Abstract
Preoperative sentinel node localization (SNL) using a subareolar injection of radiotracer technetium-99m-sulfur colloid (Tc99mSC) is associated with significant pain. Lidocaine use during SNL is not widely adopted partly due to a concern that it can obscure sentinel node identification and reduce its diagnostic accuracy. We prospectively identified women with a biopsy-proven infiltrating breast cancer who were awaiting a SNL. The women completed the McGill pain questionnaire, Visual Analog Scale, and Wong–Baker FACES Pain Rating Scale prior to and following SNL. We identified a retrospective cohort of women with similar demographic and tumor characteristics who did not receive lidocaine before SNL. We compared sentinel lymph node identification rates in the two cohorts. We used Wilcoxon rank sum tests to compare continuous measures and Fisher's exact test for categorical measures. Between January 2011 to July 2012, 110 women consented, and 105 were eligible for and received lidocaine prior to Tc99mSC injection. The post-lidocaine identification rate of SNL was 95 % with Tc99mSC, and 100 % with the addition of intraoperative methylene blue dye/saline. Pain range prior to and following the SNL was unchanged (P = 0.703). We identified 187 women from 2005 to 2009 who did not receive lidocaine during preoperative SNL. There was no significant difference in the success rate of SNL, with or without lidocaine (P = 0.194). The administration of lidocaine during SNL prevents pain related to isotope injection while maintaining the success rate. We have changed our practice at our center to incorporate the use of lidocaine during all SNL.
Keywords: Breast cancer, Sentinel node localization, Lidocaine
Introduction
Sentinel node localization (SNL) and excision is a standard procedure to stage the axilla in most patients undergoing surgical treatment for early-stage breast cancer. The concept of SNL is to selectively identify lymph nodes that would most likely harbor malignant cells, and if the sentinel node(s) is negative then the entire axillary lymph node basin is considered to be free of metastasis [1–4]. Compared to the prior method, level I and II axillary lymph node dissection, sentinel lymph node biopsy is associated with a lower morbidity rate, including the risk of lymphedema [5, 6]. SNL is conducted to selectively identify nodes that would most likely harbor malignant cells to help stage the disease.
In order for surgeons to identify the sentinel lymph nodes, an injection of technetium-99m-sulfur colloid (Tc99mSC) is injected in the subareolar area of the breast preoperatively ranging between 1.5 to 18 h prior to surgery. For patients who receive the injection the day prior to surgery, the dose of the radiotracer is increased to account for the 6 h half-life of the Tc99mSC. In the operating room, the sentinel lymph nodes are identified using a hand-held gamma probe which detects lymph nodes that uptake the radiotracer [7–9]. SNL is the preferred standard approach for axillary staging in women with early breast cancer; however, the subareolar Tc99mSC injection is associated with significant transient pain [9–11].
In 2014, an estimated 232,670 women in the United States will be diagnosed with breast cancer [12]. The majority of women will have a clinically negative axilla and are candidates for SNL and a sentinel lymph node excision. However, the subareolar Tc99mSC injection used for SNL is associated with both severe pain and anxiety. Therefore, there is an urgent need to improve current practice by reducing pain and anxiety caused by the subareolar Tc99mSC injection while ensuring that the success of SNL is maintained.
The primary objective of this study was to evaluate the success of sentinel lymph node identification rate with subareolar lidocaine injection prior to Tc99mSC injection, and to compare the results with historical data of patients who did not receive lidocaine prior to Tc99mSC injection at the Johns Hopkins Hospital. Secondary objectives were to evaluate the pain perception and pain experience during this procedure using the McGill Pain Questionnaire, the Visual Analog Scale (VAS), and the Wong–Baker FACES Pain Rating Scale.
Patients and methods
The study was approved by the Johns Hopkins Institutional Review Board. All subjects enrolled in the prospective cohort signed a written informed consent. The Board waived the need to obtain a written informed consent from individuals included in the retrospective cohort.
Prospective cohort
Eligible women were 18 years of age or older with a biopsy-proven breast cancer who were scheduled for a preoperative SNL at the Johns Hopkins Hospital in Baltimore, Maryland. A member of the research team searched for eligible patients by screening the biopsy calendar and the patients' records each week using a HIPAA waiver. We enrolled only participants who were capable of giving informed consent and who were not pregnant. Pre-defined exclusion criteria were based on possible disruption of the lymphatic pathways, which was not attributed to injection of lidocaine, including history of upper outer quadrant breast surgery, tumor size >4 cm in the upper outer quadrant on imaging, and history of neo-adjuvant chemotherapy or endocrine therapy. Patients were also excluded if they had a history consistent with a lidocaine allergy. Three radiologists administered the subareolar lidocaine and the Tc99mSC injections. Two surgeons who participated in the study used the described method of intraoperative SNL for the sentinel lymph node excision.
Retrospective control
To estimate the historical rate of sentinel lymph node identification at our institution, we retrospectively identified a separate cohort of consecutively treated historical controls using the same inclusion and exclusion criteria. We performed a retrospective chart review and included patients who received the Tc99mSC injection without the use of lidocaine from March 2005 to December 2009. This retrospective group was treated by the same two surgeons as the prospective group.
Procedures
After providing written informed consent, the patients enrolled in the prospective cohort completed baseline questionnaires including a Short-form McGill Pain Scale, VAS, and Wong–Baker FACES Pain Rating Scale. The study radiologist provided a brief explanation of the procedure. The radiologist administered 2 cc of 1 % lidocaine hydrochloride (1 % in a 1 cc volume) into the subareolar region of the breast with real-time ultrasound guidance. Ultrasound visualization enabled the administration of lidocaine and Tc99mSC to the same region in a uniform manner. All participants then received a subareolar injection of Tc99mSC per routine clinical standards (0.8–3.2 mCi in a 1 cc syringe with a 1 cc total volume) for sentinel lymph node identification. The Tc99mSC was injected as close as possible to the area that was previously anesthetized with lidocaine.
The times of lidocaine and Tc99mSC injections were recorded by a member of the research team and an ultrasound procedure assistant. Any ultrasound-guided wire localizations that were required for surgery were also inserted at that time. Participants were asked to report the pain experienced during injections by completing the same set of questionnaires immediately after the completion of all procedures.
Prior to initiating the sentinel node procedure in the operating room, the surgeon scanned the axilla with a gamma probe. If there was sufficient radioactivity to identify the sentinel lymph node, the procedure was initiated. If there was insufficient radioactivity to identify the sentinel lymph node, the surgeon injected blue dye or saline (total volume 5–10 cc) in the retroareolar area and then massaged the breast for 5–10 min. The gamma probe was again used to scan the axilla. In all cases, these maneuvers resulted in adequate radioactivity to identify the sentinel lymph node. Failure to locate the sentinel nodes was defined in the study as any patient who required an axillary dissection when Tc99mSC injection, alone or complemented with blue dye or saline, did not localize the sentinel lymph nodes.
Assessments
Study questionnaires included the McGill Pain Questionnaire, VAS, and Wong–Baker FACES Pain Rating Scale, and were administered before the radiologist came into the room and initiated any procedures and again following the procedure.
McGill Pain Questionnaire
The Short-form McGill Pain Questionnaire (SF-MPQ-2) consists of sensory, affective, and evaluative descriptors characterizing the patient's subjective appraisal of pain, and an intensity scale providing quantitative measures of pain. It is a 10-point Likert scale where 0 = no pain and 10 = worst pain. It is used to measure the major symptoms of both neuropathic and non-neuropathic pain that can be used in studies of treatment response [13].
Visual Analog Scale
The VAS is a 100-mm scale, with 0 mm representing “no pain” and 100 mm representing “most severe pain.” At baseline, patients were also asked if they had any concerns about the procedure and if they were experiencing pain. Post-procedure they were asked how long the pain lasted and if they were still having pain.
Wong–Baker FACES Pain Rating Scale
The Wong–Baker FACES Pain Rating Scale is a standardized 10-point Likert scale where 0 = no pain and 10 = worst pain [14].
Statistical considerations
We estimated that the sentinel lymph node identification rate of patients treated previously at Johns Hopkins Hospital was equal or higher than the national standard, or approximately 90–95 %. When designing this study, the acceptable sentinel lymph node identification rate nationally was 90 %. With an expected sentinel lymph node identification rate of 90–95 %, a sample size of 150–220 patients allowed us to estimate the true identification rate within ±5 %. Early termination rules were created for both futility and superiority. At pre-determined study intervals, we estimated the identification rate with an exact 99 % confidence interval. If at any point the lower bound of the interval excluded 90 %, the study would stop for superiority. Similarly, if the upper bound excluded 90 % at any point, the study would stop for futility. Baseline characteristics and outcomes were compared between the prospective cohort and the retrospective cohort with Fisher's exact test for categorical variables and Wilcoxon rank sum tests for continuous ones. Differences in pain scores from pre-injection to post-injection were calculated for each patient and tested with paired t tests, overall and by patient subgroups. Analyses were completed in R version 2.15.1.
Results
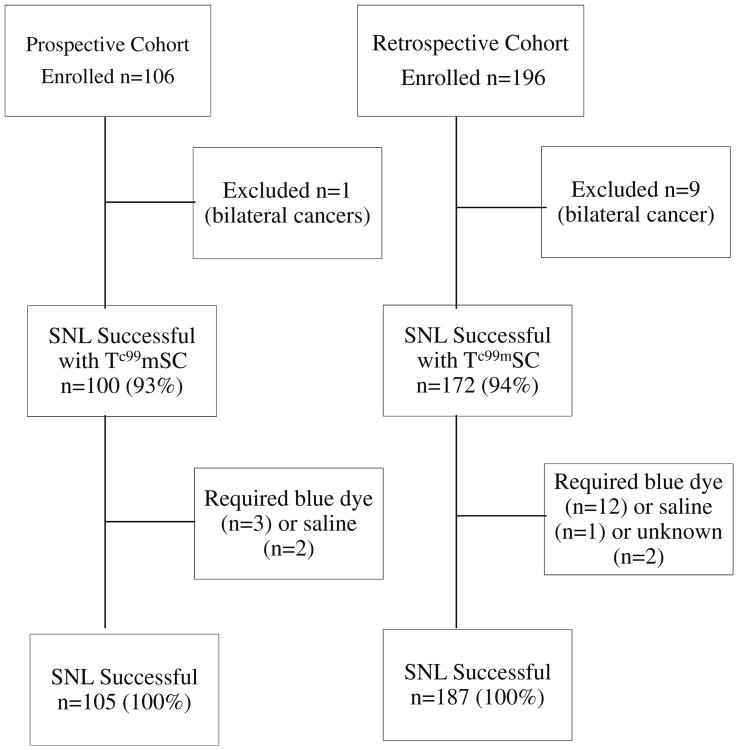
From January 2011 to July 2012, 106 women enrolled in the prospective study; one patient had bilateral breast cancer and was excluded from the analyses for a total of 105 eligible participants (Fig. 1). For the retrospective cohort, we identified 196 women using the same inclusion and exclusion criteria (Fig. 1). Of these, nine had bilateral breast cancer and were excluded from the analysis. Baseline patient demographic and tumor characteristics are similar between the prospective cohort and retrospective control groups, as reported in Table 1.
Fig. 1.
Consort diagram. SNL sentinel node localization
Table 1. Patient and tumor characteristics.
| Characteristics | Prospective cohort N = 105 | Retrospective cohort N = 187 | P value |
|---|---|---|---|
| Age—median (range) | 55 (33, 80) | 55 (28, 89) | 0.699 |
| Race—no. (%) | |||
| White | 75 (71) | 130 (72) | 0.925 |
| Black | 20 (19) | 36 (19) | |
| Other | 10 (10) | 21 (11) | |
| Tumor type—no. (%) | |||
| DCIS | 11 (10) | 29 (16) | 0.352 |
| IDC | 74 (70) | 133 (71) | |
| ILC | 7 (7) | 13 (7) | |
| IMC | 10 (10) | 8 (4) | |
| Other | 3 (3) | 4 (2) | |
| Tumor size—median (range) | 1.4 (0.4, 8) | 1.4 (0, 7.1) | 0.819 |
| Tumor grade—no. (%) | |||
| 1 | 14 (13) | 32 (18) | 0.162 |
| 2 | 65 (62) | 89 (50) | |
| 3 | 26 (25) | 57 (32) | |
| Unknown | 0 | 9 | |
| ER positive—no. (%) | 92 (88) | 158 (86) | 0.724 |
| PR positive—no. (%) | 82 (78) | 134 (73) | 0.398 |
| HER2 positive—no. (%) | 9 (10) | 20 (12) | 0.682 |
Characteristics are shown separately for the prospective cohort and for the historical control group. P values for differences between cohorts are from Wilcoxon rank sum tests comparing continuous measures and Fisher's exact test for categorical measures
DCIS ductal carcinoma in situ, ER estrogen receptor, HER2 human epidermal growth factor receptor 2, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, IMC invasive mammary carcinoma, PR progesterone receptor
Of the 105 SNL procedures prospectively performed on this study, the identification rate using lidocaine and Tc99mSC injections alone was 95 % (100 out of 105) (Fig. 1). Of the remaining five patients, three required an injection of blue dye in the operating room in order to locate the sentinel lymph nodes, and two additional patients required saline injection to successfully identify the sentinel lymph nodes. Thus, 100 % of the sentinel lymph nodes were successfully identified using Tc99mSC with or without blue dye or saline (Table 2).
Table 2. Diagnostic accuracy of sentinel lymph node identification.
| Prospective cohort N = 105 | Historical controls N = 187 | P value | |
|---|---|---|---|
| Sentinel LN identified—no. (%) | 105 (100) | 187 (100) | |
| Method used—no. (%) | |||
| Tc99mSC only | 100 (95) | 172 (93) | 0.226 |
| Blue dye | 3 (3) | 12 (6) | |
| Saline, other | 2 (2) | 1 (1) | |
| Unknown | 0 | 2 | |
| Had LN metastases—no. (%) | 11 (10) | 36 (19) | 0.067 |
| No. of SLN—median (range) | 2 (1, 12) | 2 (1, 17) | <0.001 |
| No. of positive LN—median (range) | 1 (1, 6) | 1 (1, 10) | 0.674 |
| Had more than 1 positive LN—no. (%) | 4 (36) | 10 (28) | 0.71 |
Accuracy is shown separately for the prospective cohort and for the historical control group. P values for differences between cohorts are from Wilcoxon rank sum tests comparing continuous measures and Fisher's exact test for categorical measures
LN lymph node, Tc99mSC technetium-99m-sulfur colloid
In the retrospective cohort, 187 women were eligible for analysis, and 94 % of sentinel nodes were identified using the Tc99mSC injection. The remaining 6 % required administration of blue dye or saline injection for an overall sentinel node identification rate of 100 % (Table 2). None of the participants in the prospective or retrospective cohorts required an axillary node dissection due to a failure to identify a sentinel node.
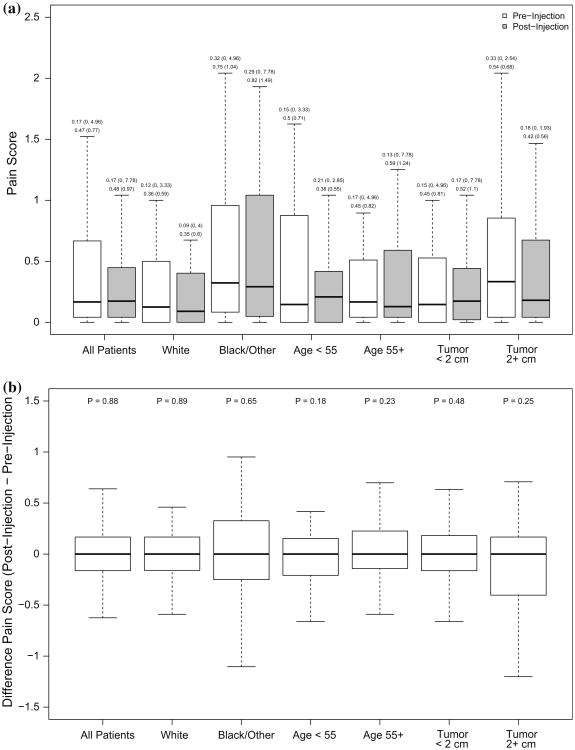
The distribution of pain scores at the pre-injection and post-injection time points and their difference for all patients and by race, age, and tumor size are shown in Fig. 2. The distributions at each time point are similar (all tests for differences within patients yielded P > 0.05), suggesting that there is no significant change in pain scores prior to and following the injection in the prospective group. Although our study was not powered to detect small differences, these results suggest that the additional injection of lidocaine was not associated with significant pain following the subareolar injection of Tc99mSC.
Fig. 2.
Distribution of pain scores. Boxplots showing, a the distribution of pain scores at the pre-injection and post-injection time point for all patients and separately by categories of age, race, and tumor size. Summary statistics [median (min, max) and mean (SD)] are shown above each box; b the distribution of the difference in pain scores (post-injection–pre-injection) for all patients and separately by categories of age, race, and tumor size. P values above each subgroup are for paired t tests for differences in patients' pain scores from pre-injection to post-injection. Outliers are not shown
Discussion
While surgical techniques and styles vary from one medical institution to another, the pain associated with the subareolar Tc99mSC injection for SNL is consistent and significant, and physicians underestimate the pain in the majority of cases [10]. Our study reveals that accurate sentinel lymph node identification is maintained when using a subareolar lidocaine injection concurrently with a subareolar Tc99mSC injection. The successful node identification rate in this study was 100 %. These results help dismiss concerns that lidocaine injections would interfere with the uptake of the Tc99mSC through the lymphatics by the lymph nodes.
A number of studies have evaluated methods to decrease pain during Tc99mSC injection. The use of lidocaine or prilocaine creams (specifically EMLA cream) has been investigated to determine the effectiveness in reducing pain prior to Tc99mSC injections. Eligible participants were randomized to receive a combination of 25 mg of lidocaine and 25 mg of prilocaine in a 5-mL syringe that contained a base cream or base cream only (control). Participants then applied the cream to the periareolar area 1 h before the Tc99mSC injection. Once the cream was applied, a plastic cling wrap barrier was used to cover the area and this remained in place until the injection [15]. Results indicate that EMLA cream was not effective in significantly reducing procedural pain during the Tc99mSC injection. While the investigators demonstrated that there appeared to be a trend toward improved pain scores with the application of EMLA cream, the findings were not significant.
Another approach to alleviate the pain during the Tc99mSC injection includes the use of a pH-adjusted and lidocaine supplemented dose of the injection. Investigators used this method in a randomized, double-blind trial, where pain was compared between patients that received the standard Tc99mSC injection or a pH-adjusted and lidocaine supplemented formula [11]. A total of 140 participants were randomly assigned equally to four groups, to receive 4 % topical lidocaine cream and the Tc99mSC injection, or to one of the three other groups: to receive topical placebo cream and Tc99mSC with either sodium bicarbonate, 1 % lidocaine, or sodium bicarbonate and 1 % lidocaine. After the injection was administered, participants completed pain rating questionnaires. A significant reduction in pain scores was observed in the groups where Tc99mSC included 1 % lidocaine. The addition of 1 % lidocaine added to the radioisotope solution can help to eliminate some of the pain experienced during this injection [11].
In an update that was published after our study was completed, the authors report that the overall sentinel lymph node identification rate was 93 % [16]. While the addition of lidocaine did not statistically significantly compromise the sentinel lymph node detection rate (control 96 %, sodium bicarbonate 97 %, lidocaine 90 %, sodium bicarbonate, and lidocaine 90 %), the sample sizes in the lidocaine groups are smaller than our study by nearly 70 %, and a decreased identification rate of 90 % compared to 96 or 97 % is arguably clinically significant if not statistically significant. While our study is not randomized, we include a large prospective cohort of carefully selected patients and clinicians. Participants completed questionnaires prior to and following the procedure. We compared the identification rate to a retrospective cohort of women who met the same eligibility criteria and who were treated by the same procedures and clinicians with exception of lidocaine use. Our study is also limited by the lack of documented pain ratings from patients in the retrospective cohort who received the injection without lidocaine.
The findings of this study demonstrate that SNL is equally as effective with or without an injection of lidocaine prior to a subareolar injection of Tc99mSC. While both methods have the same end result, the administration of lidocaine prevented a severe pain that is frequently reported by patients undergoing SNL. Because of the results of this study, we have changed our practice behavior to incorporate the use of lidocaine during all preoperative sentinel lymph node injections, unless there is a history suggesting lidocaine allergy. Breast cancer is a stressful diagnosis and many women worry about the pain associated with the Tc99mSC injection, and we hope that our results can be used to partly alleviate the stress that women associate with this procedure and also change the practice standard at the national level.
Acknowledgments
Supported in part by P30 CA06973 and a Center of Excellence Award from the Avon Foundation for Women. Vered Stearns holds the Breast Cancer Research Chair in Oncology. We thank Dr. Gary Rosner for a critical review of the manuscript, Ms. Kelly Zafman for help screening participants and administering patient-reported outcome questionnaires, and Kimberly Aguirre for help with study procedures and administering patient-reported outcome questionnaires. We thank Ms. Wagner-Smith and Ms. Alexandra Hartman for administrative support.
Abbreviations
- SF-MPQ-2
Short-form McGill Pain Questionnaire
- SNL
Sentinel node localization
- Tc99mSC
Technetium-99m-sulfur colloid
- VAS
Visual Analog Scale
Footnotes
Conflict of interest None.
Ethical standards We complied with ethical standards applicable for this study. The study was approved by the Johns Hopkins Institutional Review Board. All subjects enrolled in the prospective cohort signed a written informed consent. The Board waived the need to obtain a written informed consent from individuals included in the retrospective cohort.
References
- 1.Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, Costa A, de Cicco C, Geraghty JG, Luini A, Sacchini V, Veronesi P. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349(9069):1864–1867. doi: 10.1016/S0140-6736(97)01004-0. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15(6):2345–2350. doi: 10.1200/JCO.1997.15.6.2345. [DOI] [PubMed] [Google Scholar]
- 3.Cox CE, Pendas S, Cox JM, Joseph E, Shons AR, Yeatman T, Ku NN, Lyman GH, Berman C, Haddad F, Reintgen DS. Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg. 1998;227(5):645–651. doi: 10.1097/00000658-199805000-00005. Discussion 651–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, Feldman S, Kusminsky R, Gadd M, Kuhn J, Harlow S, Beitsch P. The sentinel node in breast cancer—a multicenter validation study. N Engl J Med. 1998;339(14):941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 5.Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, Skelly JM, Harlow SP, Weaver DL, Mamounas EP, Costantino JP, Wolmark N. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102(2):111–118. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbelen H, Gebruers N, Eeckhout FM, Verlinden K, Tjalma W. Shoulder and arm morbidity in sentinel node-negative breast cancer patients: a systematic review. Breast Cancer Res Treat. 2014;144(1):21–31. doi: 10.1007/s10549-014-2846-5. [DOI] [PubMed] [Google Scholar]
- 7.Fortunato L, Mascaro A, Amini M, Farina M, Vitelli CE. Sentinel lymph node biopsy in breast cancer. Surg Oncol Clin N Am. 2008;17(3):673–699. doi: 10.1016/j.soc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Newman EA, Newman LA. Lymphatic mapping techniques and sentinel lymph node biopsy in breast cancer. Surg Clin North Am. 2007;87(2):353–364. doi: 10.1016/j.suc.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Robertson C, Maisonneuve P, Renne G, De Cicco C, De Lucia F, Gennari R. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 10.Radowsky JS, Baines L, Howard RS, Shriver CD, Buckenmaier CC, 3rd, Stojadinovic A. Pain ratings by patients and their providers of radionucleotide injection for breast cancer lymphatic mapping. Pain Med. 2012;13(5):670–676. doi: 10.1111/j.1526-4637.2012.01374.x. [DOI] [PubMed] [Google Scholar]
- 11.Stojadinovic A, Peoples GE, Jurgens JS, Howard RS, Schuyler B, Kwon KH, Henry LR, Shriver CD, Buckenmaier CC. Standard versus pH-adjusted and lidocaine supplemented radiocolloid for patients undergoing sentinel-lymph-node mapping and biopsy for early breast cancer (PASSION-P trial): a double-blind, randomised controlled trial. Lancet Oncol. 2009;10(9):849–854. doi: 10.1016/S1470-2045(09)70194-9. [DOI] [PubMed] [Google Scholar]
- 12.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 13.Dworkin RH, Turk DC, Revicki DA, Harding G, Coyne KS, Peirce-Sandner S, Bhagwat D, Everton D, Burke LB, Cowan P, Farrar JT, Hertz S, Max MB, Rappaport BA, Melzack R. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2) Pain. 2009;144(1–2):35–42. doi: 10.1016/j.pain.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14(1):9–17. [PubMed] [Google Scholar]
- 15.Fetzer S, Holmes S. Relieving the pain of sentinel lymph node biopsy tracer injection. Clin J Oncol Nurs. 2008;12(4):668–670. doi: 10.1188/08.CJON.668-670. [DOI] [PubMed] [Google Scholar]
- 16.Arciero CA, Henry LR, Howard RS, Peoples GE, Bilchik AJ, Avital I, Buckenmaier CC, III, Stojadinovic A. Technical effects of adding 1% lidocaine to technetium sulfur colloid for sentinel lymphatic mapping in early breast cancer: analysis of data from a double-blind randomized controlled trial. Ann Surg Oncol. 2013;20(8):2548–2555. doi: 10.1245/s10434-013-2912-y. [DOI] [PubMed] [Google Scholar]