Abstract
Introduction
The presence of the blood-brain barrier (BBB) is a significant impediment to the delivery of therapeutic agents to the brain for treatment of brain diseases. Focused ultrasound (FUS) has been developed as a non-invasive method for transiently increasing the permeability of the BBB to promote drug delivery to targeted regions of the brain.
Areas Covered
The present review briefly compares the methods used to promote drug delivery to the brain and describes the benefits and limitations of FUS technology. We summarize the experimental data which shows that FUS, combined with intravascular microbubbles, increases therapeutic agent delivery into the brain leading to significant reductions in pathology in preclinical models of disease. The potential for translation of this technology to the clinic is also discussed.
Expert Opinion
The introduction of MRI guidance and intravascular administration of microbubbles to FUS treatments permits the consistent, transient, and targeted opening of the BBB. The development of feedback systems and real-time monitoring techniques improve the safety of BBB opening. Successful clinical translation of FUS has the potential to revolutionize the treatment of brain disease resulting in effective, less-invasive treatments without the need for expensive drug development.
Keywords: Focused ultrasound, blood-brain barrier, drug delivery, neurodegenerative disease, brain tumor
3. General overview of the blood-brain barrier (BBB)
The blood-brain barrier (BBB) is a selective, highly-regulated barrier created by the endothelial cells in the cerebral vasculature [1]. It was first discovered after observing that systemic injection of dye results in the staining of all organs except for the brain [2]. Since, the BBB has been characterized as a complex structure which strictly regulates molecular transport into the brain.
3.1 Cellular Structure and function
The structure of the BBB is characterized primarily by the presence of tight junctions between adjacent endothelial cells which allows the BBB to function as a physical barrier to the passage of molecules into the brain [3]. Tight junctions are created when membrane bound cell adhesion molecules such as claudins and occludins, form homodimers between adjacent cells. These protein networks prevent the paracellular passage of molecules and force molecules to take a transcellular route into the brain. However, the cerebral endothelial cells contain fewer transport vesicles than peripheral endothelial cells, thereby limiting transcellular molecular transport. Beyond the endothelial cells are the cells of the neurovascular unit which regulate and support the barrier. The neurovascular unit contains the basal lamina and a complex cellular system of astrocytes, pericytes, microglia and neurons that function together to support the BBB [3].
3.2 Barrier for Drug Delivery
The BBB has many roles which make it essential for proper brain function. Most importantly, the BBB functions to maintain a delicate homeostatic environment by regulating ion and neurotransmitter concentrations, and preventing the access of toxins, immune cells and pathogens from the peripheral circulatory system [1]. Moreover, the BBB regulates the transport of nutrients into the brain and assists with removing waste products. While these functions are necessary for maintaining health of the brain, when brain diseases develop, the BBB also prevents the access of therapeutic agents thereby making brain diseases notoriously difficult to treat [4]. It has been estimated that the BBB prevents the access of over 98% of potential therapeutics from passing into the brain [4]. As well, lipophillic and other small molecule drugs which are able to pass through the endothelial membrane are intercepted and shuttled out by the presence of efflux transporters. The BBB is the single most important factor limiting the treatment of brain diseases today. Recent studies have suggested that the financial burden of brain disease on society is double that of cancer [5].
4. Current Approaches to Drug Delivery in the Brain
Several methods have been developed to overcome the BBB and allow drugs into the brain. Surgical approaches are the most effective for direct drug delivery into the region of interest. Specific brain regions are targeted using stereotactic coordinates and needles or pumps are used to deliver drugs by direct injection. Although positive results have been seen for in clinical trials for -Parkinson’s and Alzheimer’s Diseases [6,7] the potential for damage to healthy tissue due to the needle track is great and add to the general risks associated with anaesthesia and surgery. Less invasive than surgical injection directly into the brain is injection into the cerebrospinal fluid. Intrathecal injection bypasses the BBB but drug concentrations at the target are unpredictable.
Other methods to induce temporary disruption of the BBB for drug delivery include use of chemical agents such as sugar alcohols, solvents, and vasodilators [5,8]. Mannitol, a hyperosmotic solution which shrinks the endothelial cells and stretches the tight junctions, has been often utilized to effectively promote drug delivery to the brain. However, mannitol, and other chemical permeabilization agents, induce widespread BBB disruption exposing healthy brain tissue to the therapeutic molecules and other compounds from the circulatory system [9]. As well, mannitol has the potential for renal complications, especially after repeated exposure.
Novel methods have been developed to improve drug delivery to the brain by taking advantage of the natural receptors and anatomical features of the BBB. Drug modification to increase intracellular uptake by brain endothelial cells has shown to result in bypass of the BBB however often the agents do not reach therapeutic concentrations in the brain [10]. Alternatively, intranasal administration of drugs has shown to be effective for increasing therapeutic concentrations in the brain. Recently, using fluorescent tracers, it has been shown that intranasally delivered therapeutic agents may travel through the perivascular space to reach distant brain regions [11]. However, this method requires that the drug penetrate large brain regions which may be challenging in the human brain [12].
This review will discuss the development of focused ultrasound, a novel, non-invasive, targeted method for improving drug delivery to the brain.
5. Focused Ultrasound-Mediated BBB opening
The first reports that ultrasound could be used to permeate the BBB were published in the 1950’s. The early studies showed that ultrasound could increase BBB permeability however it was accompanied by haemorrhage or thermal coagulation [13,14]. The mechanism was suggested to be both thermal and cavitation-mediated. Thermal-mechanisms were hypothesized since BBB permeability is observed in the regions surrounding a thermal lesion. Although successful using in vitro BBB models [15], investigation into the threshold for thermally-induced BBB opening in vivo indicated that thermal opening of the BBB is always associated with tissue damage [16]. Thus, while it is possible to use hyperthermia to induce BBB disruption, these approaches are currently unsafe.
High intensity focused ultrasound (HIFU) has been used to induce cavitation, the generation and collapse of bubbles within the tissue, and induce BBB opening without significant macroscopic elevation in brain temperature. In general, haemorrhage and tissue damage occurred more often as the pulse duration, pulse number and repetition frequency increased [17]. Although BBB opening was possible, the related bioeffects were unpredictable and varied extensively between studies [17,18].
The addition of preformed microbubble ultrasound contrast agent was found to reduce the acoustic pressure amplitude required for effective BBB opening, transforming the use of FUS in the brain [19]. Combining FUS and microbubbles produces consistent, reproducible and transient BBB opening without damage to the brain tissue [19]. Mechanistically, the microbubbles concentrate the ultrasound energy thereby reducing the required ultrasound power by more than 100 fold [20]. The microbubbles are important for reducing the amount of energy required to pass through the skull. The lower the energy requirements through the skull, the lower the potential for skull heating, thereby making transcranial treatments feasible and safer. When the circulating microbubbles pass through the ultrasound field, the microbubbles expand and contract, interacting with the blood vessel wall and leading to increased permeability of the BBB. Using low pressure, increased BBB permeability can be achieved and side effects are restricted to a few extravasated red blood cells [19].
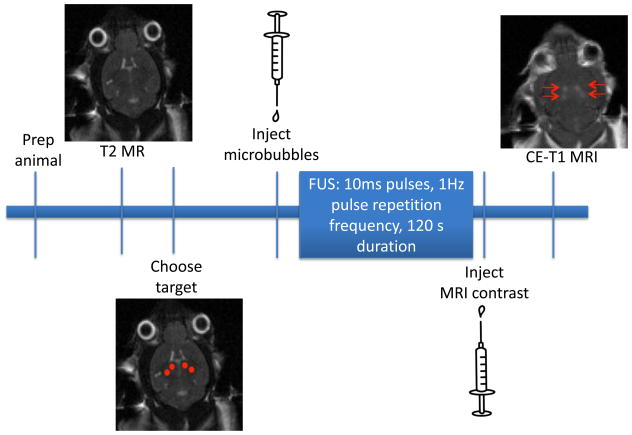
The use of magnetic resonance imaging (MRI) has been effective as a guide for targeting and as an evaluation of BBB opening. The excellent tissue contrast and ability for contrast-enhanced imaging to assess changes in BBB permeability have made MRI the primary imaging modality for FUS treatments (Figure 1).
Figure 1.
Timeline for FUS experiments. Animals are prepared for FUS treatment by using chemical depilatory to remove the hair from the head and by inserting a catheter into the tail vein. A T2-MR image is acquired and the target locations for sonication are chosen (denoted by red dots). Microbubble contrast agent is diluted and injected immediately prior to the onset of sonication. FUS is applied using standard parameters (10ms pulses, 1 Hz pulse repetition frequency, 120s total duration). Following FUS, MRI contrast agent is injected and a contrast enhanced (CE)-T1 weighted MR images is acquired. The regions of hyperintensity correspond to areas where contrast agent move from the vasculature into the brain parenchyma and is used to confirm effective BBB opening with FUS.
In the past decade, reports from many different groups have demonstrated that different ultrasound parameters can be used to open the BBB. BBB opening has been achieved using frequencies ranging from 28kHz [21] to 8MHz [22]. The range that is relevant for clinical use is between 0.2MHz and 1.5MHz. due to the large focal spot size at low frequency and high pressure requirements at high frequency [23]. In addition to frequency, other ultrasound parameters including burst duration have been shown to positively correlate with BBB opening [24–26]. With respect to pulse repetition frequency, it has been suggested that adequate time is required to allow time for reperfusion of the microbubbles [27] however, changes in burst repetition frequency did not affect changes in BBB permeability [24]. Microbubble concentration and size have been shown to be positively correlated with greater BBB opening and potential for damage [28–32].
The development of a real-time, acoustic controller has reduced the variations of BBB opening and moved on step towards optimal BBB opening using FUS [33]. The feedback controller will be discussed further in the Safety and Treatment Monitoring.
5.1 Benefits and Limitations
The benefits of using FUS and microbubbles as a method for transient BBB opening over other methods for drug delivery to the brain are numerous. First, FUS can pass through the skull and therefore does not require invasive surgical methods. Using non-invasive procedures to replace surgery reduces the risks of general anaesthetic and surgery. The administration of microbubbles at the onset of FUS is performed intravenously thereby eliminating the need for invasive intracarotid injection associated with mannitol. Second, imaging methods can be used to target the ultrasound to the brain region of interest so that drug delivery and exposure to the peripheral blood products is limited to brain regions affected by disease. This is advantageous over the widespread BBB permeabilization achieved with pharamacological inhibitors and avoids damage to healthy brain tissue which occurs with surgical interventions. The size of the FUS-treatment area is correlated to the size of the focal spot of the transducer, with higher frequencies producing a sharper focal spot. Using higher frequencies, specific brain regions have been targeted for delivery of chemotherapeutics to a brain tumour or stem cells to the striatum or hippocampus [34,35]. However, FUS can also be used to target widespread pathology as in Alzheimer’s disease. In rodents, whole-hemispheres have been treated using lower frequency transducers and multiple sonications [36] and whole brains have been treated using unfocused ultrasound transducers [37]. Targeted FUS treatments have been shown to be possible at a variety of locations in preclinical models. Third, changes in BBB permeability following FUS treatment are transient and the BBB has been shown to functionally recover within 6 hours post-treatment. The literature has varied with respect to the time that the BBB remains open following FUS [31], however it is clear that in studies when ultrasound parameters that don’t result in damage are used, the BBB is closed in 6 hours and remains impenetrable for up to 4 weeks [19]. A limitation of research using FUS and microbubbles to open the BBB is that most of the FUS studies performed to date have been on healthy animals and thus further investigation is required in order to confirm that changes in BBB permeability occur on the same time course in models of disease.
5.2 Physical and Cellular Mechanisms
The precise physical and cellular mechanisms leading to transient opening of the BBB following treatment with FUS and microbubbles remain unclear. In general, the hypothesized mechanisms can be grouped into physical effects exerted on the blood vessel or cellular changes at the level of the BBB.
Using high intensity ultrasound, bubbles are formed in the tissue and undergo a violent collapse. The threshold for this inertial cavitation is variable and often leads to tissue damage and haemorrhage [17]. Since, the addition of microbubble contrast agent, low ultrasound powers have be used to induce cavitation, resulting in microbubble oscillation and growth [19]. This predictable oscillatory behaviour of microbubbles within an ultrasound field is known as ‘stable’ cavitation and is believed to be the cause of BBB opening. Microbubble oscillations generate mechanical stress when they interact with the vessel wall [38]. Using high speed microscopy, microbubble expansion and contraction has been shown to stretch the blood vessel wall with the contraction phase imparting the greatest stress on the vessel wall [39]. Oscillating microbubbles also generate circumferential and shear stresses, which contribute to the pressure on the microvessel [40]. The combination of these mechanical stresses may activate mechanosensitive ion channels in the endothelial cells leading to BBB opening. Similar to what occurs in the absence of microbubble contrast agent, if the pressure is too high, microbubbles collapse, causing high-velocity microjetting and extreme local temperature rise [40,41]. Analysis of the harmonic and ultraharmonic emissions suggest that bubble oscillation is associated with BBB opening and that broadband emission, indicative of microbubble collapse is correlated with red blood cell extravasation and tissue damage [24,33]. The impact of the vessel wall on microbubble behaviour was recently characterized and determined that the threshold for inertial cavitation and potential for damage depends on vessel size.
The physical stresses imparted on the vessels due to interactions between the microbubble and the ultrasound lead to cellular changes at the BBB. Electron microscopy was used to demonstrate that drugs and tracer molecules are able to traverse the BBB using both paracellular and transcellular routes after FUS treatment [42,43]. Labelled endogenous IgG was found inside vesicles of endothelial cells providing evidence that molecules pass transcellularly through the brain endothelium and accumulate in the brain. Further evidence in support of transcytosis as a method for drug delivery after FUS includes an increase in the cytoplasmic channels and vesicles. Caveolin, a protein integral to endocytosis, is upregulated following FUS treatment as demonstrated by immunohistochemistry and Western blot procedures [44,45].
In addition, physical stresses of FUS and microbubbles have lead to increases in the paracellular passage of molecules via opened tight junctions [42,43]. Reduced tight junction proteins have been detected using electron microscopy. In particular, significant reductions in occludin, claudin-5 and zonula occludens were observed at 1 and 2 hours post-FUS treatment. The levels of the proteins were returned to presonication levels by 4 hours confirming that the effects of FUS on the barrier are transient [43]. These data were consistent with the use of pharmacological inhibitors such as mannitol in which occludin, claudin 5 and zonula occludens were reduced alongside BBB opening. It is possible that FUS downregulates the expression of the proteins or that FUS simply alters protein organization causing a masking of the antigen [43]. In fact, a gap junction protein, Connexin 36, which binds to zonula occludens-1, has been shown to be downregulated following FUS [46]. The protein is responsible for communication between neurons and astrocytes and one hypothesis is that reorganization of the gap junctions are part of a response to changes in BBB permeability to help protect the brain [46].
The use of two-photon microscopy for characterization of BBB opening following FUS with microbubbles has provided new insights regarding the cellular mechanisms of BBB opening [47,48]. BBB permeability was further characterized into either ‘fast leakage’ and ‘slow leakage’ based on the temporal dynamics of the leakage of the fluorescent dye from the blood vessels [48]. Fast leakage was characterized as a rapid increase to peak intensity during FUS treatment with a decrease after FUS treatment was finished. Conversely, slow leakage began 5–15 min after the FUS treatment ended occurring along the length of a vessel instead of in a localized spot. The authors hypothesized that the two leakage types correlated to paracellular transport (fast leakage) and transcellular transport (slow leakage). If future studies could combine two-photon microscopy with acoustic information, the relationship between the microbubble behaviour and the leakage type could be elucidated.
6 Drug delivery and Therapeutic Effects
6.1 Drug Delivery in Rodent Models
FUS and microbubbles have been used to effectively deliver a variety of tracers and therapeutic agents across the BBB using rodent models. MRI is most commonly used as a method for targeting and evaluation of the treatment and therefore, delivery of paramagnetic agents have been widely studied. Gadolinium-based contrast agents (500–900 Da) are the most commonly delivered agents since they can be used to confirm the success of the FUS treatment and evaluate the size of the treated region [19]. The relative amount of enhancement observed after on a contrast-enhanced T1-weighted MR scan is proportional to the relative amount of BBB opening.
Trypan blue and Evan’s blue (~70kDa when bound to albumin in vivo), are commonly used to visualize the regions of BBB opening on histology. Other tracers used to confirm BBB opening after FUS include horseradish peroxidase [42,43], MION-47 [48], Dextran conjugated fluorescent molecules [47,48], lanthanum chloride and superparamagnetic iron oxide [50,51]
FUS and microbubble mediated opening of the BBB has applications in treatment of brain tumours. The use of widespread chemotherapeutic agents have been effective for treatment of peripheral tumours however the limited ability to penetrate the BBB reduces their effectiveness in the brain. Doxorubicin has been shown to reach therapeutic concentrations when delivered to the brain using FUS [35, 52]. Follow-up studies demonstrated that treatment of brain tumours using FUS and Doxorubicin decreases tumour burden and improves median survival times [53,54]. Herceptin, an antibody which targets the HER2/neu receptor overexpressed on ~30% of breast cancers, has been effective at treating the primary disease but, like Doxorubicin, is ineffective for treatment of metastases to the brain. FUS was shown to enhance the delivery of Herceptin [55] and when delivered by FUS, Herceptin significantly reduced tumour burden and improved survival times compared to Herceptin treatment alone [56]. Other chemotherapeutic agents including Methotrexate [57], 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU)[58] and epirubicin [59] have also been delivered using FUS and have been shown to have tumour-reducing effects in the brain. Other anti-cancer agents including natural killer cells that were targeted to HER2/neu were also able to be delivered to the brain using FUS to permeabilize the BBB [60]. Despite their large size, the immune cells were found to enter the brain at quantities sufficient for tumour reduction.
Outside of cancer applications, opening the BBB with FUS and microbubbles has proven to be an effective delivery method in models of neurodegenerative disease. As a potential treatment for Huntington’s disease, FUS was used to deliver, siRNA targeted to the mutant Huntingtin protein to the striatum of rats [61]. The siRNA delivery was effective, leading to an ~30% reduction of Huntingtin protein. This level of knockdown has been suggested to be sufficient for reducing symptoms associated with the disease. In a mouse model of Alzheimer’s disease, the extent of BBB opening was determined to be similar in Alzheimer’s transgenic mice compared to wildtype controls [47,62]. Delivery of amyloid antibodies using FUS [36,47] were found to significantly reduce plaque pathology after 4 days [36]. FUS-mediated delivery of neurotrophic factors including brain-derived neurotrophic factor (BDNF)[63], neurturin [63] and glial-derived neurotrophic factor (GDNF)[64] has been shown to result in levels high enough to induce neuroprotection and survival. Activation of downstream pro-survival pathways was observed following delivery of BDNF in vivo [63]. These therapies are important for treatment of neurodegenerative disease such as Alzheimer’s and Parkinson’s diseases in which growth factor levels are reduced. Another important therapeutic option currently being investigated is the use of neural stem cells as replacement cells in diseases characterized by neuronal loss. Even with their large size, neural stem cells were capable of crossing the BBB following FUS treatment [34]. Embryonic stem cells were delivered to the striatum and the hippocampus independently and were shown to differentiate after entry into the brain.
Finally, delivery vehicles for therapeutic agents including nanoparticles, viral vectors and microbubbles themselves, have gained access to the brain following FUS [63,65–68]. have been delivered to targeted areas of the brain and have a wide range of applications for treatment of brain diseases.
6.2 BBB Opening and Drug Delivery in Non-human Primates
FUS and microbubble mediated BBB opening has been performed in non-human primates. In studies completed by two different groups, BBB opening was achieved using FUS in the presence of commercially available and custom prepared microbubbles [68–70]. In one study, the non-human primates were sonicated using a commercially available hemispherical transducer array [71]. The design of the clinical transducer reduces the aberrations in the transmitted ultrasound waves created by the variations in skull thickness and density [72]. Non-human primates were treated in multiple locations at different treatment times [71] and were found to perform equally well before and after the treatments. Similar evidence was observed using a single element transducer for ultrasound delivery [69,70]. This evidence strongly supports the safety of FUS for BBB opening in the brain.
6.3 Therapeutic Effects of Ultrasound Alone
Several studies have been designed to characterize the response of the brain to FUS treatment and therefore have been performed in the absence of exogenous drug delivery. Recent studies suggest that BBB opening due to FUS and microbubbles may have therapeutic effects in the brain, even in the absence of drug delivery. Using a mouse model of Alzheimer’s disease, FUS-mediated BBB opening was able to reduce plaque burden even in the absence of amyloid antibodies [73]. Two potential mechanisms were investigated to explain the phenomenon. First, the level of endogenous immunoglobulins (IgG and IgM) were significantly increased in the FUS-treated cortex suggesting that immunoglobulins can increase clearance of amyloid. Second, activation of astrocytes and microglia were detected in FUS-treated regions, suggesting that activated glia may internalize the amyloid and promoting clearance. Follow-up studies relating BBB opening by FUS and microbubbles to changes spatial memory behaviour are currently being completed and show promising results. This new data would support the finding that increased neurogenesis was observed in mice receiving FUS and microbubble treatment [74]. Non-transgenic mice were administered bromodeoxyuridine (BrdU) a thymidine analog which incorporates into dividing DNA, following FUS treatment. In the neurogenic regions of the hippocampus, a significant number of new neurons were observed suggesting that FUS increases neurogenesis [74]. These data correlate to previous published work indicating that FUS activates the pro-survival Akt signalling pathway [75]. Furthermore, it has been suggested that FUS without microbubbles, can increase the expression of BDNF, a neurotrophic factor which supports cell survival and differentiation [63,76]. FUS has been shown to induce neuronal activation when applied in the absence of microbubbles [76–79]. Together these data suggest that BBB opening by FUS and microbubbles may have beneficial effects in the brain in addition to its role as an effective method for drug delivery.
7 Clinical Translation of FUS Technology
Translation of FUS from rodents to the clinic presents challenges due to differences in skull shape and thickness. Clinical application of FUS will require the use of a hemispherical phased array transducer which reduces the potential for skull heating by distributing the ultrasound energy over the entire skull surface. The hemispherical arrays have a geometric focus that can be electronically steered by controlling the phase of the radiofrequency signal driving the elements in order to open the BBB in regions away from the midline [72]. Applying the ultrasound at low frequency reduces the ultrasound wave distortion induced by the skull [72]. The commercially available clinical prototype devices are available at two frequencies, 220kHz and 650kHz (Exablate 4000, Insightec, Israel). The lower frequency device has been used for effective BBB opening in nonhuman primates [71]. Using the clinical device, multiple brain regions have been targeted and sonciated in a non-human primate model with no adverse effects on behavior. These studies have demonstrated that FUS, as a method for BBB opening, is ready for clinical testing in humans.
8 Safety and Treatment Monitoring
MRI has served as the primary method for evaluating BBB opening and the effectiveness of the FUS treatment [19] however MRI is limited because it can only be performed post-treatment. In an effort to provide real-time monitoring of the FUS treatment, several groups have developed methods which detect and monitor the acoustic emissions from the microbubbles [80–82]. In particular, the development of a real-time, acoustic feedback controller program has changed FUS treatments by enabling the active monitoring, and control of the BBB opening [33]. During the sonications, the controller increases the applied ultrasound pressure in a step-wise fashion with each transmitted pulse. A hydrophone, positioned to monitor the focal spot, receives the spectral information after each pulse. The spectral information is analyzed and when the presence of ultraharmonic emissions are detected, the algorithm reduces the pressure to a user-defined percentage. In rodent models, a reduction of the pressure to 50% of the peak pressure reached has been shown to result in BBB opening but without the presence of red blood cell extravasation or other signs of associated damage [33]. The controller can be combined with a novel multi-receiver method that allows microbubble localization with super resolution and perhaps control the activity at the level of a single bubble [83]. The implementation and testing of a controller system into a clinical array would allow the operator to monitor and control the FUS treatment thereby improving the safety of clinical FUS treatments.
9 Future Directions
Despite the significant advances in FUS over the last decade, there are still hurdles for this promising technology to overcome before it can be widely used in the clinic. Before repeated applications of FUS can be considered a viable technique to treat chronic diseases, further quantified research into monitoring drug concentrations in the brain will be necessary. Quantifying delivered drug concentrations will ensure therapeutic concentrations are achieved in the brain and prevent toxicity associated with high concentrations in the periphery. These questions may be able to be addressed by the addition of tracer agents which can be monitored by MRI but the suitability of these agents needs to be addressed in future preclinical studies. Furthermore, the intravenous drug concentrations required to achieve therapeutic dose in the brain after FUS-treatment may need to be adjusted from the dosages currently used clinically. It will be important to closely monitor the potential peripheral toxicity of these agents as dosages are altered.
The incidence of brain diseases which include cancer, vascular diseases, neurodegenerative diseases, psychiatric illnesses and others, are on the rise. Globally, brain diseases serve as a growing source of emotional, practical and financial burden as the race to find effective treatments continue. While there is much work to be done to move FUS-mediated BBB opening to widespread clinical use, the studies completed thus far indicate that the technology represents a promising strategy that will be integral to the future treatment of brain disease.
10 Expert Opinion
There is considerable evidence demonstrating the efficacy of FUS as a method for drug delivery into the brain. Furthermore, using several different rodent models of disease, it has been demonstrated that FUS results in accumulation of therapeutic concentrations of drugs at the target region leading to predicted bioeffects such as reductions in tumour size or Alzheimer’s plaque pathology [36,53,54]. However, the acceptance of FUS as an alternative method for drug delivery has been slow and we believe this is due to several factors which are currently being addressed in the literature. In particular, we believe it is important to address the effects of FUS on a BBB compromised by pathology and to demonstrate using non-human primate models that the promising data reported in rodents can be achieved in a more-clinically relevant model.
For many decades, it has been known that the presence of a functioning barrier is necessary for maintaining homeostasis required for proper function of neuronal circuitry [1,3]. Adding to this, the breakdown of the BBB has been hypothesized to precipitate diseases such as Multiple Sclerosis and Alzheimer’s disease [2]. Together, we understand that these data warrant caution when proposing to open the BBB for therapeutic purposes. Recent work has begun to address these concerns as more studies are being aimed at investigating the effects of BBB opening on the brain in the absence of drug delivery [19,69–71,73,75]. In general, these studies have collectively been unable to demonstrate any adverse side effect associated with transient BBB opening in the healthy brain. In some cases, opening of the BBB in rodent models of disease may actually lead to improvements in pathology and behaviour, even in the absence of exogenous drug delivery [73,74]. FUS is advantageous over other methods that transiently open the BBB since it can be targeted to a small focal volume. When employed as a treatment strategy, this means that only areas of the brain that exhibit pathology (tumours, plaques, inclusions and other pathology) would be exposed to BBB opening and the remaining healthy brain regions will not receive the therapeutic agent.
The differences between the rodent and primate brain have led to problems with clinical translation of other treatments however recent studies performing FUS-mediated BBB opening in non-human primates suggest this isn’t the case with FUS. Studies completed by groups from Harvard and Columbia show data from non-human primates who were sonicated repeatedly at different locations in the brain [69,71]. The studies used different transducers to deliver the ultrasound, including the clinically available transducer array (Exablate 4000; Insightec, Haifa, Israel), and show no evidence of histological or functional damage after FUS treatments. These findings from non-human primates, combined with data gathered from rodent models, suggests that the transient breakdown of the BBB with FUS does not lead to deleterious effects in brain tissue. The mounting evidence in support of the safety of transient, localized BBB opening may lead to an adjustment in conventional thought whereby in brain disease, the BBB is no longer thought of as a protective barrier but as a temporary obstacle to overcome for effective treatment.
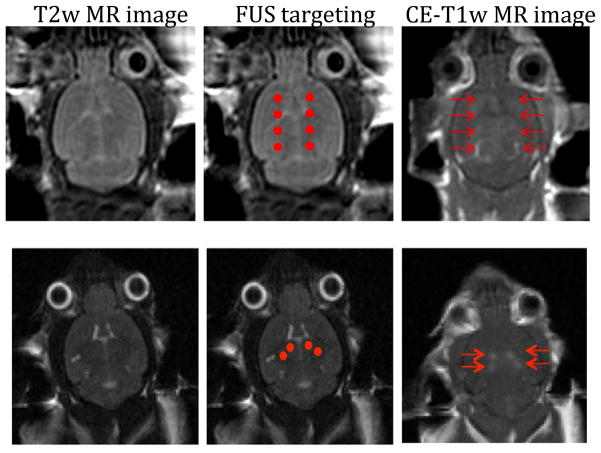
Figure 2.
MRI has been the primary imaging modality for targeting and evaluating FUS treatments. Top panel – MR images from a mouse brain were taken using a 3.0T MR scanner (GE Healthcare). T2 weighted images were used to choose 4 sonication points in each hemisphere (red dots). Contrast enhanced T1 weighted images were used to confirm BBB opening had occurred in the target locations (red arrows).
Bottom panel – MR images from a mouse brain were taken using a 7.0T MR scanner (Bruker). T2 weighted images were used to target the bilateral hippocampus using 2 sonciations in each hemisphere (red dots). T1 weighted images were used to confirm BBB opening had occurred on target (red arrows).
Table 1.
Summary of the advantages and disadvantages of current methods used to circumvent the BBB for drug delivery to the brain
| Strategy | Advantages | Disadvantages |
|---|---|---|
| Intracranial injection |
|
|
| Drug Modification |
|
|
| Intranasal Delivery |
|
|
| Global BBB disruption |
|
|
| Focused Ultrasound |
|
|
Article Highlights.
Focused ultrasound (FUS) combined with microbubbles increases the permeability of the blood-brain barrier (BBB) and improves drug delivery to the brain, leading to reduced pathology and increased survival in preclinical models of disease.
FUS-mediated increases in BBB permeability have been shown to have beneficial effects on the brain even in absence of exogenous drug delivery.
Feedback systems and real-time monitoring techniques have been developed which improve the safety of BBB opening.
The technology required for effective clinical translation of FUS for drug delivery is currently available.
Acknowledgments
Support for this work was provided by grants awarded by the National Institutes of Health (R01 EB003268) and the Canadian Institutes for Health Research (FRN 119312).
Annotated References
- 1.Abbott NJ, Patabendige AA, Doman DE, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2004;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2006;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 3.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 4•.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. This review clearly states the problem of the blood-brain barrier in development of neurotherapeutics and covers the strategies used to overcome the barrier for improved drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustavsson A, Svensson M, Jacobi F, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–79. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Marks WJ, Jr, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open label, phase I trial. Lancet Neurol. 2008;7:400–8. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 7.Gross RE, Watts RL, Hauser RA, et al. Intrastriatal transplantation of microcarrier-bound human retinal pigment epithelial cells versus sham surgery in patients with advanced Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2011;10:509–19. doi: 10.1016/S1474-4422(11)70097-7. [DOI] [PubMed] [Google Scholar]
- 8.Matsukado K, Sugita M, Black KL. Intracarotid low dose bradykinin infusion selectively increase tumor permeability through activation of bradyknin. Brain Res. 1998;792:10–5. doi: 10.1016/s0006-8993(97)01502-3. [DOI] [PubMed] [Google Scholar]
- 9.Rapoport SI. Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Expert Opin Investig Drugs. 2001;10:1809–18. doi: 10.1517/13543784.10.10.1809. [DOI] [PubMed] [Google Scholar]
- 10.Pardridge WM, Boado RJ. Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier. Methods Enzymol. 2012;503:269–92. doi: 10.1016/B978-0-12-396962-0.00011-2. [DOI] [PubMed] [Google Scholar]
- 11.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–72. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakay L, Ballantine HT, Jr, Hueter TF, Sosa D. Ultrasonically produced changes in the blood-brain barrier. AMA Arch Neurol Psychiatry. 1956;76:457–67. doi: 10.1001/archneurpsyc.1956.02330290001001. [DOI] [PubMed] [Google Scholar]
- 14.Shealy CN, Crafts D. Selective alteration of the blood-brain barrier. J Neurosurg. 1965;23:484–7. doi: 10.3171/jns.1965.23.5.0484. [DOI] [PubMed] [Google Scholar]
- 15.Ng KY, Cho CW, Henthorn TK, Tanguay RL. Effect of heat preconditioning on the uptake and permeability of R123 in brain microvessel endothelial cells during mild heat treatment. J Pharm Sci. 2004;93:896–907. doi: 10.1002/jps.20015. [DOI] [PubMed] [Google Scholar]
- 16.McDannold N, Vykhodsteva N, Jolesz FA, Hynynen K. MRI investigation of the threshold for thermally induced blood-brain barrier disruption and brain tissue damage in the rabbit. Magn Reson Med. 2004;51:913–23. doi: 10.1002/mrm.20060. [DOI] [PubMed] [Google Scholar]
- 17.Vykhodtseva N, Hynynen K, Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med Biol. 1995;21:969–979. doi: 10.1016/0301-5629(95)00038-s. [DOI] [PubMed] [Google Scholar]
- 18.Mesiwala AH, Farrell L, Wenzel HJ, et al. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med Biol. 2002;28:389–400. doi: 10.1016/s0301-5629(01)00521-x. [DOI] [PubMed] [Google Scholar]
- 19•.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. This was the initial study using microbubbles and reduced ultrasound power to cause safe, reproducible blood-brain barrier opening. [DOI] [PubMed] [Google Scholar]
- 20.Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 2010;50:221–229. doi: 10.1016/j.ultras.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Liu HL, Pan CH, Ting CY, Hsiao MJ. Opening of the blood-brain barrier by low-frequency (28kHz) ultrasound: a novel pinhole assisted mechanical scanning device. Ultrasound Med Biol. 2010;36:325–35. doi: 10.1016/j.ultrasmedbio.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Bing KF, Howles GP, Qi Y, et al. Blood-brain barrier (BBB) disruption using a diagnostic ultrasound scanner and Definity in mice. Ultrasound Med Biol. 2009;35:1298–308. doi: 10.1016/j.ultrasmedbio.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med Biol. 2008;34:834–40. doi: 10.1016/j.ultrasmedbio.2007.10.016. This study describes the relationship between frequency and pressure as they relate to the probability of BBB opening with focused ultrasound. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDannold N, Vykhodtseva N, Hynynen Effects of acoustic parameters and ultrasound. Ultrasound Med Biol. 2008;34:930–7. doi: 10.1016/j.ultrasmedbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi JJ, Selert K, Gao Z, et al. Noninvasive and localized blood-brain barrier disruption using focused ultrasound can be achieved at short pulses lengths and low repetition frequencies. J Cereb Blood Flow Metab. 2011;31:725–37. doi: 10.1038/jcbfm.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Reilly MA, Waspe AC, Ganguly M, Hynynen K. Focused ultrasound disruption of the blood-brain barrier using closely-timed short pulses: influence of sonication parameters and injection rate. Ultrasound Med Biol. 2010;37:587–94. doi: 10.1016/j.ultrasmedbio.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goertz DE, Wright C, Hynynen K. Contrast agent kinetics in the rabbit brain during exposure to therapeutic ultrasound. Ultrasound Med Biol. 2010;36:916–24. doi: 10.1016/j.ultrasmedbio.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with Definity for targeted blood-brain barrier disruption; a feasibility study. Ultrasound Med Biol. 2007;33:584–90. doi: 10.1016/j.ultrasmedbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang FY, Fu WM, Chen WS, et al. Quantitative evaluation of the use of microbubbles with transcranial focused ultrasound on blood-brain barrier disruption. Ultrason Sonochem. 2008;15:636–643. doi: 10.1016/j.ultsonch.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Weng JC, Wu SK, Yang FY, Tseng WY. Pulse sequence and timing of contrast-enhanced MRI for assessing blood-brain barrier disruption after transcranial focused ultrasound in the presence of haemorrhage. J Magn Reson Imaging. 2010;31:1323–30. doi: 10.1002/jmri.22174. [DOI] [PubMed] [Google Scholar]
- 31.Choi JJ, Feshitan JA, Baseri B, et al. Microbubble-size dependence of focused ultrasound-induced blood-brain barrier opening in mice in vivo. IEEE Trans Biomed Eng. 2010;57:145–54. doi: 10.1109/TBME.2009.2034533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlachos F, Tung YS, Konofagou EE. Permeability dependence study of the focused ultrasound-induced blood-brain barrier opening at distinct pressures and microbubble diameters using DCE-MRI. Mag Reson Med. 2011;66:821–30. doi: 10.1002/mrm.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.O’Reilly MA, Hynynen K. Blood-brain barrier: Real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller. Radiology. 2012;263:96–106. doi: 10.1148/radiol.11111417. This study discusses the development of a real-time acoustic controller which controls the ultrasound exposure thereby increasing the safety of the BBB treatments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgess A, Ayala-Grosso CA, Ganguly M, et al. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6:e27877. doi: 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Treat LH, McDannold N, Vykhodtseva N, et al. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. International journal of cancer. 2007;121:901–7. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 36.Jordão JF, Ayala-Grosso CA, Markham K, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS One. 2010;5:e10549. doi: 10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howles GP, Bing KF, Qui Y, et al. Contrast-enhanced in vivo magnetic resonance microscopy of the mouse brain enabled by noninvasive opening of the blood-brain barrier with ultrasound. Magn Reson Med. 2010;64:995–1004. doi: 10.1002/mrm.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosseinkhah N, Hynynen K. A three-dimensional model of an ultrasound contrast agent gas bubble and its mechanical effects on microvessels. Physics Med Biol. 2012;57:785–808. doi: 10.1088/0031-9155/57/3/785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen PY, Liu HL, Hua MY, et al. Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro Oncol. 2010;12:1050–60. doi: 10.1093/neuonc/noq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nyborg WL. Biological effects of ultrasound: development of safety guidelines. Part II: general review. Ultrasound Med Biol. 2001;27:301–33. doi: 10.1016/s0301-5629(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 41.Deng CX, Sieling F, Pan H, Cui J. Ultrasound-induced cell membrane porosity. Ultrasound Med Biol. 2004;30:519–526. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 42•.Sheikov N, McDannold N, Vykhodtseva N, et al. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 2004;30:979–89. doi: 10.1016/j.ultrasmedbio.2004.04.010. This study used electorn microscopy to demonstrate that tracer molecules pass through the tight junctions and are taken up in vesicles following BBB opening with focused ultrasound. [DOI] [PubMed] [Google Scholar]
- 43.Sheikov N, Mcdannold NJ, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 2008;34:1093–1104. doi: 10.1016/j.ultrasmedbio.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lionetti V, Fittipaldi A, Agostini S, et al. Enhanced caveolae-mediated endocytosis by diagnostic ultrasound in vitro. Ultrasound Med Biol. 2009;35:136–43. doi: 10.1016/j.ultrasmedbio.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Deng J, Huang Q, Wang F, et al. The role of caveolin-1 in blood-brain barrier disruption induced by focused ultrasound combined with microbubbles. Journal of molecular neuroscience. 2012;46:677–87. doi: 10.1007/s12031-011-9629-9. [DOI] [PubMed] [Google Scholar]
- 46.Alonso A, Reinz E, Fatar M, et al. Clearance of albumin following ultrasound-induced blood-brain barrier opening is mediated by glial but not neuronal cells. Brain research. 2011;1411:9–16. doi: 10.1016/j.brainres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Raymond SB, Treat LH, Dewey JD, et al. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer’s disease mouse models. PloS one. 2008;3:e2175. doi: 10.1371/journal.pone.0002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho EE, Drazic J, Ganguly M, et al. Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced blood–brain barrier opening. J Cereb Blood Flow Metab. 2011;31:1852–1862. doi: 10.1038/jcbfm.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hynynen K, McDannold N, Vykhodtseva N, et al. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105:445–54. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 50.Liu HL, Hsu PH, Chu PC, et al. Magnetic resonance imaging enhanced by superparamagnetic iron oxide particles: usefulness for distinguishing between focused ultrasound-induced blood-brain barrier disruption and brain hemorrhage. J Mag Reson Imaging. 2009;29:31–8. doi: 10.1002/jmri.21599. [DOI] [PubMed] [Google Scholar]
- 51.Liu HL, Wai YY, Hsu PH, et al. In vivo assessment of macrophage CNS infiltration during disruption of the blood-brain barrier with focused ultrasound: a magnetic resonance imaging study. J Cereb Blood Flow Metab. 2010;30:177–86. doi: 10.1038/jcbfm.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan CH, Ting CY, Lin HJ, et al. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials. 2013;34:3706–15. doi: 10.1016/j.biomaterials.2013.01.099. [DOI] [PubMed] [Google Scholar]
- 53.Treat LH, McDannold N, Zhang Y. Improved Anti-Tumor Effect of Liposomal Doxorubicin after Targeted Blood-Brain Barrier Disruption by MRI-Guided Focused Ultrasound in Rat Glioma. Ultrasound Med Biol. 2012;38:1716–25. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aryal M, Vykhodtseva N, Zhang YZ. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J Control Release. 2013;169:103–11. doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proceedings Natl Acad Sci USA. 2006;103:11719–23. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Controlled Release. 2012;163:277–84. doi: 10.1016/j.jconrel.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mei J, Cheng Y, Song Y, et al. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med. 2009;28:871–80. doi: 10.7863/jum.2009.28.7.871. [DOI] [PubMed] [Google Scholar]
- 58.Cho CW, Liu W, Cobb N, et al. Ultrasound induced mild hyperthermia as a novel approach to increase drug uptake in brain microvessel endothelial cells. Pharm Res. 2002;19:1123–29. doi: 10.1023/a:1019837923906. [DOI] [PubMed] [Google Scholar]
- 59.Liu HL, Hua MY, Yang HW, et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci USA. 2010;107:15205–10. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alkins R, Burgess A, Ganguly M, et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73:1892–1899. doi: 10.1158/0008-5472.CAN-12-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burgess A, Huang Y, Querbes W, et al. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J Controlled Release. 2012;163:125–9. doi: 10.1016/j.jconrel.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi JJ, Wang S, Brown TR, Small SA, Duff KE, Konofagou EE. Noninvasive and transient blood-brain barrier opening in the hippocampus o Alzheimer’s double transgenic mice using focused ultrasound. Ultrasonic Imaging. 2008;30:189–200. doi: 10.1177/016173460803000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baseri B, Choi JJ, Deffieux T. Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood-brain barrier using focused ultrasound and microbubbles. Phys Med Biol. 2012;57:65–81. doi: 10.1088/0031-9155/57/7/N65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang F, Shi Y, Lu L, et al. Targeted delivery of GDNF through the blood–brain barrier by MRI-guided focused ultrasound. PLoS One. 2012;7:e52925. doi: 10.1371/journal.pone.0052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Etame AB, Diaz RJ, O’Reilly MA, et al. Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomed. 2012;8:1133–1142. doi: 10.1016/j.nano.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thévenot E, Jordão JF, O’Reilly MA, et al. Targeted delivery of scAAV9 to the brain using MRI-guided focused ultrasound. Human Gene Ther. 2012;23:1144–55. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu PH, Wei KC, Huang CY, et al. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One. 2013;8:e57682. doi: 10.1371/journal.pone.0057682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Timbie K, Burke C, Nance E, et al. Ultrasound-targeted delivery of systemically administered therapeutic nanoparticles. J Acoust Soc Am. 2013;134:4047. [Google Scholar]
- 69.Tung YS, Marquet F, Teichert T, et al. Feasibility of noninvasive cavitation-guided blood-brain barrier opening using focused ultrasound and microbubbles in nonhuman primates. Applied Phys Lett. 2011;98:163704. doi: 10.1063/1.3580763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Marquet F, Tung YS, Teichert T, et al. Noninvasive, transient and selective blood-brain barrier opening in non-human primates in vivo. PLoS One. 2011;6:e22598. doi: 10.1371/journal.pone.0022598. This study was the first published evidence that focused ultrasound could be performed in non-human primates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72:3652–63. doi: 10.1158/0008-5472.CAN-12-0128. In this study, repeated sonications across several brain regions were preformed in non-human primates using a clinical transducer. The non-human primates did not display changes on visual and cognitive testing demonstrating the safety of the BBB opening procedure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Hynynen K, Clement GT, McDannold N, et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: a preliminary rabbit study with ex vivo human skulls. Magn Reson Med. 2004;52:100–107. doi: 10.1002/mrm.20118. This study describes the development of a prototype hemisphere transducer array which allows focused ultrasound technology to be translated into the clinic. [DOI] [PubMed] [Google Scholar]
- 73.Jordão JF, Thévenot E, Markham-Coultes K, et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exper Neurol. 2013;248:16–29. doi: 10.1016/j.expneurol.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scarcelli T, Jordão JF, Ellens N, et al. Effects of transcranial focused ultrasound on hippocampal neurogenesis in adult mice. Brain Stimulation. 2014 doi: 10.1016/j.brs.2013.12.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jalali S, Huang Y, Dumont DJ, Hynynen K. Focused ultrasound-mediated BBB disruption is associated with an increase in activation of AKT: experimental study in rats. BMC Neurology. 2010;10:114. doi: 10.1186/1471-2377-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tufail Y, Matyushov A, Baldwin N, et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–94. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 77.Tyler WJ, Tufail Y, Finsterwald M, et al. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS One. 2008;3:e3511. doi: 10.1371/journal.pone.0003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Legon W, Rowlands A, Opitz A, et al. Pulsed ultrasound differentially stimulates somatosensory circuits in humans as indicated by EEG and FMRI. PLoS One. 2012;7:e51177. doi: 10.1371/journal.pone.0051177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deffieux T, Younan Y, Wattiez N, et al. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Cuur Biol. 2013 doi: 10.1016/j.cub.2013.10.029. published online 2013-10-29. doi:10.1016etal.2013. [DOI] [PubMed] [Google Scholar]
- 80.Arvanitis CD, Livingstone MS, Vykhodtseva N, McDannold N. Controlled ultrasound-induced blood-brain barrier disruption using passive acoustic emissions monitoring. PLoS One. 2012;7:e45783. doi: 10.1371/journal.pone.0045783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaye EA, Chen J, Pauly KB. Rapid MR-ARFI method for focal spot localization during focused ultrasound therapy. Magn Reson Med. 2011;65:738–43. doi: 10.1002/mrm.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones RM, O’Reilly MA, Hynynen K. Transcranial passive acoustic mapping wtih hemispherical sparse arrays using CT-based skull specific aberration corrections: a simulation study. Phys Med Biol. 2013;58:4981–5005. doi: 10.1088/0031-9155/58/14/4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Reilly MA, Jones RM, Hynynen K. Transcranial bubble activity mapping for therapy and imaging. J Acous Soc Am. 2013:1343975. [Google Scholar]