PC. Prevention of autoantibody formation and prolonged survival in New Zealand black/New Zealand white F1 mice fed dehydroisoandrosterone. J Clin Invest. 1985;75:2091–3. doi: 10.1172/JCI111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vidaller A, Llorente L, Larrea F, Mendez JP, Alcocer-Varela J, Alarcon-Segovia D. T cell dysregulation in patients with hyperprolactinemia: effect of bromocriptine treatment. Clin Immunol Immunopathol. 1986;38:337–43. doi: 10.1016/0090-1229(86)90243-6. [DOI] [PubMed] [Google Scholar]
- [24].McMurray RW. Prolactin and systemic lupus erythematosus. Ann Med Intern. 1996;147:253–8. [PubMed] [Google Scholar]
- [25].Lavalle CE, Graef A, Baca V, Ramirez-Lacayo M, Blanco-Favela F, Ortiz O. Prolactin and gonadal hormones: a key relationship that may have clinical, monitoring and therapeutic implications in systemic lupus erythematosus. Lupus. 1993;2:71–5. doi: 10.1177/096120339300200202. [DOI] [PubMed] [Google Scholar]
- [26].Walker SE, McMurray RW, Houri JM, Allen SH, Keisler D, Sharp GC, et al. Effects of prolactin in stimulating disease activity in systemic lupus erythematosus. Ann NY Acad Sci. 1998;840:762–72. doi: 10.1111/j.1749-6632.1998.tb09615.x. [DOI] [PubMed] [Google Scholar]
- [27].McMurray RW, Weidensaul D, Allen SH, Walker SE. Efficacy of bromocriptine in an open label therapeutic trial for SLE. J Rheumatol. 1995;22:2084–91. [PubMed] [Google Scholar]
- [28].Allen SH, Sharp GC, Wang G, Conley C, Takeda Y, Conroy SE, et al. Prolactin levels and antinuclear antibody profiles in women tested for connective tissue disease. Lupus. 1996;5:30–7. doi: 10.1177/096120339600500107. [DOI] [PubMed] [Google Scholar]
- [29].Jara LJ, Gomez-Sanchez C, Silveira LH, Martinez-Osuna P, Vasey FB, Espinoza LR. Hyperprolactinemia in SLE: association with disease activity. Am J Med Sci. 1992;303:222–6. doi: 10.1097/00000441-199204000-00003. [DOI] [PubMed] [Google Scholar]
- [30].Cohen-Solal JF, Jeganathan V, Grimaldi CM, Peeva E, Diamond B. Sex hormones and SLE: influencing the fate of autoreactive B cells. Curr Top Microbiol Immunol. 2006;305:67–88. doi: 10.1007/3-540-29714-6_4. [DOI] [PubMed] [Google Scholar]
- [31].Tedeschi SK, Bermas B, Costenbader KH. Sexual disparities in the incidence and course of SLE and RA. Clin Immunol. 2013;149:211–8. doi: 10.1016/j.clim.2013.03.003. [DOI] [PubMed] [Google Scholar]
- [32].Bernier MO, Mikaeloff Y, Hudson M, Suissa S. Combined oral contraceptive use and the risk of systemic lupus erythematosus. Arthritis Rheum. 2009;61:476–81. doi: 10.1002/art.24398. [DOI] [PubMed] [Google Scholar]
- [33].Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2550–8. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- [34].Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:953–62. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- [35].Wu WM, Lin BF, Su YC, Suen JL, Chang BL. Tamoxifen deceases renal inflammation and alleviates disease severity in autoimmune NZB/W F1 mice. Scand J Immunol. 2000;52:393–400. doi: 10.1046/j.1365-3083.2000.00789.x. [DOI] [PubMed] [Google Scholar]
- [36].Li J, McMurray RW. Effects of estrogen receptor subtype-selective agonists on autoimmune disease in lupus-prone NZB/W F1 mouse model. Clin Immunol. 2007;132:219–26. doi: 10.1016/j.clim.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [37].Carlsten H, Nilsson N, Jonsson R, Backman K, Holmdahl R, Tarkowski A. Estrogen mediated vasculitis and sialadenitis in autoimmune MRL/lpr mice. Cell Immunol. 1992;144:190–202. doi: 10.1016/0008-8749(92)90236-i. [DOI] [PubMed] [Google Scholar]
- [38].Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmun Rev. 2012;11:A465–70. doi: 10.1016/j.autrev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- [39].Leanos A, Pascoe D, Fragra A, Blanco-Favela F. Anti-prolactin autoantibodies in systemic lupus erythematosus patients with associated hyperprolactinemia. Lupus. 1998;7:398–403. doi: 10.1191/096120398678920280. [DOI] [PubMed] [Google Scholar]
- [40].Walker SE. Bromocriptine treatment of systemic lupus erythematosus. Lupus. 2001;10:762–8. doi: 10.1191/096120301717165010. [DOI] [PubMed] [Google Scholar]
- [41].McMurray RW, Weidensaul D, Allen SH, Walker SE. Efficacy of bromocriptine in an open label therapeutic trial for systemic lupus erythematosus. J Rheumatol. 1995;22:2084–91. [PubMed] [Google Scholar]
- [42].Alvarez-Nemegyei J, Cobarrubias-Cobos A, Escalante-Triay F, Sosa-Munoz J, Miranda JM, Jara LJ. Bromocriptine in systemic lupus erythematosus: a double-blind, randomized, placebo-conrolled study. Lupus. 1998;7:414–9. doi: 10.1191/096120398678920334. [DOI] [PubMed] [Google Scholar]
- [43].McMurray R, Keisler D, Kanuckel K, Izui S, Walker SE. Prolactin influences autoimmune disease activity in the female B/W mouse. J Immunol. 1991;147:3780–7. [PubMed] [Google Scholar]
- [44].Lee TP, Chiang BL. Sex differences in spontaneous versus induced animal models of autoimmunity. Autoimmun Rev. 2012;11:A422–9. doi: 10.1016/j.autrev.2011.11.020. [DOI] [PubMed] [Google Scholar]
- [45].Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167:1886–90. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- [46].Peeva E, Michael D, Cleary J, Rice J, Chen X, Diamond B. Prolactin modulates the naïve B cell repertoire. J Clin Invest. 2003;111:275–83. doi: 10.1172/JCI16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Venkatesh J, Peeva E, Xu X, Diamond B. Cutting edge: hormonal milieu, not antigenic specificity, determines the mature phenotype of autoreactive B cells. J Immunol. 2006;176:3311–4. doi: 10.4049/jimmunol.176.6.3311. [DOI] [PubMed] [Google Scholar]
- [48].Sandel PC, Monroe JG. Negative selection of immature B cells by receptor editing or deletion is determined by site of antigen encounter. Immunity. 1999;10:289–99. doi: 10.1016/s1074-7613(00)80029-1. [DOI] [PubMed] [Google Scholar]
- [49].Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- [50].Venkatesh J, Yoshifuji H, Kawabata D, Chinnasamy P, Stanevsky A, Grimaldi CM, et al. Antigen is required for positive selection of pathogenic anti-DNA antibodies and systemic inflammation. J Immunol. 2011;186:5304–12. doi: 10.4049/jimmunol.1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ray SK, Putterman C, Diamond B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc Natl Acad Sci U S A. 1996;93:2019–24. doi: 10.1073/pnas.93.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J Exp Med. 2001;194:1151–64. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011;40:66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- [54].Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–33. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].2010. A study to evaluate the efficacy and safety of rituximab in subjects with ISN/ RPS class III or IV lupus nephritis (LUNAR)
- [56].Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109:1625–33. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Panchanathan R, Choubey D. Murine BAFF expression is up-regulated by estrogen and interferons: implications for sex bias in the development of autoimmunity. Mol Immunol. 2013;53:15–23. doi: 10.1016/j.molimm.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, et al. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–66. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Moulton VR, Holcomb DR, Zajdel MC, Tsokos GC. Estrogen upregulates cyclic AMP response element modulator a expression and downregulates interleukin-2 production by human T lymphocytes. Mol Med. 2012;18:370–8. doi: 10.2119/molmed.2011.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Panchanathan R, Duan X, Arumugam M, Shen H, Liu H, Choubey D. Cell type and gender-dependent differential regulation of the p202 and Aim2 proteins: implications for the regulation of innate immune responses in SLE. Mol Immunol. 2011;49:273–80. doi: 10.1016/j.molimm.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cutolo M, Sulli A, Straub RH. Estrogen metabolism and autoimmunity. Autoimmun Rev. 2012;11:A460–4. doi: 10.1016/j.autrev.2011.11.014. [DOI] [PubMed] [Google Scholar]
- [62].Ledesma-Soto Y, Blanco-Favela F, Fuentes-Pananá EM, Tesoro-Cruz E, Hernández-González R, Arriaga-Pizano L, et al. Increased levels of prolactin receptor expression correlate with the early onset of lupus symptoms and increased numbers of transitional-1 B cells after prolactin treatment. BMC Immunol. 2012;13:11–24. doi: 10.1186/1471-2172-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97:2063–73. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang XB, Huang WQ, Mihara M, Sinha J, Davidson A. Mechanism of action of combined short-term CTLA4Ig and anti-CD40 ligand in murine systemic lupus erythematosus. J Immunol. 2002;168:2046–53. doi: 10.4049/jimmunol.168.4.2046. [DOI] [PubMed] [Google Scholar]
- [65].Wang XB, Huang WQ, Schiffer LE, Mihara M, Akkerman A, Hiromatsu K, et al. Effects of anti-CD154 treatment on B cells in murine systemic lupus erythematosus. 2003;48:495–506. doi: 10.1002/art.10929. [DOI] [PubMed] [Google Scholar]
- [66].Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48:719–27. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- [67].Mathian A, Gallegos M, Pascual V, Banchereau J, Koutouzov S. Interferon-a induces unabated production of short-lived plasma cells in pre-autoimmune lupus-prone (NZBxNZW)F1 mice but not in BALB/c mice. Eur J Immunol. 2011;41:863–72. doi: 10.1002/eji.201040649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–26. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- [69].Yu S, Dunn R, Kehry MR, Braley-Mullen H. B cell depletion inhibits spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2008;180:7706–13. doi: 10.4049/jimmunol.180.11.7706. [DOI] [PubMed] [Google Scholar]
- [70].Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, et al. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol. 2004;14:3524–34. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- [71].Ramanujam M, Wang X, Huang W, Liu Z, Schiffer L, Tao H, et al. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;14:724–34. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mihara M, Tan I, Chuzin Y, Reddy B, Budhai L, Holzer A, et al. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. J Clin Invest. 2000;106:91–101. doi: 10.1172/JCI9244. [DOI] [PMC free article] [PubMed] [Google Scholar]